Abstract
Internal representations about the external world can be driven by the external stimuli or can be internally generated in their absence. It has been a matter of debate whether novel stimuli from the external world are instructive over the brain network to create de novo representations or, alternatively, are selecting from existing pre-representations hosted in preconfigured brain networks. The hippocampus is a brain area necessary for normal internally generated spatial–temporal representations and its dysfunctions have resulted in anterograde amnesia, impaired imagining of new experiences, and hallucinations. The compressed temporal sequence of place cell activity in the rodent hippocampus serves as an animal model of internal representation of the external space. Based on our recent results on the phenomenon of novel place cell sequence preplay, we submit that the place cell sequence of a novel spatial experience is determined, in part, by a selection of a set of cellular firing sequences from a repertoire of existing temporal firing sequences in the hippocampal network. Conceptually, this indicates that novel stimuli from the external world select from their pre-representations rather than create de novo our internal representations of the world.
Keywords: internal representation, hippocampus, preplay, temporal sequence, cellular assemblies, temporal order diagrams
1. Role of prior experience in the expression of internally generated representations
One of the most fascinating functions of the brain is to form and express internal representations about the external world. Some of these representations are driven online by the current features of objects and events from the external environment and take the form of perceptions. Other representations are internally generated; they are mental representations of objects and events that are not currently present. The internally generated representations are sophisticated forms that include mental travel [1] through virtual space and time. The most common form of internally generated representation occurs when our mind travels back in time into our past as is the case with episodic memory retrieval [2]. Other types of internally generated representations are projections into the future and take the form of imagining [1,3,4]. Probably the most genuine form of internally generated representation occurs during sleep, particularly during dreaming, when the brain is virtually disconnected from the external world. Under abnormal conditions, the internally generated representations can take the form of hallucinations in certain pathological states, like schizophrenia, when the subjects internally generate representations of objects and events that they firmly believe are currently present but that cannot be perceived by the other observers. It is not entirely clear what role prior knowledge plays in the formation of internally generated representations.
The role of prior knowledge and previous experience in generating internal representations about the external world has been a highly debated topic in philosophy. On the one hand, Aristotle and later the British empiricist Locke have argued that at birth our mind has no innate ideas, it is blank, tabula rasa and that we cannot represent beyond our experience [5]. Learning is then a progressive accumulation of facts and experiences leading to knowledge. A diametrically opposed view led Plato [6] to propose ‘ideal forms’ that exist independent of the learner. During learning, the subject selects relevant information about the external world that gets assimilated into preconfigured mental schemata. Later on, Kant [7] argued that the concepts of space and time are not derived from experience but rather are its preconditions. To introduce these debates in the field of neuroscience and start looking for their answers, we first ask: what brain areas are implicated in the formation and expression of internally generated representations about the external world?
Located in the medial temporal lobe, the hippocampus is a brain area that has been intimately involved with multiple aspects of internally generated representations, primarily with the spatial–temporal ones. Bilateral removal of the hippocampus to alleviate untreatable epilepsy left patient H.M. with a severe form of anterograde amnesia [8], a case that first indicated the role of the hippocampus in the formation of new memories. Interestingly, the same patients that suffer from anterograde amnesia following bilateral hippocampal dysfunction are deficient in their ability to imagine new experiences [3,4,9], indicating that the same brain structure is necessary to internally represent past as well as future experiences. Furthermore, electrophysiological recordings of neuronal ensemble activity from the rodent hippocampus showed a similar pattern of temporal firing sequences during exploratory run and during subsequent periods of rapid eye movement sleep [10] which are associated with dreaming in human [11]. Finally, various anatomical and physiological deficits in the hippocampus have been reported in patients suffering from schizophrenia [12,13], a medical condition associated with abnormal forms of internally generated representations, such as delusions and hallucinations. Altogether, it appears that the hippocampus is a brain structure that is necessary for internally generated representations and, consequently, the study of the functional activity of ensembles of hippocampal cells could offer insight into the neuronal mechanisms underlying such representations.
