Abstract
Acquired dyslexia offers a unique window on to the nature of the cognitive and neural architecture supporting skilled reading. This paper provides an integrative overview of recent empirical and computational work on acquired dyslexia within the context of the primary systems framework as implemented in connectionist neuropsychological models. This view proposes that damage to general visual, phonological or semantic processing abilities are the root causes of different forms of acquired dyslexia. Recent case-series behavioural evidence concerning pure alexia, phonological dyslexia and surface dyslexia that supports this perspective is presented. Lesion simulations of these findings within connectionist models of reading demonstrate the viability of this approach. The commitment of such models to learnt representations allows them to capture key aspects of performance in each type of acquired dyslexia, particularly the associated non-reading deficits, the role of relearning and the influence of individual differences in the premorbid state of the reading system. Identification of these factors not only advances our understanding of acquired dyslexia and the mechanisms of normal reading but they are also relevant to the complex interactions underpinning developmental reading disorders.
Keywords: reading, primary systems, connectionist neuropsychology, pure alexia, phonological dyslexia, surface dyslexia
1. Introduction
Your ability to read the words on this page must rely upon your capacity to process their component letters and then access their associated sounds and meanings. This seemingly effortless process relies on a complex set of cognitive processes housed within a distributed set of neural regions [1]. Reading is of course an essential skill in modern society, and hence the mechanisms underpinning skilled reading have been the subject of much behavioural research, which has led to the development of detailed computational models of the reading process [2,3]. Yet there is still ongoing debate about the fundamental assumptions embodied by competing perspectives [4–7]. In recent times, neuroimaging data have been applied to attempt to support different theoretical perspectives on reading [8–10], but to date this has proved less than decisive. This is at least partly because, while data from normal neuroimaging can reveal what processes are involved in skilled reading aloud, they cannot determine which aspects are necessary. In this sense, data from acquired dyslexia, which are disorders of reading in previously literate individuals as a result of brain damage, constitute a unique and essential source of evidence to enhance our understanding of the reading system.
Through considering the striking reading deficits observed after different kinds of lesions, we can see what elements are necessary for fluent reading and where these processes are housed in the brain. While a number of models can account for basic facts about acquired dyslexia, recent case-series data have indicated that these disorders are in fact more multi-faceted, dynamic and variable than previously thought [11–13]. This paper aims to demonstrate that these case-series data on acquired dyslexia can be best accommodated within connectionist models. Lesioning of these models to capture the effects of brain damage is termed connectionist neuropsychology, an approach that has been most thoroughly developed in the context of acquired dyslexia. This paper provides an integrative overview of this work with reference to three well-studied forms of acquired dyslexia, namely pure alexia, phonological dyslexia and surface dyslexia. For each type of dyslexia, a theme emerges that relates to the assumptions and capabilities of connectionist neuropsychology, namely domain specificity, recovery and relearning, and individual differences in the premorbid reading system. Each of these themes relates directly to the promise of connectionist models to capture key aspects of developmental reading disorders.
2. The primary systems view
Connectionist models of reading aloud are framed within the primary systems view of acquired reading disorders [14]. This approach proposes that, as reading is a phylogenetically and ontogenetically late acquired ability, the mechanisms supporting literacy must rely upon more basic underlying representational systems. By this account, letters are simply a particular class of visual stimuli with specific perceptual requirements [15]. The process of learning to read requires that these novel symbols are mapped onto existing representations of speech sounds [3], which themselves emerge from the interplay of acoustics and articulation [16], and onto semantic knowledge, which is developed via detection of higher level correlations between the features of concepts across different modalities [17]. When brain damage impinges upon any of these primary visual, phonological or semantic systems, then a particular form of acquired dyslexia will result.
In the case of damage to areas supporting visual processing, the resultant disorder is pure alexia, as high-acuity visual information is particularly relevant to the demands of reading. This contrasts with the attribution of this disorder to disconnection of reading-specific orthographic representations [18]. In the case of damage to areas supporting phonological processing, the resultant disorder is phonological dyslexia, as subword phonology is particularly important when pronouncing novel strings of phonemes. This interpretation differs from models that attribute this to a selective deficit of reading-specific subword mappings from graphemes to phonemes [19]. Lastly, damage to areas representing semantic knowledge produces surface dyslexia, because meaning supports the reading aloud of words with exceptional spelling-to-sound mappings. This perspective challenges the attribution of this disorder to damage to reading-specific orthographic lexical representations [4].
It is a clear prediction of the primary systems view that the areas damaged in patients suffering from pure alexia, phonological and surface dyslexia should map onto those areas known to be involved in vision, phonology and semantics, respectively, as identified by functional neuroimaging of normal participants. This leads to the behavioural prediction that an association should be observed between the extent of impairments in visual, phonological or semantic processing and the degree of the reading deficit. To assess these kinds of relationships requires a case-series approach, where the performance of a number of patients across a variety of comparable assessments is considered [20–22].
3. Connectionist neuropsychology
The commitment of connectionist neuropsychology of dyslexia to the primary systems framework means that there is a focus on associations between patient performance on different tasks that contrasts with a more traditional neuropsychological emphasis on dissociation between abilities within a single case. Associations have traditionally been distrusted in cognitive neuropsychology as they may arise from the anatomical contiguity of regions supporting particular processes rather than to any functional link between the processes supporting task performance [4,23,24]. While this is certainly a valid concern, with recent increases in the availability of patient neuroimaging and advances in our understanding of the functional neuroanatomy of the language processing system [1,25], this interpretation of associations can now often be discounted. It should be emphasized that the connectionist neuropsychological focus on association does not entail disregarding dissociations, but rather that their relative frequency is important in guiding interpretation concerning functional independence of underlying cognitive components.
In addition to a different methodological emphasis, connectionist neuropsychology also challenges a number of key assumptions of the traditional cognitive neuropsychological approach [2]. The first challenge is to the assumption of modularity, both functional and structural. One of the defining features of modularity is that cognitive components are domain specific [26], hence with respect to reading, this entails a model that includes elements devoted to the processing of letter strings [2]. This contrasts with the notion of domain generality that is at the heart of the primary systems account of reading disorders. Connectionist models assume that the components of the cognitive and neural architecture respond to multiple different types of stimuli, such as words and faces [15]. While this approach does incorporate a degree of learned specialization within the system, this is graded and corresponds to the processing demands associated with particular characteristics of the input, rather than mapping categorically on to its domain.
Another key assumption in traditional neuropsychology is that of subtractivity [2,27], whereby it is assumed that the only effect of brain damage is to remove a component of the normal system. Yet the highly interactive and dynamic nature of the reading system makes this assumption unlikely to be true in most cases. In acquired dyslexia, it is rarely the case that we simply see an isolated deficit for a particular word type. Rather, the reading deficit is often accompanied by an upregulation of other aspects of the reading system. Nor is acquired dyslexia a static disorder, as we know that even in the case of a punctate damage event, for example stroke, there is significant recovery and relearning. Similarly, in dyslexia arising from dementia, there is a continuing decline over time. Connectionist models are well placed to capture these changes, as processing in such models is both interactive and dynamic, and indeed the changes that occur in the operation of the reading system in acquired dyslexia can be extremely illuminating.
The final key assumption of cognitive neuropsychology is that of uniformity of the cognitive architecture across different individuals [2]. With the increasing availability of patient neuroimaging, we can now start to appreciate that the consequences of a similar lesion are quite variable across cases. In the domain of reading, connectionist neuropsychology has provided an account of some of this variation in terms of premorbid state determining the nature of post-damage deficits observed. Connectionist models can address all of these aspects of acquired dyslexia that challenge traditional assumptions because these models rely upon learnt, and hence flexible, processing. While the data can be simulated within static localist models, this provides a re-description of the phenomenon rather than an explanation in terms of more general properties of the reading system [5,28].
