Abstract
Sharp wave/ripple (SWR, 150–250 Hz) hippocampal events have long been postulated to be involved in memory consolidation. However, more recent work has investigated SWRs that occur during active waking behaviour: findings that suggest that SWRs may also play a role in cell assembly strengthening or spatial working memory. Do such theories of SWR function apply to animal learning? This review discusses how general theories linking SWRs to memory-related function may explain circuit mechanisms related to rodent spatial learning and to the associated stabilization of new cognitive maps.
Keywords: memory consolidation, place cell, sleep, cognitive map
1. Introduction
There is increasing evidence suggesting that network oscillatory patterns play major roles in the memory-related function of the hippocampus. Among these, the so-called sharp wave/ripple (SWR) patterns have drawn special attention because these are linked to memory consolidation. These SWR events are best marked by the transiently occurring 150–250 Hz ripple oscillations near the CA1 pyramidal cell layer [1–3]. They are usually present in inactive behavioural periods such as waking immobility and slow-wave sleep but they also occur during consummatory behaviour, grooming and brief interruptions in locomotion [4,5].
The possible role of SWRs originated from theories suggesting a specific role of the hippocampus in memory consolidation during sleep [6]. According to this theory, the hippocampus transiently stores recently learned memory traces, which are spontaneously reactivated during sleep. This process could enable the transfer of memory traces to extra-hippocampal locations, where they are ultimately stored. Because large number of neurons synchronously fire action potentials together during SWRs, it has been suggested that this network state is optimal for the transfer of these memory traces to extra-hippocampal locations [7,8]. Therefore, it has been suggested that during SWRs, previous waking neuronal activity is reactivated, which represent memory traces that might undergo a process of consolidation. Indeed, a large number of studies have confirmed the reactivation of waking neuronal activity patterns during sleep, particularly during SWRs [5,9–12]. It was also shown that the disruption of sleep SWRs through electrical stimulation leads to mild spatial memory impairments [13,14], although it is unclear whether SWR-coupled electrical stimulation caused plastic changes of synaptic weights in these experiments.
More recent work focused on the role of SWRs that occur during active waking periods, suggesting additional roles for SWRs beyond memory consolidation. It was proposed that the overlay of sensory-driven activity with the underlying network burst during these SWRs enables plastic processes to strengthen cell assemblies [5]. However, because the blockade of waking SWRs impairs spatial working memory [15], they are suggested to represent recall of memory traces that could be used for working memory.
This review discusses work suggesting roles for SWRs in learning and associated mnemonic functions from rodent experiments. It argues that the stabilization of cognitive maps in the hippocampus and beyond may be an underlying process through which spatial memories might be stabilized and examines the possibility that SWRs might facilitate such map stabilization.
2. Contribution of sharp wave/ripples in spatial learning and map stabilization
The simplest form of spatial learning is related to the ability of the animal to recognize the degree of familiarity of an environment, which is closely related to the recall of stable hippocampal place maps. This ability to detect place-associated novelty has been suggested to involve the hippocampus [16,17]. Moreover, the ability of animals to recognize changes of spatial configuration, for example the misplacement of local object cues, requires the hippocampus [18]. Place cells reorganize rapidly when the animal is placed into a new environment: typically new place fields appear, existing place fields disappear or move to different locations (for review: [19]). This remapping of place fields leads to the formation of an entirely new representation of that environment. Importantly, this map is reinstated later, when the animal is placed back into the same environment. The successful reinstatement of new hippocampal maps requires their stabilization, a process which is N-methyl-d-aspartate (NMDA) receptor-dependent and requires protein synthesis [20,21], and long-term potentiation induction triggers the remapping of hippocampal place fields [22]. Hence, the stabilization of newly formed maps in a novel environment is closely related to the ability of the animal to recognize this environment and this process requires circuit reorganization involving NMDA-dependent synaptic plasticity. In order to examine whether SWRs play a role in the spatial recognition of entire environments, one needs to explore whether SWRs occurring during sleep or waking periods promote the stabilization of new place maps.
Since, many studies have clearly indicated that sleep promotes memory consolidation [23,24], sleep SWRs are likely candidates for place map stabilization. Although a direct link has not been established between SWR-related reactivation and map stabilization, the reactivation of neuronal patterns representing novel environments supports such a role. In comparing the reactivation of familiar and novel environments, it was revealed that reactivation of newly formed maps is stronger. Moreover, the time period necessary for the animal to spend at a given location so that this location is reliably reactivated is similar to that needed for the stabilization of place fields [25,26].