2. Internal representation of past and future spatial experiences in the hippocampus
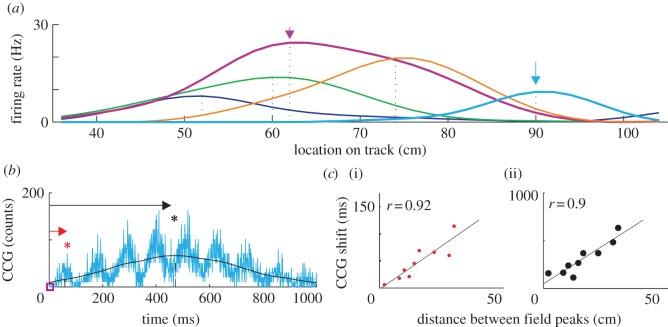
Individual hippocampal pyramidal cells are active at specific locations along the trajectory of an animal, and thus are called place cells [14]. Given that most place cells have unimodal spatial tuning curves (i.e. single place fields), the sequence of an ensemble of place cells depicts the trajectory of the animal in real time (figure 1a; [15,16]). Euclidian spatial distances between any two locations along the trajectory of the animal can be derived by multiplying the velocity of the animal and the relative timing between the firing spikes of any two place cells with place field centres in the respective two locations, as measured by the temporal bias (temporal shift from zero) of the maximum peak of the cross-correlation between the spikes of the two place cells (figure 1b, black arrow) [15]. Owing to the oscillatory nature of the place cell spiking activity in the theta band (7–11 Hz), the cross-correlation between any two place cells active during run is also oscillatory and displays multiple peaks in the time domain (figure 1b). Interestingly, the temporal bias of the maximum peak of the cross-correlogram (figure 1b, black arrow) correlates with the temporal bias of the nearest-to-zero peak of the same cross-correlogram (figure 1b, red arrow), which occurs at a one order of magnitude faster timescale [15]. Thus, spatial distance can be represented online in time at two different timescales, one being the real-time timescale as derived from the velocity of the animal movement through the physical space (i.e. temporal bias of the maximum peak; figure 1c(i)) while the other is temporally compressed 8–16 times (temporal bias of the closest-to-zero peak; figure 1c(ii)) [15]. We propose that an animal model of internal representation of the external space is the compressed temporal sequence of place cell activity in the rodent hippocampus as represented by the closest-to-zero temporal bias on the cross-correlogram during run. This compressed representation occurs online as the animal moves through the spatial environment [15–18] and is dependent on the synaptic weights within the hippocampal network [19,20].
Figure 1.
Representation of place cell sequences by temporal correlation at two timescales. (a) Encoding of sequential locations by individual place cells. Arrows mark Euclidian distance between two colour-coded locations, magenta and cyan. (b) Temporal correlation of the two cells marked in (a) assessed at two timescales by the temporal bias on the cross-correlogram (CCG) and measured by the red (compressed timescale) and black (real-time timescale) arrows. Asterisks mark the first (red) and absolute (black) maxima on the CCG. (c) Spatial distance between the five cells in (a) encoded at compressed ((i), red dots) and real-time ((ii), black dots) timescales during run. Reproduced from Dragoi & Buzsaki [15] with permission from Cell Press.
A similar form of compressed temporal sequence of place cell firing has been repeatedly shown to occur offline, during the sleep period following the spatial experience [21–24], particularly in association with sharp-wave ripples in the CA1 area of the hippocampus. The detection of such temporal sequences during sleep was greatly facilitated by the property of hippocampal pyramidal cells to synchronously fire in ‘frames’ of activity [23] flanked by short epochs of neuronal silence [23–25]. As this type of compressed representation was initially recorded during a sleep session following the spatial experience, it was considered to represent a reactivation or replay of the previously experienced activity [22]. This finding gave experimental support to the previously formulated two-stage model of memory formation [26]. According to this model, memory formation occurs in two stages, the encoding stage and the consolidation stage. First, novel information is encoded in the place cell activity associated with theta oscillation in the CA1 area of the hippocampus during exploratory behaviour [26,27]. Second, the encoded information is consolidated and transferred to neocortical areas for long-term storage during epochs of increased synchrony when reactivation/replay was believed to occur in association with sharp-wave ripples in the CA1 [28,29]. Subsequent studies have shown that such replay events also occur during brief periods of awake resting states [24,25,30–34]. More importantly, the existence of the offline activity structured into compressed temporal sequences creates an opportunity for experimentally testing a long-standing debate: prior to a new spatial experience, is the hippocampal network tabula rasa or is it a preconfigured network?