4. Pure alexia
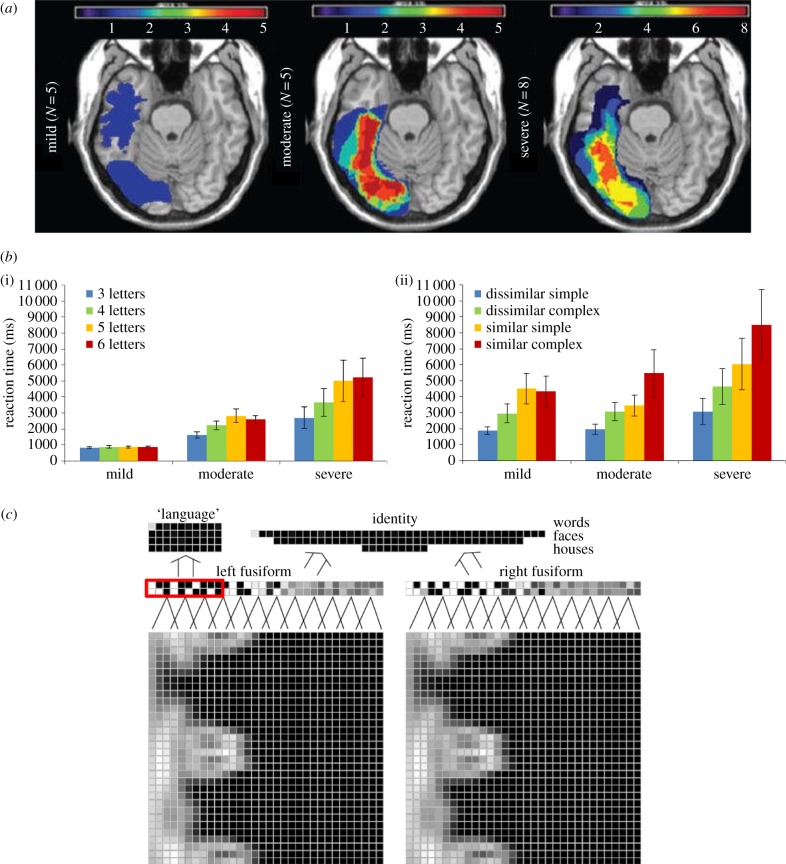
The defining feature of pure alexia is markedly slow reading aloud combined with an abnormally large effect of the number of letters. The neural damage underpinning this disorder encompasses the posterior portion of the fusiform gyrus (pFG) in the left hemisphere (figure 1a) which overlaps with the area activated in normal participants when viewing words relative to viewing checkerboards [30]. As can be seen in figure 1b(i), patients with a severe reading disorder as a result of pFG lesions show very slow reading speeds that are unduly affected by letter length, in contrast to the rapid reading and negligible length effects shown by normal participants [31]. Although stroke lesions are variable and often extend beyond the pFG, voxel-based lesion symptom mapping has revealed that the length effect in reading is indeed associated with damage specifically to the pFG [11]. In terms of theoretical accounts of this disorder, debates have centred around the domain specificity of the functional cause, with some accounts proposing damage to reading-specific cognitive components housed in pFG, whereas the connectionist primary systems perspective holds that the disorder arises as a consequence of the involvement of this area in the kind of visual processing that is particularly salient for word reading.
Figure 1.
(a) Lesion overlap maps for the case series of patients with left posterior fusiform gyrus lesions according to the severity of their reading disorders (slope of the length effect) from [11], with the scale representing the number of participants out of the total in that group with lesions in a given area. (b) For the same patients, reading performance according to word length (i) and matching performance for unfamiliar Kanji symbols according to complexity and foil similarity (ii) from [11]. (c) The connectionist model from [29], which accepts retinotopic visual input (bottom layer of units, within each block, central on left and peripheral on right), with red indicating the set of units where the damage produced deficits in both word and face processing.
(a). Domain specificity
There is clear agreement across functional imaging studies that the left pFG is consistently activated in response to words, although there has been considerable debate about the extent to which this activation is specific to these stimuli [32,33]. The performance of pure alexic patients has in fact been one of the strongest forms of evidence for the modality specificity of this region, because as the name suggests, such patients are portrayed as having a highly selective deficit in the processing of letter strings, without concomitant disorders of spelling (dysgraphia) or spoken language processing (aphasia) [32,34]. Careful evaluation of this claim in light of recent evidence, however, suggests quite a different interpretation, more consistent with the connectionist primary systems account in terms of a more general visual deficit.
Pure alexia is unusual among the acquired dyslexias in that it is defined in terms of speed, rather than accuracy, of reading. Of those reports of pure alexic patients who had intact processing of non-linguistic stimuli, many assessed performance purely in terms of accuracy, and hence likely lacked sensitivity to more general visual processing deficits. Indeed, in one of the first direct considerations of this issue, Behrmann et al. [35] found five pure alexic patients to be abnormally slow in the naming of pictures, but only when those pictures were visually complex. Similar deficits have been observed when processing entirely novel stimuli, as visual processing deficits may be underestimated when using familiar stimuli that offer top-down support. Mycroft et al. [36] found in a case series of seven pure alexic patients that there were impairments in processing strings of novel letter-like visual symbols. Moreover, they explored the ability of these patients to match checkerboards and found them to show a disproportionate impact of the complexity of the stimulus, relative to normal participants.
More recently, in the largest case-series study of this issue to appear to date, Roberts et al. [11] considered the performance of 20 patients with pFG damage both in reading aloud and other visual processing tasks. Not only did we find slowed object naming and spoken word-to-picture matching, we additionally demonstrated that performance on these tasks was quantitatively related to the extent of the reading deficit as measured by the size of the length effect in reading reaction times. This visual deficit extended to matching performance for novel non-linguistic stimuli, including both checkerboards and Kanji characters (figure 1b(ii)), and was most apparent in those cases where the stimuli were complex and the distractors were similar. Moreover, it was the reaction times for these complex stimuli with similar distractors that were most strongly related to the length effect in reading aloud. These associations between performances on different types of visual stimuli are precisely what would be predicted according the primary systems account.
The pattern of visual processing deficits seen across these studies also provides a suggestion as to the underlying mechanism of impairment. Words are a very particular type of visual stimuli, in that they are a small and easily confusable set of symbols that draw on high spatial frequency information. Indeed, Woodhead et al. [37] demonstrated that the area of the left pFG that responded to words over scrambled words also responded particularly to gratings of high over low spatial frequency. This observation is highly congruent with Roberts et al.'s [11] findings that in a subset of their patients tested on measures of visual acuity, there was a clear deficit specifically for the medium to high spatial frequency range. These findings fit well with proposals for a retinotopic organization of visual cortex, with words mapping onto the high-acuity areas involved in foveal vision [38–40].
It is interesting to note that a rather different kind of visual stimulus, namely faces, also partially relies on high spatial frequency information for efficient processing. Woodhead et al. [37] found considerable overlap between the left pFG areas that were active for word processing/high spatial frequency gratings and those that were active for face processing, with a homologous right hemisphere region active for both face processing and low spatial frequency gratings. This accords closely with the functional neuroimaging results of Hasson et al. [38], which showed left pFG activation for face processing proximal to activation for word processing. There is therefore a clear prediction from this account that pure alexic patients should also manifest difficulties in face processing tasks, which has recently been confirmed by Behrmann & Plaut [41].
Hence, although pure alexia was once considered the existence proof for cognitive and neural representations specifically dedicated to reading, more recent case-series data have demonstrated more general deficits for complex visual stimuli that share particular characteristics with letter strings. When assessed, the pure alexic patients demonstrate deficits in the medium to high spatial frequencies necessary for processing such items, consistent with the association of their lesion site to such frequencies across different visual stimuli in normal functional neuroimaging. The recent development of computational models that accept retinotopic visual input (figure 1c) has meant that these more general visual processing deficits for words and faces can be simulated [29]. Within these models, graded specialization of visual cortex according to the properties of the stimulus emerges over the course of learning [15,29].
