Abstract
The hippocampal region contains several principal neuron types, some of which show distinct spatial firing patterns. The region is also known for its diversity in neural circuits and many have attempted to causally relate network architecture within and between these unique circuits to functional outcome. Still, much is unknown about the mechanisms or network properties by which the functionally specific spatial firing profiles of neurons are generated, let alone how they are integrated into a coherently functioning meta-network. In this review, we explore the architecture of local networks and address how they may interact within the context of an overarching space circuit, aiming to provide directions for future successful explorations.
Keywords: entorhinal cortex, presubiculum, parasubiculum, hippocampus, development
1. Introduction
The hippocampal–parahippocampal region contains a diversity of neural circuits and functionally specialized cell-types involved in the representation of self-location. Hippocampal networks, particularly those of CA3 and CA1, embed the place cells, encoding locations in specific environments. Parahippocampal networks in the medial entorhinal cortex (MEC) and the associated presubiculum (PrS) and parasubiculum (PaS) provide anchorage for grid cells, head-direction cells and border cells. The latter three functionally defined neuron types universally map directions and positions, irrespective of the environment. As yet, little is known about the mechanisms or network properties by which these functionally specific firing profiles are generated, let alone how they are integrated into a coherently functioning meta-network.
From the maiden exploratory voyages through the navigational system in the brain, the system appeared neatly organized, with individual functional cell-types belonging to unique neural networks. Place cells were described in the hippocampus [1], whereas head-direction neurons were associated with the dorsal PrS [2,3]. In MEC, grid cells were subsequently discovered as the third main component [4,5]. Similar to how sirens lured ship's navigators to their treacherous coast, these observations lured the field to the concept that each functional cell-type was associated with a certain network architecture and that there was likely a hierarchical relationship between these three functional cell-types. This induced attempts to model upstream or downstream relationships between these cell-types [6–10]. Subsequent studies showed that although grid cells are prominent in layer II of MEC, they co-localize, particularly in deeper layers III–VI, with head-direction cells and with yet another cell-type, the border or boundary vector cells. Neurons that showed combinations of some features were also described in MEC [5,11–13]. The same constellation of neuron types exists in PrS and PaS [11], casting doubts on whether functional cell-types could be related to specific neural architectures. An alternative interpretation is that the neural signals do reflect neural computations of a specific neural architecture, and that emerging local signals are transferred to adjacent networks. Although this might provide a suitable and attractive explanation for the presence of for example border or boundary vector cells in subiculum, in MEC and in PrS and PaS [14], it does not explain why grid cells, head-direction cells and border cells are not present in the CA-fields, and vice versa, why the typical CA1 place cells with narrow fields are only sparsely present in the subiculum and any of the parahippocampal areas [15–18]. In this review, we explore our current knowledge about the architecture of local networks and how networks interact, providing possibilities for input interactions or interactions between inputs and local circuit operations [19–21].
2. Classical network
We focus on MEC as the core structure around which to centre the remaining (para)hippocampal structures. The by now accepted ‘classical’ network [22–24] is already more elaborate than that proposed at the time of the trisynaptic pathway [25]. Neurons in layers II and III of MEC give rise to projections to all constituents of the hippocampus. Layer II cells project to DG and CA3, whereas cells in layer III project to CA1 and the subiculum. The layer II projection to DG is the entry point of the trisynaptic pathway, which subsequently includes the mossy fibre projection from DG to CA3 and the Schaffer collateral projection from CA3 to CA1 (figure 1). The two entorhinal inputs have become known as the direct (layer III to CA1) and the indirect (layer II via the trisynaptic pathway) pathways to CA1. A substantial part of the inputs to MEC layers II and III originate from PrS and PaS [26]. Efferent MEC projections to cortical and subcortical domains originate mainly from layers V and VI, the recipients of hippocampal output from CA1 and the subiculum. Although the reciprocal entorhinal–CA1 network shows a complicated topographical organization, it has a high connectional fidelity in that any point source in MEC that originates projections to CA1 will receive output from that recipient part of CA1 [27]. This reciprocal connectivity provides a clear hierarchical perspective in that grid cells in MEC are downstream to head-direction cells in pre- and parasubiculum, and upstream to place cells. The latter is true for both CA3 and CA1, because grid cells are present in both layers II and III of MEC. Model studies have shown that the sum of many grid cells with different orientations and spatial phases or the summation of border cells can result in place cell firing [8,9,19,20,28–30]. Place cell firing can be transformed into grid cell firing [31], potentially explaining the presence of grid cells in MEC layer V. Adding the projections from layer V neurons to superficial layers II and III [32,33] closes the loop between place cells in CA1 and grid cells in layers II and III (figures 1, 2 and 3).