Most of the studies focusing on the offline hippocampal replay of the previous run activity used experienced animals and did not thoroughly investigate the activity of the hippocampal network prior to the run experience [21,25,31]. The remaining part of the studies on replay investigated the offline neuronal activity prior to the run experience, but only prior to runs on already visited (i.e. familiar) spaces in experienced animals. In addition, the latter studies either based their analysis on the activity of pairs of cells [35–37], which is not sensitive enough to detect extended sequences [22,24], or have insufficiently sampled spiking events during the sleep or rest epochs preceding the run experience [21–23,25,31]. In order to detect significantly correlated temporal sequences using spatial template matching [22] or Bayesian decoding [33] analysis of spiking events during sleep/rest a good coverage of the spatial environment by place cells is needed, whereas extended sleep periods are required to elicit sufficient spiking event ‘frames’, as discussed before [24]. Most importantly, all of these studies focused only on the activity of pyramidal cells that were active as place cells during the previous spatial experience, and in addition described the existence of significant replay events in no more than 50% of the total spiking events that could be detected during hippocampal frames of activity, in sleep or rest.
However, a more comprehensive look at all temporal sequences during sleep and rest (marked here with small letters) with respect to the place cell sequences (capitalized letters) recorded during run on a track (e.g. A-B-C-D-E; letters depict individual cells) will detect: (i) a subset of correlated temporal sequences (e.g. a-b-c-d-e; same place cells, same order of firing), and (ii) an equally large subset of uncorrelated sequences contributed by the same cells (e.g. e-b-c-a-d; same place cells, different order) but also (iii) a significant subset of temporal sequences contributed by the ‘silent’ cells [38,39] that are active during sleep or rest, but not while the animals explore the track (e.g. f-g-h-i-j, silent cells only; c-f-g-h-a-i, mix of place cells and silent cells). Within this overall distribution of temporal sequences, the subset of replay events represents a minority. Indeed, recent more conservative estimations of the incidence of replay events out of the total number of events detected using both silent and place cells report that about 15% of all detected events during sleep or rest are representations of the recent run experience [24,33]. This rather low percentage raises several questions. Why is the hippocampal network devoting 85% of its energy and time during sleep and rest to an activity that has little to do with the recent spatial experience of the animal or with the memory of the recently encoded information? Is it the case that the remaining, non-replay temporal sequences rather reflect the default organization of the hippocampal formation into sequential cellular assemblies [40]? Finally, in experimentally naive animals, is the order in which hippocampal cells fire during the sleep or rests epochs that precede a novel spatial experience predictive or correlated with the order in which they will fire as place cells during the experience? An affirmative answer will demonstrate that the brain of experimentally naive animals is not tabula rasa.
3. Distinct preplay of multiple novel spatial experiences in the hippocampus
In a recent set of experiments, we investigated whether temporally structured activity exists in the hippocampal network of experimentally naive animals during sleep and rest and whether the temporal pattern of that activity is predictive or correlated with the pattern of cellular activation during repeated runs on a novel linear track [24,41]. Prior to our experiments, the dominant model of hippocampal place cell ensemble activity had postulated that place cell firing order is established for the first time during the exploration to encode the spatial experience, and is subsequently replayed during sleep or rest perhaps to enable the consolidation of the encoded experience [26,27,32,35]. In contrast with this view, we found that during a significant proportion (approx. 10%) of awake resting epochs, the temporal order of firing of a mix of silent cells and previously active place cells was predictive of their future order of firing as place cells on a novel linear track [24]. We called this novel phenomenon preplay [24,41] because the predictive temporal sequences preceded the matching place cell sequences on the novel track. The preplay sequences were not a replay of the recent activity because the order in which the previously active place cells fired on the novel track was different from the order in which they had fired during the previous exploration of the familiar track, and the preplay sequences were not correlated with the place cell sequences active on the familiar track [24]. The preplay sequences occur prior to the exploration of novel spaces and in that respect they are different from the forward replay sequences that occur in anticipation of runs in already visited, familiar linear [25] and two-dimensional spaces [42].