5. Phonological dyslexia
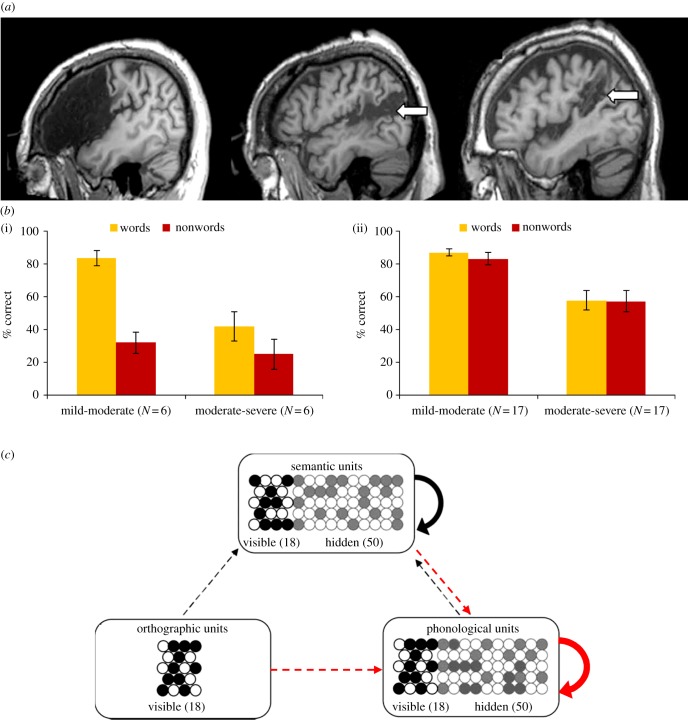
The reading profile seen in phonological dyslexia is a relatively selective deficit in reading aloud novel letter strings, or non-words, which are often lexicalized (e.g. gat > ‘cat’). This deficit for novel letters strings causes a disproportionate lexicality effect, or advantage for words over nonwords, in reading accuracy, which mirrors the reaction time effects observed in normal healthy readers [42]. These patients rely heavily on meaning to support their reading, as performance for words is strongly determined by the imageability of their referent [43]. Patients with phonological dyslexia usually come to attention owing to a concomitant non-fluent aphasia, or difficulty in processing spoken language, primarily in terms of production [43]. Phonological dysgraphia, a relatively selective difficulty in nonword spelling is usually also observed. Phonological dyslexia is often seen after damage to the left perisylvian regions ([44]; figure 2a) associated with phonological processing in normal neuroimaging studies [46]. Some have proposed that phonological dyslexia is caused by damage to a dedicated system of subword mappings that exist to permit reading of novel letter strings [19]. The co-occurrence of phonological dyslexia and non-fluent aphasia associated with left perisylvian damage is precisely what would be predicted according to the primary systems view, which attributes this reading disorder to the damage to general phonological processes that are particularly important when dealing with novel strings [47], as these do not have recourse to any of the semantic support available for words.
Figure 2.
(a) Three example cases of lesions associated with phonological dyslexia from [44], encompassing posterior inferior frontal gyrus/Broca's area/precentral Gyrus (left), superior temporal gyrus (middle, arrow) and supramarginal gyrus (right, arrow). (b) Reading performance for 12 chronic stroke aphasic patients from [43] on the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA) 31 low-frequency words and PALPA 36 nonwords, with severity determined by picture-naming ability (i) and 34 observations of progressive non-fluent aphasia patients’ reading from [13] on the Surface List low-frequency words and nonwords, with severity determined by picture-naming ability (ii). (c) The connectionist model from [45] with red indicating the connections damaged to simulate phonological dyslexia (dotted and solid arrows indicate 30 and 80% connectivity, respectively).
(a). Recovery and relearning
The primary systems account of phonological dyslexia predicts that there should be a correlation between performance on phonological processing tests and nonword reading performance. A number of case-series studies to date have validated this prediction. The first report of such an association was provided by Patterson & Marcel [48] who found that six phonologically dyslexic patients with intact nonword repetition were all severely impaired when asked to remove or add phonemes to spoken strings, particularly when this required a nonword response (e.g. crall > ‘rall’). In a subsequent study of 12 cases that replicated the association between nonword reading and phoneme manipulation abilities, Crisp & Lambon Ralph [43] also revealed effects of lexicality and word imageability of comparable magnitude across reading aloud and delayed repetition. Although a few case studies have reported intact phonological processing in phonological dyslexia [23], case-series studies have demonstrated general phonological processing deficits in those selected for their nonword reading deficits.
In a case series that selected patients according to presence of a left perisylvian lesion, evidence of extensive impairments in reading and spelling, most pronounced for nonwords, was found across 27 patients [44]. Reading performance correlated strongly with performance on a phonological processing battery including both repetition and phoneme manipulation tasks. In terms of underlying damage, the size of the lexicality effect correlated with damage in left inferior frontal and precentral gyri, but lesions to any of five perisylvian areas associated with phonological processing in functional imaging studies [46] was sufficient to produce phonological dyslexia (figure 2a). This is consistent with a view in which the general function of phonological processing is supported by the coordinated operation of a network of distributed perisylvian cortical areas. Large lesions to this network cause deep dyslexia [49], which is characterized by severe nonword reading deficits combined with semantic errors in word reading (e.g. heart > ‘blood’). These patients show clear phonological processing impairments and also deficits on some semantic assessments [43], hence it remains unclear whether their semantic errors arise owing to the removal of phonological constraints on normal semantic activation [50,51] or additional disruption to semantic processing [43].
Damage to the left perisylvian phonological processing network is also seen in progressive non-fluent aphasia [52,53], and results in deficits on repetition and phoneme manipulation tests [54,55]. The primary systems view would therefore seem to predict that progressive non-fluent aphasia should be associated with phonological dyslexia. While is it the case that progressive non-fluent aphasia patients do indeed show nonword reading deficits, they also have difficulty in reading aloud exception words that contain atypical mappings between spelling and sound [13]. A similar pattern is seen irrespective of whether damage is focused more anteriorly on the insula or more posteriorly on the intraparietal sulcus [56–58], consistent with the perisylvian network view. The reading problems seen in progressive non-fluent aphasia do seem to stem directly from the phonological processing deficits, as performance for both nonwords and exception words are predicted by the rate of phonological errors in picture naming [13]. The stronger influence of lexicality upon reading performance in chronic non-fluent stroke aphasia than progressive non-fluent aphasia is illustrated in figure 2b. How are we to reconcile the different reading profiles seen in these two disorders, given both are associated with phonological deficits owing to left perisylvian damage?
Connectionist neuropsychological simulations suggest that the answer lies in differences between the two disorders in the opportunity for recovery and relearning. Lesioning phonological output representations in connectionist models by disrupting within-level connections and/or incoming connections (figure 3c) does indeed impair nonword reading, but it also impairs word reading, particularly for uncommon words with exceptional mappings between spelling and sound [60,61]. Nonwords are susceptible to damage as they lack semantic support, whereas exception words are vulnerable as activation of the correct pronunciation is weaker as a result of conflicting spelling-to-sound mappings. Hence, phonological damage alone produces a good approximation of performance in progressive non-fluent aphasia, where the opportunity for recovery and relearning is minimal [61]. By contrast, lesion simulations that have allowed the model a period of relearning [45,60] have shown that the performance for words improves, with the remaining connection weights adjusting to increase the contribution of the semantic pathway, although very severe damage can limit capacity for relearning. It seems plausible to assume, in the context of a punctate damage event, for example stroke, that relearning will occur for words as a consequence of their presence in the patients’ environment, and hence that the lexicality effect that defines phonological dyslexia is an emergent phenomenon.
Figure 3.