Figure 1.
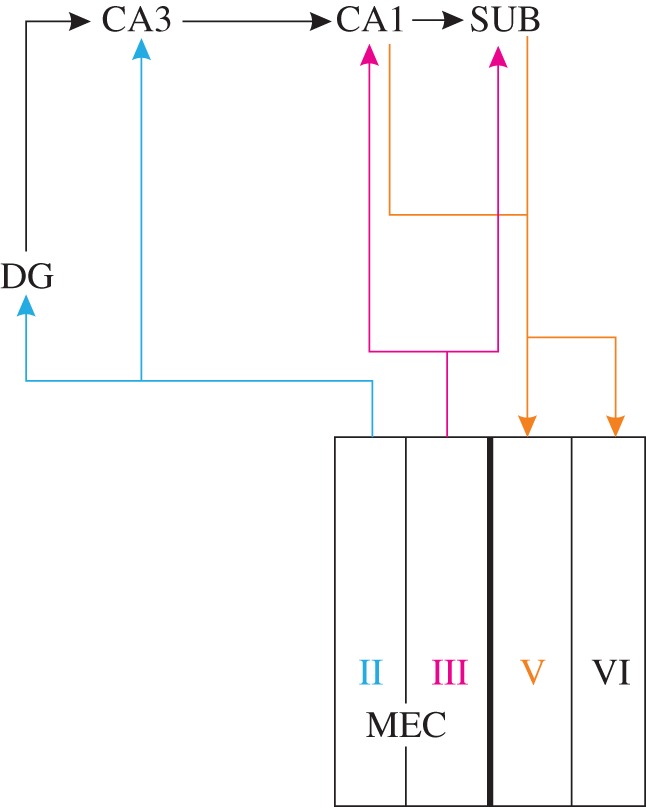
The ‘classical’ hippocampal–parahippocampal network. Neurons in MEC layer II provide inputs DG and CA3, feeding into the trisynaptic pathway that includes projections from DG to CA3 to CA1. Layer III neurons project to CA1 and SUB, which both reciprocate with projections to layer V (and weaker to VI). (Online version in colour.)
Figure 2.

Extended hippocampal–parahippocampal network. The ‘classical’ scheme with added local connectivity of PrS and PaS and the reciprocal layer II–CA2 pathway. (Online version in colour.)
Figure 3.

Medial entorhinal circuits. Principal cells in all layers receive monosynaptic convergent inputs from PrS and PaS. Axons of layer V pyramidal cells target superficial layers, making synaptic contacts onto principal cells in layers II and III. These layer V cells are likely contacted by local axons of layer II and III cells, and cells in layer II presumably contact layer III cells. Although no neurons are indicated in layer VI, synaptic contacts from PrS and PaS have been reported [34] and are schematically indicated. (Online version in colour.)
3. Place cells and grid cells
The fact that place cells mature earlier than grid cells during postnatal development [35,36] defies the concept that place cells emerge from convergence of grid cell outputs onto a pyramidal cell, although disrupting the inputs from MEC layer III to CA1 lowers the specificity of place cell firing [37]. Even more challenging is fact that inputs from grid cells in MEC layer III, of which combined output result in CA1 place cells, likely project to pyramidal cells in the subiculum as well, yet resulting in very different spatial properties of subiculum neurons [15,16]. The difference between CA1 and subiculum neural firing, narrow place fields in CA1 and more broader ones with a higher background firing in subiculum, might result from two different network architectures. First, CA1 single pyramidal cells receive convergent input from MEC and CA3. However, removing the output from CA3 does not strongly effect place fields recorded in CA1 [38,39]. In the subiculum, such convergence between CA1 and MEC inputs is non-existent [40]. Second, neurons in the MEC recipient portion of the subiculum, the distal part that borders PrS, differ markedly in their electrophysiological properties from those in CA1 [41]. Particularly, the distal neurons may combine hippocampal spatial codes with contextual information into spatial-rich signal to be used by other brain regions [16].