The preplay sequences occurred more frequently during resting periods in the spatial locations adjacent to the novel track compared with the more remote locations [24]. This indicates that in the awake resting state, the external cues from the environment as well as factors internal to the animal (i.e. planning, imagining) modulate which specific cell assembly sequences are active at given times and locations [24]. A recent study [42] using experienced rats showed that an animal's intention to reach a known nearby goal location is associated with a number of temporal sequences that, when decoded, depict future paths ending in that familiar goal location. As the goal location was daily assigned to one of the 36 possible goal locations on the familiar two-dimensional arena, the spiking events depicting the paths specifically ending in that location represented a relatively small, though significant, proportion out of all possible temporal sequences recorded in the hippocampus during that session (2–3%) [42]. As in our initial study [24] the awake resting preplay was associated with animals' prior exposure to part of the experimental apparatus (i.e. room context and the familiar track) and possibly with undetermined visual access to the area of the future trajectory, it was not entirely clear whether the prior experience on the familiar track was necessary for preplay to occur.
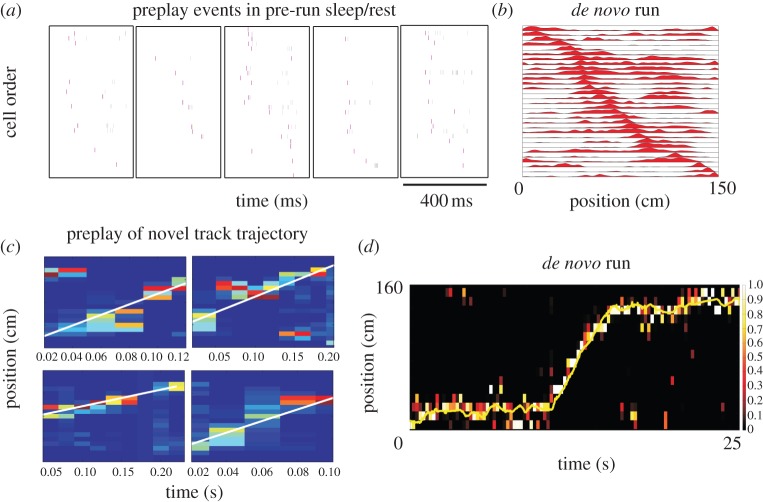
In order to address this necessity issue, we recently recorded 1–2 h of sleep/rest from naive rats (figure 2a) before they had any visual or physical access to a linear track [41]. Subsequently, the naive animals ran for the very first time on a linear track and the newly established place cell sequences (figure 2b) were correlated with the temporal sequences fired during the preceding sleep/rest session (figure 2a). Intriguingly, we found significant preplay sequences (figure 2a,c) for the future place cell sequences (figure 2b) and future spatial trajectories (figure 2d) on the novel track in 7–10% of all spiking events recorded during the preceding sleep in the naive rats [41]. This result demonstrates that the hippocampal network, and likely the remaining brain, is not tabula rasa in the naive animals (figure 3a) but it is preconfigured by default into sequentially active cellular assemblies (figure 3b), possibly as a result of previous, spatially unstructured experiences. This indicates that a novel spatial experience does not ‘instruct’ a blank hippocampal network as previously thought (figure 3a), but rather ‘selects’ an ensemble of cells whose preconfigured sequential activation is associated with a given spatial experience (figure 3c). The specific external cues from the environment and factors internal to the animal (e.g. imagining) active at the time of the spatial experience likely contribute to the selection of the specific ensemble of cells. It is not entirely clear whether the preconfigured sequences originate from preconfigured maps or, conversely, neurons have an inherent temporal bias in between them that might influence place map formation. Given the similar range of proportions for the ‘putatively intended’ preplay events during awake rest [24,42] in experienced animals and the preplay events during sleep/rest [24,41] in naive animals, it is not entirely clear whether in the two experimental conditions (i.e. awake rest and sleep) we are detecting a neuronal organization that goes beyond the default preconfigured architecture of the hippocampal network.
Figure 2.
Examples of preplay (a) of a future novel place cell sequence (b) using template matching method and of preplay (c) of a future novel spatial trajectory (d) using Bayesian decoding method in the sleeping rat. Reproduced with permission from Dragoi & Tonegawa [41].