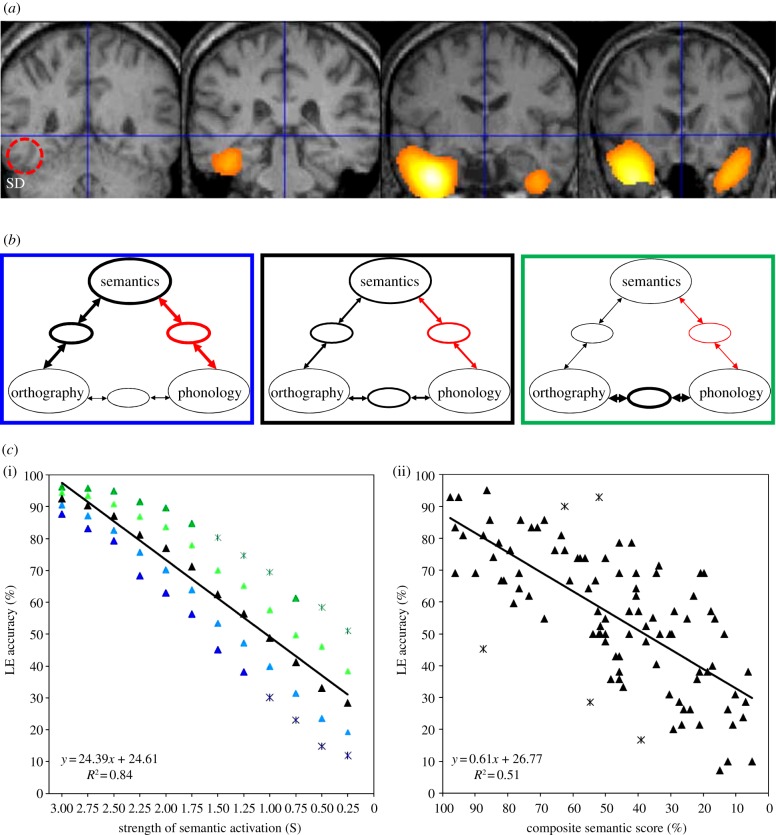
(a) Areas of hypometabolism for the semantic dementia patients considered by [59]—note the absence of abnormality in BA37, within the red dashed circle in the leftmost slice. (b) A schematic of the connectionist model from [12] with versions of the model trained with decreasing levels of semantic support from left to right and red indicating the damage used to simulate surface dyslexia. The weight of the lines indicate the strength of connections developed during training, from a more semantic model on the left (blue) to a more direct model on the right (green). (c) Simulation of low-frequency exception word reading (LE) according to degree of semantic damage in this model, with blue corresponding to greater and green corresponding to lesser premorbid semantic reliance (i) and 100 observations of low-frequency exception word reading from 51 semantic dementia patients according to average of performance on picture naming and spoken word-to-picture matching (ii) from [12], with asterisks indicating outliers.
6. Surface dyslexia
The hallmark of surface dyslexia is a deficit in reading aloud words with exceptional spelling-to-sound correspondences (e.g. brooch), particularly for those lower in frequency. These exceptional items are usually regularized, in that they are pronounced according to more common spelling-to-sound mappings (e.g. ‘brewch’). The deficits for exception words cause an enhanced regularity effect in reading accuracy, paralleling those seen in normal readers in reaction times [62]. Spelling of exception words is usually undermined in a parallel fashion to reading [63]. Surface dyslexia is most commonly seen in semantic dementia, a form of fronto-temporal dementia characterized by progressive deterioration of conceptual knowledge owing to atrophy and hypometabolism of anterior temporal lobe regions ([59,64,65]; figure 3a) that have recently been shown to be active in normal participants both while completing semantic processing tasks [66,67] and when reading aloud exceptional items [68]. As localist models invoke lexical knowledge to support exception word reading, the integrity of semantic information is irrelevant, and therefore surface dyslexia has been simulated as the result of damage to whole-word orthographic representations [4]. In connectionist models, the semantic system provides whole-word knowledge able to support exception word reading, consistent with observations of imageability effects for exception words among normal readers [69–71]. The co-occurrence of surface dyslexia with semantic deficits and anterior temporal lobe damage is exactly what would be expected according to the primary systems view.
(a). Individual differences
The primary systems view of reading disorders predicts an association between the degree of semantic impairment and the severity of the exception word reading deficit. Indeed, many studies have demonstrated a relationship between integrity of semantic knowledge and exception word reading performance in semantic dementia both across patients [12,55,63,72,73] and for particular words within a patient [74–76]. This relationship is sufficiently reliable that in fact surface dyslexia has come to represent a supportive feature in the diagnosis of semantic dementia [77–79].
Nevertheless, advocates of localist models have suggested that the association between semantic impairment and surface dyslexia in semantic dementia is spurious [4]. The basis for this assertion is the existence of rare single cases of dissociation between impaired knowledge of meaning and intact exception word reading [12]. By this account, the prevalence of surface dyslexia in semantic dementia is attributed to the spread of atrophy from the left anterior fusiform gyrus involved in semantic processing to the posterior fusiform regions thought to house the orthographic lexicon [4,24]. While it is certainly true that atrophy can spread to the left pFG in semantic dementia [80], there have been at least seven cases of surface dyslexia in semantic dementia who have shown no structural or functional damage to this region ([5]; figure 3a).
Hence another explanation must be found for dissociations between knowledge of meaning and exception word reading. Plaut [81] suggested that premorbid individual differences may be at play. Within connectionist computational models of reading aloud, there is a graded division of labour across the direct pathway between orthography and phonology and the semantically mediated pathway. In principle, the direct pathway can learn to correctly map regular words, non-words and exception words. In practice, it is more efficient for the model to partially rely on semantic activation for words that are uncommon and/or exceptional in their spelling-to-sound correspondences. If the degree of division of labour can vary over individuals, this could result in dissociations between reading and meaning after brain damage.
Woollams et al. [12] explored this issue by creating a population of models that varied in the amount of semantic support provided during training (figure 3b), then lesioning these models by reducing the quantity and clarity of semantic activation to simulate reading in semantic dementia (figure 3c(i)). We then compared these results to a large case series of reading data from 51 semantic dementia patients (figure 3c(ii)). The patient data showed a very strong relationship between the degree of semantic impairment, as measured by the non-reading tasks of picture naming and spoken word-to-picture matching, and exception word reading accuracy. The connectionist simulation of the effects of semantic damage upon reading aloud matched these data very closely, with the model's predictions accounting for 93% of the variance in the patients’ reading. This strong similarity between the model and patient data suggest that premorbid individual differences exert an appreciable effect on the manifestation of acquired surface dyslexia.
7. Intervention for acquired dyslexia
By placing the reading system in its evolutionary and developmental context, the primary systems view allows us to consider the ultimate causes [82] of reading deficits, which in turn entails clear predictions concerning which interventions are likely to prove successful. Although remediation of the underlying deficit is the general principle, more severe damage to a primary system may entail a focus on increasing reliance on other intact abilities to compensate. Pure alexia has proved difficult to remediate through a focus on visual word recognition, but promising results have been obtained in more severe cases using kinaesthetic information to supplement degraded visual input [83–85]. In phonological dyslexia, word and nonword reading performance has been found to improve as a consequence of working on phonological processing capacity [54,86,87], but when such deficits are severe, as in deep dyslexia, then increasing reliance on semantic processing has also proved effective [87–89]. To date, the few studies concerning therapy for surface dyslexia have focused on linking orthography with meaning [90,91], however the primary systems view suggests that improvements in semantic knowledge should be accompanied by improved reading.
It should be emphasized that the implication of the primary systems view is not that a therapy directed specifically toward reading itself will be ineffective. Rather, because reading is parasitic upon more primary systems, treatment of deficits within those systems should yield generalization across tasks to reading. Connectionist neuropsychology has also allowed simulation of therapeutic interventions, which have shown that gains and degree of generalization depends upon lesion site [92] and timing of intervention after damage [93]. Such simulations have also considered the extent to which one can opt to treat particular items in order to maximize generalization to untreated items. This has suggested that function along the semantic pathway is enhanced more by treating semantically atypical items (e.g. penguin) than typical items (e.g. dog) [92], which has been confirmed in subsequent interventions for anomia [94]. By contrast, function along the direct pathway between orthography and phonology should be enhanced more by training with regular items (e.g. black) than exception items (e.g. blood) [93]), which appears to be the case in therapy [95]. These simulations demonstrate how connectionist neuropsychology can improve our understanding of the factors that determine treatment efficacy.