4. Layer II medial entorhinal cortex network generates grid cell firing
Among the theoretical models for grid cells in layer II, one class, the attractor models, emphasizes the internal connectivity in MEC, assuming that between grid cells a precisely formed connectivity pattern exists that includes both excitatory and inhibitory connections [6,7,42]. The other class, the oscillatory interference models, postulates interactions between two independent oscillators to be responsible for grid cell properties. In the case of grid cells in layer II, the interference is between field theta oscillations or network oscillations, and cell-specific subthreshold membrane oscillations [43–45]. Both models converge on the basic assumption that grid cells emerge from certain local network features with the added presence of directional input. In two recent studies, the local network in layer II was scrutinized using an in vitro approach [46,47]. The focus was on stellate cells, which are the most likely candidates to be grid cells in layer II [48–50]. Both studies showed that stellate cells are exclusively interconnected via fast-spiking inhibitory interneurons. Model attractor networks demonstrated that stable grid firing can emerge from a simple recurrent inhibitory network, thus suggesting that the observed inhibitory microcircuitry between stellate cells is sufficient to generate grid-cell firing. The models rely on the presence of head-directional and velocity-tuned inputs in addition to an excitatory input that exceeds the local inhibition. The two studies differ with respect to the type of excitatory input used in the model, constant [46] or oscillatory in the theta range [47]. There is experimental evidence that removing either type of input disrupts grid cell properties in vivo [51–53]. The excitatory input from the hippocampus is thought to reach the grid cells in layer II via an intermediary synapse in layer V, following the scheme outlined above (figure 3). A direct projection from CA2 to layer II of MEC has been suggested as a monosynaptic alternative (figure 2) [54], but this projection has not been confirmed by others [55]. Whether or not to welcome CA2 as a new player in the field of place-to-grid cell interactions remains thus to be seen, although the unique integrative responses of CA2 neurons make them likely key players in the grid-to-place cell interactions [56,57]. The required excitatory drive may also come from local excitatory networks in MEC, embedded in layer II through non-stellate cells or feedback loops between the layers [32,33,58,59]. Interestingly, a comparable local architecture with principal cells being connected almost exclusively through an inhibitory interneuron network that receives excitatory inputs is also found in DG [60,61]. The fact that grid cells have not been reported in DG thus points to head-directional and velocity-tuned inputs as relevant for grid cell firing to emerge.
It is well known that layer II of MEC not only contains stellate cells and fast-spiking interneurons, but also pyramidal cells and low threshold-spiking interneurons [46,62]. Our data indicated that reciprocal connectivity between fast-spiking interneurons and stellate cells was high, and stellate cell connectivity to fast-spiking interneurons was much higher than to low threshold-spiking interneurons. Stellate cells connect to pyramidal neurons, whereas the reverse connection was not found [46]. Among the fast-spiking neurons are parvalbumin-positive basket cells providing perisomatic inhibition onto stellate cells and pyramidal cells [63,64]. Further detailing this network ([65]; own unpublished observations, 2013), we postulate the presence of two, independently modulated networks. The first, made up by hippocampus-projecting, reelin-positive stellate cells and fast-spiking parvalbumin-positive interneurons, and a second, comprising non-hippocampal projecting pyramidal cells and parvalbumin–cholecystokinin (CCK)-positive interneurons (figure 4). The interactions between these two networks and the position of the low threshold-spiking interneurons remain to be established. It also remains to be seen whether the pyramidal cells in this second network do express grid-like firing. Although current in vivo recording data seem to indicate the opposite [48,49], pyramidal grid cells cannot be ruled out [46,48].
Figure 4.
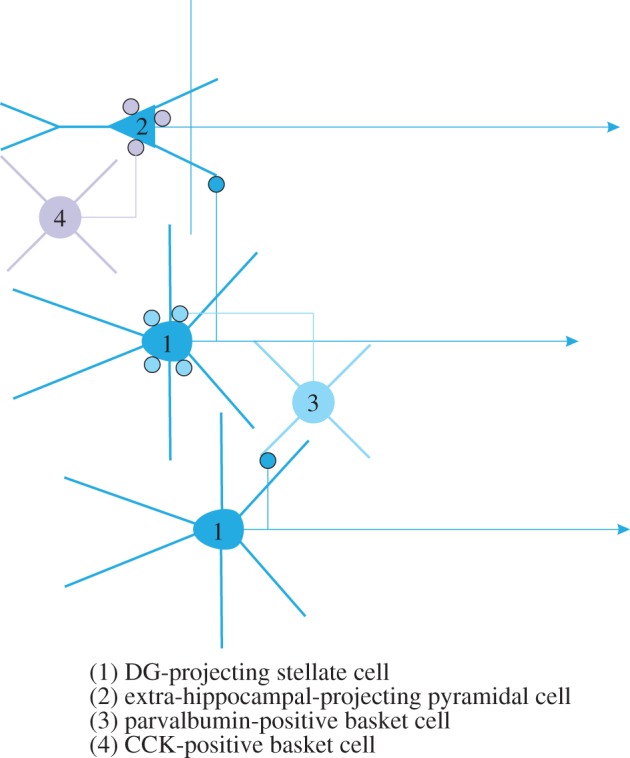
Proposed local circuits in medial entorhinal layer II. (1) DG projecting stellate cells make synaptic contacts with fast-spiking, (3) parvalbumin-positive interneurons that innervate the somata of stellate cells. Stellate-to-stellate synaptic contacts are non-existent. (2) Non-hippocampal projecting neurons, likely pyramidal cells receive input from stellate cells but do not reciprocate that input. The pyramidal cells are innervated by (4) CCK-positive basket cells. Other interneuron types, such as chandelier, goblet and multipolar cells, are not indicated. CCK, cholecystokinin. (Online version in colour.)