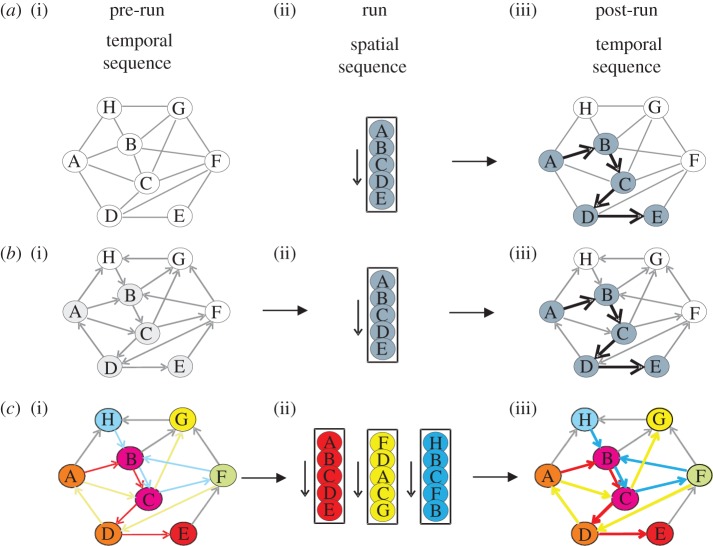
Figure 3.
Temporal order diagrams emphasizing order of firing of hippocampal cells during the sleep/rest epochs preceding (i) and following (iii) a de novo run session on a linear track (ii). (a) Earlier interpretations of externally driven hippocampal network emphasizing the lack of temporal sequences during pre-run sleep and the predominant replay of the recent experience during post-run sleep. (b) Internally driven temporal sequences during pre-run (preplay) and post-run sleep. (c) Selection of specific preplay sequences during run from a repertoire of temporal sequences existing in the preconfigured hippocampal network during pre-run sleep. Circled letters represent individual cells; arrows represent order of firing on individual tracks. Colours mark cells specifically active during run on individual tracks (red, yellow and blue) or on a combination of tracks (remaining colours).
The preconfigured hippocampal network during sleep/rest was able to preplay multiple, distinct, parallel spatial experiences as early as 6–8 h before the actual spatial experiences would occur [41]. Using three individual tracks connected in a letter ‘U’ shape, we were able to show the existence of three separate clusters of temporal sequences that individually preplayed each of the three tracks with very high specificity, 90% on a pair of novel tracks [41]. The basic unit that uniquely encoded each of the tracks was represented by the sequence of place cells on each track, rather than the identity of individual place cells, 90% of which were shared between the individual tracks. The hippocampal architecture was known to support the formation of novel individual place cells in any novel environment [27,43–46]. However, it was previously not known whether the place cell sequences on a novel track are created de novo during the experience [22,36,47] or whether and to what extent the naive hippocampal network was preconfigured into sequential cellular assemblies (i.e. Hebbian phase sequences) that could rapidly be used to encode future novel spatial experiences [24,41]. We further estimated the capacity of the naive hippocampal network that we recorded during sleep/rest to preplay future novel experiences of similar complexity and distinctiveness. We propose that at least 15 novel linear tracks could be separately encoded by the sets of sequential cellular assemblies as recorded during the preceding sleep/rest state [41]. This does not mean that during an animal's entire lifetime, the hippocampus can only encode 15 different spatial experiences. Rather, just like in the case of working memory that can actively hold 7 ± 2 items at a time [48,49], the 15 experiences refer to the number of linear trajectories that can be simultaneously preplayed with 90% specificity by the recorded group of neurons (up to 55 neurons/animal) in the current spatial context [50]. If more neurons are recorded simultaneously and larger overlaps are allowed between the decoded spatial experiences, and if multiple spatial contexts are considered, then one would expect a higher capacity for the hippocampal network to encode multiple spatial experiences. However, as very low percentages of individually significant preplay events may not reach an overall significant incidence level under those conditions, the practical experimental demonstration of large hippocampal network capacities might technically face a detectability limit.