8. Promise for developmental dyslexia
The implementation of the primary systems view within connectionist models makes this perspective ideally suited for application to reading development and its disorders. Individuals with developmental dyslexia form a heterogeneous population, and with reading problems that arise from a variety of causes [96], some of which may align with those seen in acquired dyslexia. For example, a recent case study of a developmentally dyslexic participant showed the enhanced length effects seen in pure alexia along with dysfunction in the left ventral occipito-temporal cortex [97]. This is consistent with other reports of abnormalities of this area in developmental dyslexia in terms of both function and structure [98–100]. Many developmentally dyslexic individuals have associated phonological processing deficits [96,101] in line with the prevalence of non-word reading deficits in this sample [102,103]. This profile fits with observations that developmentally dyslexic individuals show disruptions of the structure and function of the left perisylvian language network [104–107] that is implicated in acquired phonological dyslexia. There is also evidence that children with inefficient semantic processing or smaller spoken vocabularies not only show poor reading comprehension but also weaknesses in exception word reading that are reminiscent of those seen in acquired surface dyslexia [108,109].
Although there have been suggestions that there are subtypes of developmental dyslexia that map onto acquired phonological and surface dyslexia [110,111], this does not seem to be true in the majority of cases [112,113], where a more mixed profile of deficits across nonwords and exception words is observed. This is hardly surprising given that developmental dyslexia is likely to have a series of complex and interacting causes over time. Connectionist models are well placed to capture this phenomenon, and simulations within an orthography to phonology model have shown that prereading phonological deficits cause problems with both nonword and exception word reading [114]. Developmental phonological dyslexia would emerge if a child comes to rely heavily on semantic activation to support the reading aloud of known words, as seems to be indicated in some cases [115]. By contrast, developmental surface dyslexia would emerge if there was compromised semantic activation of phonology in an otherwise normal reading system.
In terms of intervention for developmental dyslexia, the general principle of remediation of primary systems must be tempered by consideration of interactions between components of the reading system over time. To date, many studies have shown reading gains from training in phonological awareness, particularly in younger children, but they have found additional benefits when a reading element is also included, particularly in older children [116–118]. While this may seem to run against the predictions of the primary systems view, it illustrates the dynamic nature of reading deficits, which has in fact been simulated in connectionist models [119]. Specifically, disruption to phonological representations before the onset of reading development will cause suboptimal mappings to develop between orthography and phonology. Once these mappings have been formed, remediation of the phonological representations alone will not directly affect them, hence why inclusion of a reading component in interventions is particularly important in older children. This work provides an elegant demonstration of the way in which connectionist modelling has the potential to influence the formulation of remediation strategies, thereby helping to optimize treatment outcomes.
9. Summary and conclusion
This paper has provided an integrative overview of the current literature on acquired dyslexia according to a connectionist neuropsychological approach. This rests on the primary systems view of reading disorders which assumes that the cognitive and neural mechanisms involved in reading are also involved in other more basic language functions. Recent research on three subtypes of acquired dyslexia has demonstrated strong support for the predictions of connectionist neuropsychological models. The presence of visual processing deficits in pure alexia, phonological processing deficits in phonological dyslexia and semantic deficits in surface dyslexia are all in line with the predictions of the primary systems view. Moreover, consideration of performance in acquired dyslexia has highlighted key aspects of processing that do not fall naturally from other approaches, namely graded specialization, recovery and relearning, and the impact of normal variation on performance after damage. Connectionist neuropsychological models are able to capture these three dimensions of performance in acquired dyslexia as a consequence of their commitment to learnt representations. The success of the connectionist neuropsychological approach in uncovering the ultimate causes of acquired dyslexia clearly demonstrates its potential for revealing the cognitive and neural mechanisms involved in developmental disorders of reading in the future.
References
- 1.Price CJ. 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage 62, 816–847. ( 10.1016/j.neuroimage.2012.04.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coltheart M. 2001. Assumptions and methods in cognitive neuropsychology. In Handbook of cognitive neuropsychology (ed. Rapp B.). Philadelphia, PA: Psychology Press. [Google Scholar]
- 3.Harm MW, Seidenberg MS. 2004. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychol. Rev. 111, 662–720. ( 10.1037/0033-295X.111.3.662) [DOI] [PubMed] [Google Scholar]
- 4.Coltheart M, Tree JJ, Saunders SJ. 2010. Computational modeling of reading in semantic dementia: comment on Woollams, Lambon Ralph, Plaut, and Patterson (2007). Psychol. Rev. 117, 256–271. ( 10.1037/a0015948) [DOI] [PubMed] [Google Scholar]
- 5.Woollams AM, Lambon Ralph MA, Plaut DC, Patterson K. 2010. SD-squared revisited: reply to Coltheart, Tree, and Saunders (2010). Psychol. Rev. 117, 273–281. ( 10.1037/a0017641) [DOI] [PubMed] [Google Scholar]
- 6.Rastle K, Coltheart M. 2006. Is there serial processing in the reading system; and are there local representations? In From inkmarks to ideas: current issues in lexical processing (ed. Andrews S.), pp. 3–24. Hove, UK: Psychology Press. [Google Scholar]
- 7.Chang YN, Furber S, Welbourne S. 2012. ‘Serial’ effects in parallel models of reading. Cog. Psychol. 64, 267–291. ( 10.1016/j.cogpsych.2012.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. 2010. Neural systems for reading aloud: a multiparametric approach. Cereb. Cortex 20, 1799–1815. ( 10.1093/cercor/bhp245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobard G, Crivello F, Tzourio-Mazoyer N. 2003. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage 20, 693–712. ( 10.1016/S1053-8119(03)00343-4) [DOI] [PubMed] [Google Scholar]
- 10.Taylor JSH, Rastle K, Davis MH. 2012. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 139, 766–791. ( 10.1037/a0030266) [DOI] [PubMed] [Google Scholar]
- 11.Roberts DJ, Woollams AM, Kim E, Beeson PM, Rapcsak SZ, Lambon Ralph MA. 2012. Efficient visual object and word recognition relies on high spatial frequency coding in the left posterior fusiform gyrus: evidence from a case-series of patients with ventral occipito-temporal cortex damage. Cereb. Cortex. 23, 2568–2580. ( 10.1093/cercor/bhs224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woollams AM, Ralph MAL, Plaut DC, Patterson K. 2007. SD-squared: on the association between semantic dementia and surface dyslexia. Psychol. Rev. 114, 316–339. ( 10.1037/0033-295X.114.2.316) [DOI] [PubMed] [Google Scholar]