5. Grid cells in medial entorhinal cortex deep layers and in pre-parasubiculum
Grid cells are not unique for layer II of MEC; layers III, V and VI of MEC, as well as PrS and PaS, also contain grid cells together with stable percentages of head-direction, conjunctive and border cells [11–13]. The architecture in these layers and domains, although less well studied compared with layer II of MEC, is unlikely to be similar to the latter [63,66]. This raises the question whether grid cell properties in these networks are independently generated, as suggested above, through a local pyramidal cell network, or inherited from MEC layer II. The latter scenario, in the case of MEC, requires an intrinsic network, connecting neurons in layer II to those in deeper layers. Although it is well established that neither axons of layer II stellate cells nor of pyramidal cells extensively target deep layers [59,62,67,68], superficial-to-deep connections do exist [69]. These are likely mediated by way of the apical dendrites of pyramidal cells in layers III and V (figure 3) [62,70]. Known connectivity does not make it plausible that the grid cells in PrS and PaS depend on inputs from MEC layer II. Projections from MEC to PrS and PaS are not dense and they mainly originate from layer V of MEC [71]. It thus seems likely that grid cells in PrS and PaS are locally generated.
6. Grid cells and head-direction cells
Directional information is apparently relevant for grid cell firing and is represented in all layers of MEC. Most models for grid cells in layer II of MEC postulated velocity-dependent directional information as relevant for the emergence of grid cell properties [6,7,44,46,47]. Supporting this postulate is the finding that layer II grid cells reveal directional tuning after removal of hippocampal inputs [51]. Grid cells in all layers show remarkably consistent orientations and directionally tuned neurons are present in layers III, V and VI [12]. Directional information is likely not generated within the network, because necessary inputs to MEC from the vestibular system, mediated by way of the lateral mammillary–anterior thalamic route [72–74], are either sparse and restricted to the extreme dorsocaudal part [75,76], or absent [26]. Vestibular inputs however specifically influence PrS and PaS [77], which are known to give rise to about 35% of the hippocampal–parahippocampal input and around 15% of the total cortical inputs to MEC [26].
Projections from PrS and PaS show a strikingly laminar terminal distribution in superficial MEC [78,79]. Both inputs form synaptic contacts with principal neurons and interneurons that reside in the targeted layers [80–82]. In line with the above, proposed architecture mediating superficial to deep intrinsic connectivity in MEC, PrS and PaS inputs contact layer V pyramidal neurons on their apical dendrites [83]. We recently showed electrophysiologically that principal neurons in all layers of MEC receive convergent monosynaptic inputs from both PaS and PrS (figure 3) [34]. These shared inputs thus provide a parsimonious substrate for the prominent directional tuning of head-direction cells and the coherent orientational tuning of grid cells in all layers of MEC.
The postnatal timing of emergent head-directional and grid cell properties in the network may be indicative as well for the relevance of head-direction cells. In vivo recordings in freely behaving animals, from the moment after eye-opening (postnatal day (P) 14/15), when they start to actively leave the nest, showed that the latter are the first to be present. The percentage of head-direction cells as well as the degree of directional tuning were similar to that in adults, and the cells were stable between sessions [35,36]. Data obtained in brain slices with preserved connectivity between PaS, PrS and MEC showed that projections from PaS and PrS to MEC become functional around P9/P10 before grid cell firing is apparent in MEC. After P14/P15, the connectivity from PaS and PrS to MEC becomes more adult-like even though minor changes still occur from P15 to P30 [35,84].