4. Selection of preconfigured pre-representations: functional significance of preplay
Before the preplay phenomenon was described in the hippocampus, the existing models of neuronal ensemble organization across different animal behaviours and brain states and their role in processing different stages of memory formation were largely focusing on the encoding part and on the subsequent sleep/rest period [22,26,51,52]. It has been assumed that in experienced rats prior to a new spatial experience, the activity of the hippocampal network was noisy and did not exhibit any form of organization in sequential cellular assemblies, particularly if no run on a familiar or novel track occurred in the past 24 h [22,53]. However, a single run session on a novel or familiar track was considered sufficient to instruct the CA1 network of the hippocampus to repeatedly fire in unique spatial–temporal sequences during run, in association with theta oscillations (figure 3a). This sequential activity was repeatedly shown to be replayed in compressed temporal sequences during the sleep or resting period following the run, for 1–2 h, possibly owing to an increase in the firing rate of the previously active place cells [54,55]. It was hypothesized that this replay process facilitated memory consolidation as well as the transfer of the encoded information to neocortical brain areas during the postrun sleep, particularly during epochs of synchronous hippocampal ripples and neocortical spindles [26,28]. By contrast, the demonstration of preplay sequences indicated that the hippocampal network of naive mice and rats was preconfigured before the novel experience and further argued that the firing sequences on the novel track were not entirely created de novo in response to the external stimuli (figure 3b) [24,34,39]. Instead, the internal neuronal dynamics during resting and sleep organized the hippocampal cellular assemblies into temporal sequences that contributed to the encoding of the novel experience occurring in the future. Furthermore, the existence of distinct preplay sequences for multiple novel spatial experiences indicated that the place cell sequence of a novel spatial experience is determined, in part, by an online selection of a subset of cellular firing sequences from a larger repertoire of pre-existing temporal firing sequences in the hippocampal cellular assembly network which become rapidly bound to the novel experience (figure 3c) [41]. Conceptually, this indicates that novel stimuli from the external world select from their pre-representations rather than construct de novo our internal representations of the world.
What could be the functional significance of a preconfigured hippocampal network? Both episodic memory and imagining are sequential processes embedded in the spatial–temporal continuum. The existence of temporally compressed neuronal sequences that are independent of the recent experience of the animal could support its ability to mentally travel into its past as well as into the future, a sophisticated process that may underlie higher cognitive functions like memory recollection [8,56,57], navigational planning [42,58,59], imagining [3,4,9], cognitive map formation [58,60] and schema-based rapid learning [61,62]. Recent work that demonstrated that mice and rats are able to successfully navigate in virtual environments [63–67] reinforces the idea that rodents are capable of generating internal representations. A hippocampal network that is preconfigured by default could also accelerate the encoding of novel spatial information into the hippocampus, a brain area known to support rapid animal learning [68] and spatial memory [69–71]. The preconfigured network of the hippocampus could be supported by the highly autoassociative nature of the neuronal communications in the area CA3 which are passed onto downstream area CA1. Anatomical support for the cellular assemblies [15,19,40] generation in neocortical networks has been recently reported in the rat [72].
Funding statement
We acknowledge support from the RIKEN Brain Science Institute.
Reference
- 1.Nyberg L, Kim AS, Habib R, Levine B, Tulving E. 2010. Consciousness of subjective time in the brain. Proc. Natl Acad. Sci. USA 107, 22 356–22 359. ( 10.1073/pnas.0908453107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulving E. 1972. Episodic and semantic memory. In Organization of memory (ed. Tulving E.), pp. 382–403. New York, NY: Academic Press. [Google Scholar]
- 3.Schacter DL, Addis DR, Buckner RL. 2008. Episodic simulation of future events: concepts, data, and applications. Ann. NY Acad. Sci. 1124, 39–60. ( 10.1196/annals.1440.001) [DOI] [PubMed] [Google Scholar]
- 4.Hassabis D, Kumaran D, Vann SD, Maguire EA. 2007. Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl Acad. Sci. USA 104, 1726–1731. ( 10.1073/pnas.0610561104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke J. 1690. An essay concerning human understanding. London, UK: Thomas Basset. [Google Scholar]
- 6.Plato 1894. The republic. Oxford, UK: Clarendon Press. [Google Scholar]