- 13.Woollams AM, Patterson K. 2012. The consequences of progressive phonological impairment for reading aloud. Neuropsychologia 50, 3469–3477. ( 10.1016/j.neuropsychologia.2012.09.020) [DOI] [PubMed] [Google Scholar]
- 14.Patterson K, Lambon Ralph MA. 1999. Selective disorders of reading? Curr. Opin. Neurobiol. 9, 235–239. ( 10.1016/S0959-4388(99)80033-6) [DOI] [PubMed] [Google Scholar]
- 15.Behrmann M, Plaut DC. 2013. Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn. Sci. 17, 210–219. ( 10.1016/j.tics.2013.03.007) [DOI] [PubMed] [Google Scholar]
- 16.Plaut DC, Kello CT. 1999. The emergence of phonology from the interplay of speech comprehension and production: a distributed connectionist approach. In The emergence of language (ed. MacWhinney B.), pp. 381–415. Mahwah, NJ: Erlbaum. [Google Scholar]
- 17.Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. 2004. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 111, 205–235. ( 10.1037/0033-295X.111.1.205) [DOI] [PubMed] [Google Scholar]
- 18.Bowers JS, Arguin M, Bub DN. 1996. Fast and specific access to orthographic knowledge in a case of letter-by-letter surface alexia. Cogn. Neuropsychol. 13, 525–567. ( 10.1080/026432996381917) [DOI] [Google Scholar]
- 19.Nickels L, Biedermann B, Coltheart M, Saunders S, Tree JJ. 2008. Computational modelling of phonological dyslexia: how does the DRC model fare? Cogn. Neuropsychol. 25, 165–193. ( 10.1080/02643290701514479) [DOI] [PubMed] [Google Scholar]
- 20.Nickels LA. 2002. Theoretical and methodological issues in the cognitive neuropsychology of spoken word production. Aphasiology 16, 3–19. ( 10.1080/02687040143000645) [DOI] [Google Scholar]
- 21.Patterson K, Plaut DC. 2009. ‘Shallow draughts intoxicate the brain’: lessons from cognitive science for cognitive neuropsychology. Topics Cogn. Sci. 1, 39–58. ( 10.1111/j.1756-8765.2008.01012.x) [DOI] [PubMed] [Google Scholar]
- 22.Lambon Ralph MA, Patterson K, Plaut DC. 2011. Finite case series or infinite single-case studies? Comments on ‘Case series investigations in cognitive neuropsychology’ by Schwartz and Dell (2010). Cogn. Neuropsychol. 28, 466–474. ( 10.1080/02643294.2012.671765) [DOI] [PubMed] [Google Scholar]
- 23.Coltheart M. 2006. Acquired dyslexias and the computational modelling of reading. Cogn. Neuropsychol. 23, 96–109. ( 10.1080/02643290500202649) [DOI] [PubMed] [Google Scholar]
- 24.Noble K, Glosser G, Grossman M. 2000. Oral reading in dementia. Brain Lang. 74, 48–69. ( 10.1006/brln.2000.2330) [DOI] [PubMed] [Google Scholar]
- 25.Price CJ. 2013. Current themes in neuroimaging studies of reading. Brain Lang. 125, 131–133. ( 10.1016/j.bandl.2013.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coltheart M. 1999. Modularity and cognition. Trends Cogn. Sci. 3, 115–120. ( 10.1016/S1364-6613(99)01289-9) [DOI] [PubMed] [Google Scholar]
- 27.Caramazza A. 1984. The logic of neuropsychological research and the problem of patient classification in aphasia. Brain Lang. 21, 9–20. ( 10.1016/0093-934X(84)90032-4) [DOI] [PubMed] [Google Scholar]
- 28.Seidenberg MS, Plaut DC. 2006. Progress in understanding word reading: data fitting versus theory building. In From inkmarks to ideas: current issues in lexical processing (ed. Andrews S.), pp. 25–49. Hove, UK: Psychology Press. [Google Scholar]
- 29.Plaut DC, Behrmann M. 2011. Complementary neural representations for faces and words: a computational exploration. Cogn. Neuropsychol. 28, 251–275. ( 10.1080/02643294.2011.609812) [DOI] [PubMed] [Google Scholar]
- 30.Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. 2002. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125, 1054–1069. ( 10.1093/brain/awf094) [DOI] [PubMed] [Google Scholar]
- 31.Weekes BS. 1997. Differential effects of number of letters on word and nonword naming latency. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 50, 439–456. [Google Scholar]
- 32.Cohen L, Dehaene S. 2004. Specialization within the ventral stream: the case for the visual word form area. NeuroImage 22, 466–476. ( 10.1016/j.neuroimage.2003.12.049) [DOI] [PubMed] [Google Scholar]
- 33.Price CJ, Devlin JT. 2003. The myth of the visual word form area. NeuroImage 19, 473–481. ( 10.1016/S1053-8119(03)00084-3) [DOI] [PubMed] [Google Scholar]
- 34.Dehaene S, Cohen L. 2011. The unique role of the visual word form area in reading. Trends Cogn. Sci. 15, 254–262. ( 10.1016/j.tics.2011.04.003) [DOI] [PubMed] [Google Scholar]
- 35.Behrmann M, Nelson J, Sekuler EB. 1998. Visual complexity in letter-by-letter reading: ‘Pure’ alexia is not pure. Neuropsychologia 36, 1115–1132. ( 10.1016/S0028-3932(98)00005-0) [DOI] [PubMed] [Google Scholar]
- 36.Mycroft RH, Behrmann M, Kay J. 2009. Visuoperceptual deficits in letter-by-letter reading? Neuropsychologia 47, 1733–1744. ( 10.1016/j.neuropsychologia.2009.02.014) [DOI] [PubMed] [Google Scholar]
- 37.Woodhead ZVJ, Wise RJS, Sereno M, Leech R. 2011. Dissociation of sensitivity to spatial frequency in word and face preferential areas of the fusiform gyrus. Cereb. Cortex 21, 2307–2312. ( 10.1093/cercor/bhr008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasson U, Levy I, Behrmann M, Hendler T, Malach R. 2002. Eccentricity bias as an organizing principle for human high-order object areas. Neuron 34, 479–490. ( 10.1016/S0896-6273(02)00662-1) [DOI] [PubMed] [Google Scholar]
- 39.Malach R, Levy I, Hasson U. 2002. The topography of high-order human object areas. Trends Cogn. Sci. 6, 176–184. ( 10.1016/S1364-6613(02)01870-3) [DOI] [PubMed] [Google Scholar]
- 40.Levy I, Hasson U, Avidan G, Hendler T, Malach R. 2001. Center-periphery organization of human object areas. Nat. Neurosci. 4, 533–539. [DOI] [PubMed] [Google Scholar]
- 41.Behrmann M, Plaut DC. 2012. Bilateral hemispheric representation of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb. Cortex. ( 10.1093/cercor/bhs390) [DOI] [PubMed] [Google Scholar]
- 42.Forster KI, Chambers SM. 1973. Lexical access and naming time. J. Verbal Learn. Verbal Behav. 12, 627–635. ( 10.1016/S0022-5371(73)80042-8) [DOI] [Google Scholar]
- 43.Crisp J, Lambon Ralph MA. 2006. Unlocking the nature of the phonological-deep dyslexia continuum: the keys to reading aloud are in phonology and semantics. J. Cogn. Neurosci. 18, 348–362. ( 10.1162/089892906775990543) [DOI] [PubMed] [Google Scholar]
- 44.Rapcsak SZ, Beeson PM, Henry ML, Leyden A, Kim E, Rising K, Anderson S, Cho H. 2009. Phonological dyslexia and dysgraphia: cognitive mechanisms and neural substrates. Cortex 45, 575–591. ( 10.1016/j.cortex.2008.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welbourne SR, Woollams AM, Crisp J, Ralph MAL. 2011. The role of plasticity-related functional reorganization in the explanation of central dyslexias. Cogn. Neuropsychol. 28, 65–108. ( 10.1080/02643294.2011.621937) [DOI] [PubMed] [Google Scholar]
- 46.Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. 2006. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage 30, 1414–1432. ( 10.1016/j.neuroimage.2005.11.002) [DOI] [PubMed] [Google Scholar]
- 47.Farah MJ, Stowe RM, Levinson KL. 1996. Phonological dyslexia: loss of a reading-specific component of the cognitive architecture? Cogn. Neuropsychol. 13, 849–868. ( 10.1080/026432996381836) [DOI] [Google Scholar]
- 48.Patterson KM, Marcel A. 1992. Phonological ALEXIA or PHONOLOGICAL alexia? In Analytic approaches to human cognition (eds Alegria J, Holender D, Junca de Morais J, Radeau M.), pp. 259–274. New York, NY: Elsevier. [Google Scholar]