Grid cell properties continue to develop from P16 until at least P34. Their periodic properties evolve between P16 and P34, reaching adult levels around the end of that period [35]. The gradually increasing precision of grid cells was found to be paralleled with developmental changes in the local layer II network [35,46]. There was a notable increase in the synchrony of spontaneous subthreshold changes in membrane potentials of stellate cells from P16 to P29. These membrane potential changes are considered a hallmark feature of MEC layer II stellate cells and have been implicated in the oscillatory interference models as one of the two oscillators needed for the formation of grid cells [44,85,86]. All data thus suggest that head-directional representations are established and reach MEC before grid-like firing properties emerge. These inputs are fine-tuned during the subsequent two weeks, in parallel to a further development of the intrinsic connectivity of the layer II network.
7. Concluding remarks
Networks in divisions of the hippocampal region provide the substrate for many complex integrative processes, resulting from local and more complex, higher-order interactions. The focus on either these local interactions or the more complex, partially hierarchical processes has contributed to a mechanistic description of the functionally different spatially modulated cells. Now, it is time to move forward to abandon the classic hierarchical view and to encompass the many parallel and converging routes present in the region and the importance of feedback and feed-forward balance. A similar shift in emphasis in visual research away from a pure hierarchical conceptualization has led to the notion that feedback from downstream and lateral processing systems contribute to a sharpened upstream processing [87]. The balance between feed-forward and feedback processing in the visual system partially depends on differential distributions of various glutamate receptors [88]. In a parallel manner, a differential distribution of NMDA receptors onto two different types of MEC layer II interneurons has been proposed to result in the switching between slow and fast gamma oscillations [89]. Adding this level of detail to network descriptions is one of the future challenges.
Funding statement
The preparation of this manuscript was supported by the Kavli Foundation and a Centre of Excellence grant from the Norwegian Research Council.
References
- 1.O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. ( 10.1016/0006-8993(71)90358-1) [DOI] [PubMed] [Google Scholar]
- 2.Ranck JB. 1985. Head direction cells in the deep cell layer of dorsal presubiculum in freely moving rats. In Electrical activity of the archicortex (eds Buzsaki G, Vanderwolf CH.), pp. 217–220. Budapest, Hungary: Akademiai Kiado. [Google Scholar]
- 3.Taube JS, Muller RU, Ranck JB., Jr 1990. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fyhn M, et al. 2004. Spatial representation in the entorhinal cortex. Science 305, 1258–1264. ( 10.1126/science.1099901) [DOI] [PubMed] [Google Scholar]
- 5.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. ( 10.1038/nature03721) [DOI] [PubMed] [Google Scholar]
- 6.Fuhs MC, Touretzky DS. 2006. A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276. ( 10.1523/JNEUROSCI.4353-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B. 2006. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 7, 663–678. ( 10.1038/nrn1932) [DOI] [PubMed] [Google Scholar]
- 8.Solstad T, Moser EI, Einevoll GT. 2006. From grid cells to place cells: a mathematical model. Hippocampus 16, 1026–1031. ( 10.1002/hipo.20244) [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J, Burgess N. 2005. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus 15, 853–866. ( 10.1002/hipo.20115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molter C, Yamaguchi Y. 2008. Entorhinal theta phase precession sculpts dentate gyrus place fields. Hippocampus 18, 919–930. ( 10.1002/hipo.20450) [DOI] [PubMed] [Google Scholar]
- 11.Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser M-B. 2010. Grid cells in pre- and parasubiculum. Nat. Neurosci. 13, 987–994. ( 10.1038/nn.2602) [DOI] [PubMed] [Google Scholar]
- 12.Sargolini F, et al. 2006. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762. ( 10.1126/science.1125572) [DOI] [PubMed] [Google Scholar]