- 7.Kant I. 1781. Critique of pure reason. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. ( 10.1136/jnnp.20.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulving E. 2002. Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25. ( 10.1146/annurev.psych.53.100901.135114) [DOI] [PubMed] [Google Scholar]
- 10.Louie K, Wilson MA. 2001. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–156. ( 10.1016/S0896-6273(01)00186-6) [DOI] [PubMed] [Google Scholar]
- 11.Dresler M, Wehrle R, Spoormaker VI, Koch SP, Holsboer F, Steiger A, Obrig H, Sämann PG, Czisch M. 2012. Neural correlates of dream lucidity obtained from contrasting lucid versus non-lucid REM sleep: a combined EEG/fMRI case study. Sleep 35, 1017–1020. ( 10.5665/sleep.1974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamminga CA, Stan AD, Wagner AD. 2010. The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178–1193. ( 10.1176/appi.ajp.2010.09081187) [DOI] [PubMed] [Google Scholar]
- 13.Schobel SA, et al. 2013. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93. ( 10.1016/j.neuron.2013.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. ( 10.1016/0006-8993(71)90358-1) [DOI] [PubMed] [Google Scholar]
- 15.Dragoi G, Buzsaki G. 2006. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50, 145–157. ( 10.1016/j.neuron.2006.02.023) [DOI] [PubMed] [Google Scholar]
- 16.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172. () [DOI] [PubMed] [Google Scholar]
- 17.Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. 2006. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus 16, 785–794. ( 10.1002/hipo.20202) [DOI] [PubMed] [Google Scholar]
- 18.Huxter JR, Senior TJ, Allen K, Csicsvari J. 2008. Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat. Neurosci. 11, 587–594. ( 10.1038/nn.2106) [DOI] [PubMed] [Google Scholar]
- 19.Dragoi G, Harris KD, Buzsaki G. 2003. Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39, 843–853. ( 10.1016/S0896-6273(03)00465-3) [DOI] [PubMed] [Google Scholar]
- 20.Muller RU, Stead M, Pach J. 1996. The hippocampus as a cognitive graph. J. Gen. Physiol. 107, 663–694. ( 10.1085/jgp.107.6.663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. ( 10.1016/S0896-6273(02)01096-6) [DOI] [PubMed] [Google Scholar]
- 23.Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. ( 10.1038/nn1825) [DOI] [PubMed] [Google Scholar]
- 24.Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401. ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diba K, Buzsaki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242. ( 10.1038/nn1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzsaki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 27.Wilson MA, McNaughton BL. 1993. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058. ( 10.1126/science.8351520) [DOI] [PubMed] [Google Scholar]
- 28.Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128. ( 10.1016/S0896-6273(00)80629-7) [DOI] [PubMed] [Google Scholar]
- 29.Sirota A, Csicsvari J, Buhl D, Buzsaki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl Acad. Sci. USA 100, 2065–2069. ( 10.1073/pnas.0437938100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neill J, Senior T, Csicsvari J. 2006. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49, 143–155. ( 10.1016/j.neuron.2005.10.037) [DOI] [PubMed] [Google Scholar]
- 31.Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683. ( 10.1038/nature04587) [DOI] [PubMed] [Google Scholar]
- 32.Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918. ( 10.1038/nn.2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. 2010. Hippocampal replay is not a simple function of experience. Neuron 65, 695–705. ( 10.1016/j.neuron.2010.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. ( 10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 35.Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873. ( 10.1126/science.271.5257.1870) [DOI] [PubMed] [Google Scholar]
- 36.Gerrard JL, Burke SN, McNaughton BL, Barnes CA. 2008. Sequence reactivation in the hippocampus is impaired in aged rats. J. Neurosci. 28, 7883–7890. ( 10.1523/JNEUROSCI.1265-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson LT, Best PJ. 1989. Place cells and silent cells in the hippocampus of freely-behaving rats. J. Neurosci. 9, 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epsztein J, Brecht M, Lee AK. 2011. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120. ( 10.1016/j.neuron.2011.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson TJ, Kloosterman F, Wilson MA. 2009. Hippocampal replay of extended experience. Neuron 63, 497–507. ( 10.1016/j.neuron.2009.07.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebb DO. 1949. The organization of behavior: a neuropsychological theory. New York, NY: Wiley. [Google Scholar]
- 41.Dragoi G, Tonegawa S. 2013. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl Acad. Sci. USA 110, 9100–9105. ( 10.1073/pnas.1306031110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill AJ. 1978. First occurrence of hippocampal spatial firing in a new environment. Exp. Neurol. 62, 282–297. ( 10.1016/0014-4886(78)90058-4) [DOI] [PubMed] [Google Scholar]