- 49.Price CJ, Howard D, Patterson K, Warburton EA, Friston KJ, Frackowiak RSJ. 1998. A functional neuroimaging description of two deep dyslexic patients. J. Cogn. Neurosci. 10, 303–315. ( 10.1162/089892998562753) [DOI] [PubMed] [Google Scholar]
- 50.Hillis AE, Caramazza A. 1991. Mechanisms for accessing lexical representations for output: evidence from a category-specific semantic deficit. Brain Lang. 40, 106–144. ( 10.1016/0093-934X(91)90119-L) [DOI] [PubMed] [Google Scholar]
- 51.Ciaghi M, Pancheri E, Miceli G. 2010. Semantic paralexias: a group-case study on the underlying functional mechanisms, incidence and clinical features in a consecutive series of 340 Italian aphasics. Brain Lang. 115, 121–132. ( 10.1016/j.bandl.2010.06.003) [DOI] [PubMed] [Google Scholar]
- 52.Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. 2003. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126, 2406–2418. ( 10.1093/brain/awg240) [DOI] [PubMed] [Google Scholar]
- 53.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. 2004. Cognition and anatomy in three variants of primany progressive aphasia. Ann. Neurol. 55, 335–346. ( 10.1002/ana.10825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louis M, Espesser R, Rey V, Daffaure V, Di Cristo A, Habib M. 2001. Intensive training of phonological skills in progressive aphasia: a model of brain plasticity in neurodegenerative disease. Brain Cogn. 46, 197–201. ( 10.1016/S0278-2626(01)80065-8) [DOI] [PubMed] [Google Scholar]
- 55.Patterson K, Graham NL, Lambon Ralph MA, Hodges JR. 2006. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology 20, 1018–1034. ( 10.1080/02687030600739463) [DOI] [Google Scholar]
- 56.Brambati SM, Ogar J, Neuhaus J, Miller BL, Gorno-Tempini ML. 2009. Reading disorders in primary progressive aphasia: a behavioral and neuroimaging study. Neuropsychologia 47, 1893–1900. ( 10.1016/j.neuropsychologia.2009.02.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry ML, Beeson PM, Alexander GE, Rapcsak SZ. 2012. Written language impairments in primary progressive aphasia: a reflection of damage to central semantic and phonological processes. J. Cogn. Neurosci. 24, 261–275. ( 10.1162/jocn_a_00153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. 2010. Connected speech production in three variants of primary progressive aphasia. Brain 133, 2069–2088. ( 10.1093/brain/awq129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nestor PJ, Fryer TD, Hodges JR. 2006. Declarative memory impairments in Alzheimer's disease and semantic dementia. NeuroImage 30, 1010–1020. ( 10.1016/j.neuroimage.2005.10.008) [DOI] [PubMed] [Google Scholar]
- 60.Welbourne SR, Lambon Ralph MA. 2007. Using parallel distributed processing models to simulate phonological dyslexia: the key role of plasticity-related recovery. J. Cogn. Neurosci. 19, 1125–1139. ( 10.1162/jocn.2007.19.7.1125) [DOI] [PubMed] [Google Scholar]
- 61.Plaut DC, McClelland JL, Seidenberg MS, Patterson K. 1996. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychol. Rev. 103, 56–115. ( 10.1037/0033-295X.103.1.56) [DOI] [PubMed] [Google Scholar]
- 62.Baron J, Strawson C. 1976. Use of orthographic and word-specific knowledge in reading words aloud. J. Exp. Psychol. Hum. Percept. Perform. 2, 386–393. ( 10.1037/0096-1523.2.3.386) [DOI] [Google Scholar]
- 63.Graham NL, Patterson K, Hodges JR. 2000. The impact of semantic memory impairment on spelling: evidence from semantic dementia. Neuropsychologia 38, 143–163. ( 10.1016/S0028-3932(99)00060-3) [DOI] [PubMed] [Google Scholar]
- 64.Adlam ALR, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, Hodges JR. 2006. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 129, 3066–3080. ( 10.1093/brain/awl285) [DOI] [PubMed] [Google Scholar]
- 65.Mion M, et al. 2010. What the left and right anterior fusiform gyri tell us about semantic memory. Brain 133, 3256–3268. ( 10.1093/brain/awq272) [DOI] [PubMed] [Google Scholar]
- 66.Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. 2010. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb. Cortex 20, 2728–2738. ( 10.1093/cercor/bhq019) [DOI] [PubMed] [Google Scholar]
- 67.Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MAL. 2010. The inferior, anterior temporal lobes and semantic memory clarified: novel evidence from distortion-corrected fMRI. Neuropsychologia 48, 1689–1696. ( 10.1016/j.neuropsychologia.2010.02.016) [DOI] [PubMed] [Google Scholar]
- 68.Wilson MA, Joubert S, Ferré P, Belleville S, Ansaldo AI, Joanette Y, Rouleau I, Brambati SM. 2012. The role of the left anterior temporal lobe in exception word reading: reconciling patient and neuroimaging findings. NeuroImage 60, 2000–2007. ( 10.1016/j.neuroimage.2012.02.009) [DOI] [PubMed] [Google Scholar]
- 69.Strain E, Patterson K, Seidenberg MS. 1995. Semantic effects in single-word naming. J. Exp. Psychol. Learn. Mem. Cogn. 21, 1140–1154. ( 10.1037/0278-7393.21.5.1140) [DOI] [PubMed] [Google Scholar]
- 70.Woollams AM. 2005. Imageability and ambiguity effects in speeded naming: convergence and divergence. J. Exp. Psychol. Learn. Mem. Cogn. 31, 878–890. ( 10.1037/0278-7393.31.5.878) [DOI] [PubMed] [Google Scholar]
- 71.Shibahara N, Zorzi M, Hill MP, Wydell T, Butterworth B. 2003. Semantic effects in word naming: evidence from English and Japanese Kanji. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 56A, 263–286. ( 10.1080/02724980244000369) [DOI] [PubMed] [Google Scholar]
- 72.Patterson K, Hodges JR. 1992. Deterioration of word meaning: implications for reading. Neuropsychologia 30, 1025–1040. ( 10.1016/0028-3932(92)90096-5) [DOI] [PubMed] [Google Scholar]
- 73.Jefferies E, Ralph MAL, Jones R, Bateman D, Patterson K. 2004. Surface dyslexia in semantic dementia: a comparison of the influence of consistency and regularity. Neurocase 10, 290–299. ( 10.1080/13554790490507623) [DOI] [PubMed] [Google Scholar]
- 74.Graham KS, Hodges JR, Patterson K. 1994. The relationship between comprehension and oral reading in progressive fluent aphasia. Neuropsychologia 32, 299–316. ( 10.1016/0028-3932(94)90133-3) [DOI] [PubMed] [Google Scholar]
- 75.Funnell E. 1996. Response biases in oral reading: an account of the co-occurrence of surface dyslexia and semantic dementia. Q. J. Exp. Psychol. A Hum. Exp. Psychol. 49, 417–446. [DOI] [PubMed] [Google Scholar]
- 76.McKay A, Castles A, Davis C, Savage G. 2007. The impact of progressive semantic loss on reading aloud. Cogn. Neuropsychol. 24, 162–186. ( 10.1080/02643290601025576) [DOI] [PubMed] [Google Scholar]
- 77.Hodges JR, Patterson K, Oxbury S, Funnell E. 1992. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 115, 1783–1806. ( 10.1093/brain/115.6.1783) [DOI] [PubMed] [Google Scholar]
- 78.Neary D, et al. 1998. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. ( 10.1212/WNL.51.6.1546) [DOI] [PubMed] [Google Scholar]
- 79.Gorno-Tempini ML, et al. 2011. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. ( 10.1212/WNL.0b013e31821103e6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC. 2009. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 72, 1562–1569. ( 10.1212/WNL.0b013e3181a4124e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plaut DC. 1997. Structure and function in the lexical system: insights from distributed models of word reading and lexical decision. Lang. Cogn. Process. 12, 765–805. ( 10.1080/016909697386682) [DOI] [Google Scholar]
- 82.Alessi G. 1992. Models of proximate and ultimate causation in psychology. Am. Psychol. 47, 1359–1370. ( 10.1037/0003-066X.47.11.1359) [DOI] [PubMed] [Google Scholar]
- 83.Starrfelt R, Ólafsdóttir RR, Arendt IM. 2013. Rehabilitation of pure alexia: a review. Neuropsychol. Rehabil. 23, 755–779. ( 10.1080/09602011.2013.809661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maher LM, Clayton MC, Barrett AM, Schober-Peterson D, Gonzalez Rothi LJ. 1998. Rehabilitation of a case of pure alexia: exploiting residual abilities. J. Int. Neuropsychol. Soc. 4, 636–647. ( 10.1017/S1355617798466128) [DOI] [PubMed] [Google Scholar]