- 13.Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI. 2008. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. ( 10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 14.Lever C, Burton S, Jeewajee A, O'Keefe J, Burgess N. 2009. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 29, 9771–9777. ( 10.1523/JNEUROSCI.1319-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp PE, Green C. 1994. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J. Neurosci. 14, 2339–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SM, Ganguli S, Frank LM. 2012. Spatial information outflow from the hippocampal circuit: distributed spatial coding and phase precession in the subiculum. J. Neurosci. 32, 11 539–11 558. ( 10.1523/JNEUROSCI.5942-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargreaves EL, et al. 2005. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308, 1792–1794. ( 10.1126/science.1110449) [DOI] [PubMed] [Google Scholar]
- 18.Taube JS. 1995. Place cells recorded in the parasubiculum of freely moving rats. Hippocampus 5, 569–583. ( 10.1002/hipo.450050608) [DOI] [PubMed] [Google Scholar]
- 19.Rolls ET, Stringer SM, Elliot T. 2006. Entorhinal cortex grid cells can map to hippocampal place cells by competitive learning. Network 17, 447–465. ( 10.1080/09548980601064846) [DOI] [PubMed] [Google Scholar]
- 20.Savelli F, Knierim JJ. 2010. Hebbian analysis of the transformation of medial entorhinal grid-cell inputs to hippocampal place fields. J. Neurophysiol. 103, 3167–3183. ( 10.1152/jn.00932.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee D, Lin BJ, Lee AK. 2012. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853. ( 10.1126/science.1221489) [DOI] [PubMed] [Google Scholar]
- 22.van Strien NM, Cappaert NL, Witter MP. 2009. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 10, 272–282. ( 10.1038/nrn2614) [DOI] [PubMed] [Google Scholar]
- 23.Somogyi P. 2010. Hippocampus: intrinsic organization. In Handbook of brain microcircuits (eds Shepherd GM, Grillner S.), pp. 148–164. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Witter MP. 2011. Connectivity of the Hippocampus. In Hippocampal microcircuits (ed. Cutsuridis VEA.), pp. 5–26. Springer series in Computational Neuroscience Berlin, Germany: Springer. [Google Scholar]
- 25.Andersen P, Bliss TV, Skrede KK. 1971. Unit analysis of hippocampal polulation spikes. Exp. Brain Res. 13, 208–221. ( 10.1007/BF00234086) [DOI] [PubMed] [Google Scholar]
- 26.Kerr KM, Agster KL, Furtak SC, Burwell RD. 2007. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus 17, 697–708. ( 10.1002/hipo.20315) [DOI] [PubMed] [Google Scholar]
- 27.Naber PA, Lopes Da Silva FH, Witter MP. 2001. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus 11, 99–104. ( 10.1002/hipo.1028) [DOI] [PubMed] [Google Scholar]
- 28.de Almeida L, Idiart M, Lisman JE. 2009. The input–output transformation of the hippocampal granule cells: from grid cells to place fields. J. Neurosci. 29, 7504–7512. ( 10.1523/JNEUROSCI.6048-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Keefe J, Burgess N. 1996. Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. ( 10.1038/381425a0) [DOI] [PubMed] [Google Scholar]
- 30.Barry C, et al. 2006. The boundary vector cell model of place cell firing and spatial memory. Rev. Neurosci. 17, 71–97. ( 10.1515/REVNEURO.2006.17.1-2.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kropff E, Treves A. 2008. The emergence of grid cells: intelligent design or just adaptation? Hippocampus 18, 1256–1269. ( 10.1002/hipo.20520) [DOI] [PubMed] [Google Scholar]
- 32.Kloosterman F, van Haeften T, Witter MP, Lopes da Silva FH. 2003. Electrophysiological characterization of interlaminar entorhinal connections: an essential link for re-entrance in the hippocampal–entorhinal system. Eur. J. Neurosci. 18, 3037–3052. ( 10.1111/j.1460-9568.2003.03046.x) [DOI] [PubMed] [Google Scholar]
- 33.Van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. 2003. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus 13, 943–952. ( 10.1002/hipo.10144) [DOI] [PubMed] [Google Scholar]
- 34.Canto CB, Koganezawa N, Beed P, Moser EI, Witter MP. 2012. All layers of medial entorhinal cortex receive presubicular and parasubicular inputs. J. Neurosci. 32, 17 620–17 631. ( 10.1523/JNEUROSCI.3526-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser M-B. 2010. Development of the spatial representation system in the rat. Science 328, 1576–1580. ( 10.1126/science.1188210) [DOI] [PubMed] [Google Scholar]
- 36.Wills TJ, Cacucci F, Burgess N, O'Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328, 1573–1576. ( 10.1126/science.1188224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brun VH, Leutgeb S, Wu H-Q, Schwarcz R, Witter MP, Moser EI, Moser M-B. 2008. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron 57, 290–302. ( 10.1016/j.neuron.2007.11.034) [DOI] [PubMed] [Google Scholar]
- 38.Brun VH, et al. 2002. Place cells and place recognition maintained by direct entorhinal–hippocampal circuitry. Science 296, 2243–2246. ( 10.1126/science.1071089) [DOI] [PubMed] [Google Scholar]
- 39.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. 2008. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264. ( 10.1126/science.1151120) [DOI] [PubMed] [Google Scholar]