- 44.Muller RU, Kubie JL. 1987. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. 2005. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309, 619–623. ( 10.1126/science.1114037) [DOI] [PubMed] [Google Scholar]
- 46.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 13, 995–1002. ( 10.1038/nn.2599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudrimoti HS, Barnes CA, McNaughton BL. 1999. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller GA. 1956. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol. Rev. 63, 81–97. ( 10.1037/h0043158) [DOI] [PubMed] [Google Scholar]
- 49.Lisman JE, Idiart MA. 1995. Storage of 7±2 short-term memories in oscillatory subcycles. Science 267, 1512–1515. ( 10.1126/science.7878473) [DOI] [PubMed] [Google Scholar]
- 50.Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. 2010. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J. Neurosci. 30, 7414–7422. ( 10.1523/JNEUROSCI.6021-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNaughton BL, et al. 1996. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 199, 173–185. [DOI] [PubMed] [Google Scholar]
- 52.Cheng S, Frank LM. 2008. New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313. ( 10.1016/j.neuron.2007.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsuno M, Lipa P, McNaughton BL. 2006. Methodological considerations on the use of template matching to study long-lasting memory trace replay. J. Neurosci. 26, 10 727–10 742. ( 10.1523/JNEUROSCI.3317-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavlides C, Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9, 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battaglia FP, Sutherland GR, Cowen SL, Mc Naughton BL, Harris KD. 2005. Firing rate modulation: a simple statistical view of memory trace reactivation. Neural Netw. 18, 1280–1291. ( 10.1016/j.neunet.2005.08.011) [DOI] [PubMed] [Google Scholar]
- 56.Squire LR. 1992. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231. ( 10.1037/0033-295X.99.2.195) [DOI] [PubMed] [Google Scholar]
- 57.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. 1999. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209–226. ( 10.1016/S0896-6273(00)80773-4) [DOI] [PubMed] [Google Scholar]
- 58.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford, UK: Oxford University Press. [Google Scholar]
- 59.Johnson A, Redish AD. 2007. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 27, 12 176–12 189. ( 10.1111/j.1460-9568.2007.05978.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tolman EC. 1948. Cognitive maps in rats and men. Psychol. Rev. 55, 189–208. ( 10.1037/h0061626) [DOI] [PubMed] [Google Scholar]
- 61.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. 2007. Schemas and memory consolidation. Science 316, 76–82. ( 10.1126/science.1135935) [DOI] [PubMed] [Google Scholar]
- 62.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RGM. 2011. Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895. ( 10.1126/science.1205274) [DOI] [PubMed] [Google Scholar]
- 63.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. 2010. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440. ( 10.1038/nn.2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harvey CD, Collman F, Dombeck DA, Tank DW. 2009. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946. ( 10.1038/nature08499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsaki G. 2012. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775. ( 10.1038/nn.3077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G, King JA, Burgess N, O'Keefe J. 2013. How vision and movement combine in the hippocampal place code. Proc. Natl Acad. Sci. USA 110, 378–383. ( 10.1073/pnas.1215834110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravassard P, Kees A, Willers B, Ho D, Aharoni DA, Cushman J, Aghajan ZM, Mehta MR. 2013. Multisensory control of hippocampal spatiotemporal selectivity. Science 340, 1342–1346. ( 10.1126/science.1232655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. 2003. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38, 305–315. ( 10.1016/S0896-6273(03)00165-X) [DOI] [PubMed] [Google Scholar]
- 69.Olton DS, Wible CG, Shapiro ML. 1986. Mnemonic theories of hippocampal function. Behav. Neurosci. 100, 852–855. ( 10.1037/0735-7044.100.6.852) [DOI] [PubMed] [Google Scholar]
- 70.Handelmann GE, Olton DS. 1981. Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res. 217, 41–58. ( 10.1016/0006-8993(81)90183-9) [DOI] [PubMed] [Google Scholar]
- 71.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. 2000. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27, 623–633. ( 10.1016/S0896-6273(00)00071-4) [DOI] [PubMed] [Google Scholar]
- 72.Perin R, Berger TK, Markram H. 2011. A synaptic organizing principle for cortical neuronal groups. Proc. Natl Acad. Sci. USA 108, 5419–5424. ( 10.1073/pnas.1016051108) [DOI] [PMC free article] [PubMed] [Google Scholar]