- 85.Sage K, Hesketh A, Lambon Ralph MA. 2005. Using errorless learning to treat letter-by-letter reading: contrasting word versus letter-based therapy. Neuropsychol. Rehabil. 15, 619–642. ( 10.1080/09602010443000155) [DOI] [PubMed] [Google Scholar]
- 86.Beeson PM, Rising K, Kim ES, Rapcsak SZ. 2010. A treatment sequence for phonological alexia/agraphia. J. Speech Lang. Hear. Res. 53, 450–468. ( 10.1044/1092-4388(2009/08-0229)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kendall DL, Conway T, Rosenbek J, Gonzalez-Rothi L. 2003. Phonological rehabilitation of acquired phonologic alexia. Aphasiology 17, 1073–1095. ( 10.1080/02687030344000355) [DOI] [Google Scholar]
- 88.Stadie N, Rilling E. 2006. Evaluation of lexically and nonlexically based reading treatment in a deep dyslexic. Cogn. Neuropsychol. 23, 643–672. ( 10.1080/02643290500538364) [DOI] [PubMed] [Google Scholar]
- 89.Ska B, Garneau-Beaumont D, Chesneau S, Damien B. 2003. Diagnosis and rehabilitation attempt of a patient with acquired deep dyslexia. Brain Cogn. 53, 359–363. ( 10.1016/S0278-2626(03)00143-X) [DOI] [PubMed] [Google Scholar]
- 90.Scott C, Byng S. 1989. Computer assisted remediation of a homophone comprehension disorder in surface dyslexia. Aphasiology 3, 301–320. ( 10.1080/02687038908248996) [DOI] [Google Scholar]
- 91.Coltheart M, Byng S. 1989. A treatment for surface dyslexia. In Cognitive approaches in neuropsychological rehabilitation (eds Seron X, Deloche G.), pp. 159–174. Hillsdale, NJ: Erlbaum. [Google Scholar]
- 92.Plaut DC. 1996. Relearning after damage in connectionist networks: toward a theory of rehabilitation. Brain Lang. 52, 25–82. ( 10.1006/brln.1996.0004) [DOI] [PubMed] [Google Scholar]
- 93.Welbourne SR, Lambon Ralph MA. 2005. Using computational, parallel distributed processing networks to model rehabilitation in patients with acquired dyslexia: an initial investigation. Aphasiology 19, 789–806. ( 10.1080/02687030500268811) [DOI] [Google Scholar]
- 94.Kiran S, Thompson CK. 2003. The role of semantic complexity in treatment of naming deficits: training semantic categories in fluent aphasia by controlling exemplar typicality. J. Speech Lang. Hear. Res. 46, 608–622. ( 10.1044/1092-4388(2003/048)) [DOI] [PubMed] [Google Scholar]
- 95.Kiran S, Thompson CK, Hashimoto N. 2001. Training grapheme to phoneme conversion in patients with oral reading and naming deficits: a model-based approach. Aphasiology 15, 855–876. ( 10.1080/02687040143000258) [DOI] [Google Scholar]
- 96.Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. 2003. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain 126, 841–865. ( 10.1093/brain/awg076) [DOI] [PubMed] [Google Scholar]
- 97.Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. 2010. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PLoS ONE 5, e12073 ( 10.1371/journal.pone.0012073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richlan F, Kronbichler M, Wimmer H. 2011. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 56, 1735–1742. ( 10.1016/j.neuroimage.2011.02.040) [DOI] [PubMed] [Google Scholar]
- 99.Kronbichler M, Wimmer H, Staffen W, Hutzier F, Mair A, Ladurner G. 2008. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum. Brain Mapp. 29, 613–625. ( 10.1002/hbm.20425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raschle NM, Chang M, Gaab N. 2011. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage 57, 742–749. ( 10.1016/j.neuroimage.2010.09.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liberman IY, Shankweiler D. 1985. Phonology and the problems of learning to read and write. Remedial Spec. Educ. 6, 8–17. ( 10.1177/074193258500600604) [DOI] [Google Scholar]
- 102.Herrmann JA, Matyas T, Pratt C. 2006. Meta-analysis of the nonword reading deficit in specific reading disorder. Dyslexia 12, 195–221. ( 10.1002/dys.324) [DOI] [PubMed] [Google Scholar]
- 103.Rack JP, Olson RK. 1993. Phonological deficits, IQ, and individual differences in reading disability: genetic and environmental influences. Dev. Rev. 13, 269–278. ( 10.1006/drev.1993.1013) [DOI] [Google Scholar]
- 104.Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. 2004. Regional reductions of gray matter volume in familial dyslexia. Neurology 63, 742–745. ( 10.1212/01.WNL.0000134673.95020.EE) [DOI] [PubMed] [Google Scholar]
- 105.Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. 2001. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology 56, 781–783. ( 10.1212/WNL.56.6.781) [DOI] [PubMed] [Google Scholar]
- 106.Paulesu E, et al. 2001. Dyslexia: cultural diversity and biological unity. Science 291, 2165–2167. ( 10.1126/science.1057179) [DOI] [PubMed] [Google Scholar]
- 107.Shaywitz SE, et al. 1998. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl Acad. Sci. USA 95, 2636–2641. ( 10.1073/pnas.95.5.2636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nation K, Snowling MJ. 1998. Semantic processing and the development of word-recognition skills: evidence from children with reading comprehension difficulties. J. Mem. Lang. 39, 85–101. ( 10.1006/jmla.1998.2564) [DOI] [Google Scholar]
- 109.Ricketts J, Nation K, Bishop DVM. 2007. Vocabulary is important for some, but not all reading skills. Sci. Stud. Read. 11, 235–257. ( 10.1080/10888430701344306) [DOI] [Google Scholar]
- 110.Castles A, Coltheart M. 1993. Varieties of developmental dyslexia. Cognition 47, 149–180. ( 10.1016/0010-0277(93)90003-E) [DOI] [PubMed] [Google Scholar]
- 111.Castles A, Bates TC, Coltheart M. 2006. John Marshall and the developmental dyslexias. Aphasiology 20, 871–892. ( 10.1080/02687030600738952) [DOI] [Google Scholar]
- 112.Manis FR, Seidenberg MS, Doi LM, McBride-Chang C, Petersen A. 1996. On the bases of two subtypes of development dyslexia. Cognition 58, 157–195. ( 10.1016/0010-0277(95)00679-6) [DOI] [PubMed] [Google Scholar]
- 113.Peterson RL, Pennington BF, Olson RK. 2013. Subtypes of developmental dyslexia: testing the predictions of the dual-route and connectionist frameworks. Cognition 126, 20–38. ( 10.1016/j.cognition.2012.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harm MW, Seidenberg MS. 1999. Phonology, reading acquisition, and dyslexia: insights from connectionist models. Psychol. Rev. 106, 491–528. ( 10.1037/0033-295X.106.3.491) [DOI] [PubMed] [Google Scholar]
- 115.Hennessey NW, Deadman A, Williams C. 2012. Semantic effects on word naming in children with developmental dyslexia. J. Res. Read. 35, 267–286. ( 10.1111/j.1467-9817.2010.01458.x) [DOI] [Google Scholar]
- 116.Bus AG, Van Ijzendoorn MH. 1999. Phonological awareness and early reading: a meta-analysis of experimental training studies. J. Educ. Psychol. 91, 403–414. ( 10.1037/0022-0663.91.3.403) [DOI] [Google Scholar]
- 117.Ehri LC, Nunes SR, Stahl SA, Willows DM. 2001. Systematic phonics instruction helps students learn to read: evidence from the national reading panel's meta-analysis. Rev. Educ. Res. 71, 393–447. ( 10.3102/00346543071003393) [DOI] [Google Scholar]
- 118.Ehri LC, Nunes SR, Willows DM, Schuster BV, Yaghoub-Zadeh Z, Shanahan T. 2001. Phonemic awareness instruction helps children learn to read: evidence from the national reading panels meta-analysis. Read. Res. Q. 36, 250–283. ( 10.1598/RRQ.36.3.2) [DOI] [Google Scholar]
- 119.Harm MW, McCandliss BD, Seidenberg MS. 2003. Modeling the successes and failures of interventions for disabled readers. Sci. Stud. Read. 7, 155–182. ( 10.1207/S1532799XSSR0702_3) [DOI] [Google Scholar]