- 40.Cappaert NL, Wadman WJ, Witter MP. 2007. Spatiotemporal analyses of interactions between entorhinal and CA1 projections to the subiculum in rat brain slices. Hippocampus 17, 909–921. ( 10.1002/hipo.20309) [DOI] [PubMed] [Google Scholar]
- 41.Graves AR, Moore SJ, Bloss EB, Mensh BD, Kath WL, Spruston N. 2012. Hippocampal pyramidal neurons comprise two distinct cell types that are countermodulated by metabotropic receptors. Neuron 76, 776–789. ( 10.1016/j.neuron.2012.09.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burak Y, Fiete IR. 2009. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 5, e1000291 ( 10.1371/journal.pcbi.1000291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welday AC, Shlifer IG, Bloom ML, Zhang K, Blair HT. 2011. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16 157–16 176. ( 10.1523/JNEUROSCI.0712-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess N, Barry C, O'Keefe J. 2007. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812. ( 10.1002/hipo.20327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zilli EA, Hasselmo ME. 2010. Coupled noisy spiking neurons as velocity-controlled oscillators in a model of grid cell spatial firing. J. Neurosci. 30, 13 850–13 860. ( 10.1523/JNEUROSCI.0547-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couey JJ, et al. 2013. Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 16, 318–324. ( 10.1038/nn.3310) [DOI] [PubMed] [Google Scholar]
- 47.Pastoll H, Solanka L, van Rossum MCW, Nolan MF. 2013. Feedback inhibition enables theta-nested gamma oscillations and grid firing fields. Neuron 77, 141–154. ( 10.1016/j.neuron.2012.11.032) [DOI] [PubMed] [Google Scholar]
- 48.Domnisoru C, Kinkhabwala AA, Tank DW. 2013. Membrane potential dynamics of grid cells. Nature 495, 199–204. ( 10.1038/nature11973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgalossi A, Herfst L, von Heimendahl M, Haskic K, Schmidt M, Brecht M. 2011. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70, 773–786. ( 10.1016/j.neuron.2011.04.003) [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Hieber C, Hausser M. 2013. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 16, 325–331. ( 10.1038/nn.3340) [DOI] [PubMed] [Google Scholar]
- 51.Bonnevie T, et al. 2013. Grid cells require excitatory drive from the hippocampus. Nat. Neurosci. 16, 309–317. ( 10.1038/nn.3311) [DOI] [PubMed] [Google Scholar]
- 52.Brandon MP, Bogaard R, Libby CP, Connerney MA, Gupta K, Hasselmo ME. 2011. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599. ( 10.1126/science.1201652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. 2011. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595. ( 10.1126/science.1201685) [DOI] [PubMed] [Google Scholar]
- 54.Rowland DC, et al. 2013. Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J. Neurosci. 33, 14 889–14 898. ( 10.1523/JNEUROSCI.1046-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui Z, Gerfen CR, Young WS., 3rd 2012. Hypothalamic and other connections with the dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 521, 1844–1866. ( 10.1002/cne.23263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevaleyre V, Siegelbaum SA. 2010. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66, 560–572. ( 10.1016/j.neuron.2010.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones MW, McHugh TJ. 2011. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 34, 526–535. ( 10.1016/j.tins.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 58.Beed P, Bendels MHK, Wiegand HF, Leibold C, Johenning FW, Schmitz D. 2010. Analysis of excitatory microcircuitry in the medial entorhinal cortex reveals cell-type-specific differences. Neuron 68, 1059–1066. ( 10.1016/j.neuron.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 59.Quilichini P, Sirota A, Buzsaki G. 2010. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J. Neurosci. 30, 11 128–11 142. ( 10.1523/JNEUROSCI.1327-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan RJ, Santhakumar V, Soltesz I. 2007. Modeling the dentate gyrus. Prog. Brain Res. 163, 639–658. ( 10.1016/S0079-6123(07)63035-0) [DOI] [PubMed] [Google Scholar]
- 61.Acsady L, et al. 1998. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 18, 3386–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canto CB, Witter MP. 2012. Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus 22, 1277–1299. ( 10.1002/hipo.20993) [DOI] [PubMed] [Google Scholar]
- 63.Canto CB, Wouterlood FG, Witter MP. 2008. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008, 381243 ( 10.1155/2008/381243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones RS, Buhl EH. 1993. Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci. Lett. 149, 35–39. ( 10.1016/0304-3940(93)90341-H) [DOI] [PubMed] [Google Scholar]
- 65.Varga C, Lee SY, Soltesz I. 2010. Target-selective GABAergic control of entorhinal cortex output. Nat. Neurosci. 13, 822–824. ( 10.1038/nn.2570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhillon A, Jones RS. 2000. Laminar differences in recurrent excitatory transmission in the rat entorhinal cortex in vitro. Neuroscience 99, 413–422. ( 10.1016/S0306-4522(00)00225-6) [DOI] [PubMed] [Google Scholar]
- 67.Jones RS. 1994. Synaptic and intrinsic properties of neurons of origin of the perforant path in layer II of the rat entorhinal cortex in vitro. Hippocampus 4, 335–353. ( 10.1002/hipo.450040317) [DOI] [PubMed] [Google Scholar]
- 68.Klink R, Alonso A. 1997. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus 7, 571–583. () [DOI] [PubMed] [Google Scholar]
- 69.Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. 1996. Entorhinal–hippocampal interactions revealed by real-time imaging. Science 272, 1176–1179. ( 10.1126/science.272.5265.1176) [DOI] [PubMed] [Google Scholar]
- 70.Hamam BN, et al. 2000. Morphological and electrophysiological characteristics of layer V neurons of the rat medial entorhinal cortex. J. Comp. Neurol. 418, 457–472. () [DOI] [PubMed] [Google Scholar]
- 71.Kohler C. 1986. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J. Comp. Neurol. 246, 149–169. ( 10.1002/cne.902460202) [DOI] [PubMed] [Google Scholar]
- 72.Taube JS. 1995. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 15, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stackman RW, Taube JS. 1998. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J. Neurosci. 18, 9020–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vann SD. 2010. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia 48, 2316–2327. ( 10.1016/j.neuropsychologia.2009.10.019) [DOI] [PubMed] [Google Scholar]
- 75.Shibata H. 1993. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J. Comp. Neurol. 337, 431–445. ( 10.1002/cne.903370307) [DOI] [PubMed] [Google Scholar]
- 76.Van Groen T, Wyss JM. 1995. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J. Comp. Neurol. 358, 584–604. ( 10.1002/cne.903580411) [DOI] [PubMed] [Google Scholar]
- 77.Taube JS. 2007. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181–207. ( 10.1146/annurev.neuro.29.051605.112854) [DOI] [PubMed] [Google Scholar]
- 78.Kohler C. 1985. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J. Comp. Neurol. 236, 504–522. ( 10.1002/cne.902360407) [DOI] [PubMed] [Google Scholar]
- 79.Caballero-Bleda M, Witter MP. 1993. Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat. J. Comp. Neurol. 328, 115–129. ( 10.1002/cne.903280109) [DOI] [PubMed] [Google Scholar]
- 80.Caballero-Bleda M, Witter MP. 1994. Projections from the presubiculum and the parasubiculum to morphologically characterized entorhinal–hippocampal projection neurons in the rat. Exp. Brain Res. 101, 93–108. ( 10.1007/BF00243220) [DOI] [PubMed] [Google Scholar]
- 81.Van Haeften T, et al. 1997. GABAergic presubicular projections to the medial entorhinal cortex of the rat. J. Neurosci. 17, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tolner EA, Frahm C, Metzger R, Gorter JA, Witte OW, Lopes da Silva FH, Heinemann U. 2007. Synaptic responses in superficial layers of medial entorhinal cortex from rats with kainate-induced epilepsy. Neurobiol. Dis. 26, 419–438. ( 10.1016/j.nbd.2007.01.009) [DOI] [PubMed] [Google Scholar]
- 83.Wouterlood FG, van Haeften T, Eijkhoudt M, Baks-te-Bulte L, Goede PH, Witter MP. 2004. Input from the presubiculum to dendrites of layer-V neurons of the medial entorhinal cortex of the rat. Brain Res. 1013, 1–12. ( 10.1016/j.brainres.2004.03.017) [DOI] [PubMed] [Google Scholar]
- 84.Koganezawa N, Canto CB, Witter MP.2010. Postnatal development of functional connectivity from pre- and parasubiculum to medial entorhinal cortex. FENS abstr., p. 087.30.
- 85.Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. 2007. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722. ( 10.1126/science.1139207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hasselmo ME, Giocomo LM, Zilli EA. 2007. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252–1271. ( 10.1002/hipo.20374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gilbert CD, Sigman M. 2007. Brain states: top-down influences in sensory processing. Neuron 54, 677–696. ( 10.1016/j.neuron.2007.05.019) [DOI] [PubMed] [Google Scholar]
- 88.Self MW, Kooijmans RN, Super H, Lamme VA, Roelfsema PR. 2012. Different glutamate receptors convey feedforward and recurrent processing in macaque V1. Proc. Natl Acad. Sci. USA 109, 11 031–11 036. ( 10.1073/pnas.1119527109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Middleton S, Jalics J, Kispersky T, LeBeau FEN, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO. 2008. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc. Natl Acad. Sci. USA 105, 18 572–18 577. ( 10.1073/pnas.0809302105) [DOI] [PMC free article] [PubMed] [Google Scholar]