How might reactivation during SWRs contribute to the stabilization of maps? SWR events have been shown to enable synaptic potentiation in vivo between those cells that are active within the same SWRs [27]. During reactivation, cells that encode similar locations fire together with high temporal synchrony. Therefore, reactivation during SWRs could enable the potentiation of synapses between those cells that code for similar locations, which can promote the strengthening of cell assemblies coding for similar locations. It is possible that place field stabilization can take place without SWRs; however, even if this were the case, it is likely that SWRs may yet strengthen place-related assemblies during sleep and ultimately promote their accurate expression during subsequent re-exposure to those environments. SWRs and associated sleep reactivation may also help the slow refinement of maps, for example those observed when animals were repeatedly exposed to environments with similar geometric layouts, which ultimately led to the divergence of maps representing these environments [28,29]. Reactivation may trigger plastic processes that facilitate the separation of maps by strengthening strongly associated cell assemblies, and at the same time weakening the joint firing of those cells that belong to different assemblies, a process that can lead to map divergence [25]. Moreover, sleep may enable the interactions of cell assemblies linked to different environments. Although the most recently explored environment is reactivated the strongest in sleep, other environments that the animal might have visited, or will visit, are also reactivated [25,30]. Therefore, sleep might provide a substrate for a wider process of consolidation in which different experiences are compared.
In terms of the sleep reactivation theory, the transfer of reactivated patterns to extra-hippocampal locations is a key step. Can the extra-hippocampal transfer of place cell assembly patterns play a role in the strengthening of maps? The interrelated nature of place maps in the hippocampus and those occurring in the entorhinal cortex (EC) is increasingly recognized. In fact, hippocampal place cell output is required for the normal activation of grid cells in the EC [31]. Therefore, the reactivation of hippocampal assemblies representing places could strengthen interrelated assemblies between the hippocampus and the EC. This can potentially contribute to the tuning refinement of grid cell firing fields as the environment becomes more familiar [32].
Those SWRs that occur during waking exploration periods have also been suggested to promote the stabilization of place maps. They have drawn considerable interest because neuronal activity in these SWRs can be influenced by ongoing place-related sensory inputs [33,34]. These exploratory-related SWRs that occur during brief immobility periods are influenced by the current location of the animal, unlike those occurring in longer quiet immobility periods which are not influenced by the location of the animal per se [5]. These SWRs are well placed to emphasize certain locations. During these SWRs, place cell firing is stronger when the animal is located inside the cell's place field as compared to outside, and there is a nonlinear increase of the location-specific SWR firing rate as compared with that during theta periods [5]. This can promote plasticity beyond that expected in areas in which SWRs did not occur, enabling SWRs to highlight locations of behavioural importance, for example those where food can be found, as we will soon describe. Overall, the exploration of novel environments has been associated with strengthened pyramidal firing responses during SWRs [35], which can further promote the stabilization of cell assemblies in this condition.
Both waking and sleep SWRs suggest a similar mechanism for map stabilization by strengthening spatial cell assemblies. Such strengthening of assemblies can enable more consistent place cell activation, and consequently the more accurate coding of places, and facilitate pattern completion processes when only partial or ambiguous sensory cues are available. In exploration of mazes, frequent reactivation of place cell firing sequences may also promote accurate map activation [12,36,37]. The repetition of these sequences during SWRs may help the formation of associations between assemblies encoding locations along the path through spike-timing-dependent plasticity. However, given that both the forward and reverse reactivation of firing sequences is observed in waking SWRs, such strengthening of sequences may be weak.
3. Role of sharp wave/ripples during goal-directed learning
Multiple studies have shown that many place maps reorganize their place fields as animals learn locations of behavioural valence; for example, place cells cluster their place fields near the escape platform of an annular version of the Morris water maze [38]. Similarly, when animals learn the location of rewards on the cheeseboard maze (figure 1), many place cells in the CA1 region, but not in CA3, fire near locations where food was hidden [39]. Previously, the relevance of goal-related remapping was questioned because changes in the animals’ stereotyped movement patterns can cause remapping. It was suggested that changes in path stereotypy could cause the remapping and it may not merely be due to goal-coding. However, on the cheeseboard maze, goal-oriented remapping occurs in the allocentric learning context only; it did not take place when guideposts mark the goals even though the movement path of the animal was identical in both conditions. Therefore, the preferred representations of goal locations are expected to help efficient navigation to goals and may therefore form a substrate for goal-related spatial memory traces. Moreover, recent findings are now revealing that reorganization of place cell activity patterns underlies the formation of a neural representation where distinct goal locations are embedded within a unique spatial schema [40].
Figure 1.
(a) The movement pattern of the animal on the cheeseboard maze. The example shows the animal's movement path during learning trials and in memory probe sessions before and after learning. Animals learned the location of three food rewards during the learning sessions which changed every day. Animals were able to learn the task within a few trials and they frequently crossed the previously learned locations in the probe trials in which food was no longer available. (b) Goal-related remapping of place cells. The examples show the rate maps of place cells during the probe sessions and in blocks of consecutive learning trials. (Adapted from [39].) (Online version in colour.)
Although goal-oriented learning leads to the remapping of place cells as in novel environments, there are differences between map formations in these two conditions. While novel environments can cause remapping in the entire hippocampus, newly learned goals on the cheeseboard are represented by CA1 cells only. The dynamical expression of new goal-oriented maps is also different. While new maps are rapidly formed in novel environments and remain stable later, the newly formed goal-oriented maps flicker initially with that of the previously learned goal representations [41]. Therefore, the role of SWRs in the stabilization of goal-oriented maps and their ultimate role in the consolidation of spatial memory traces necessitate further scrutiny.
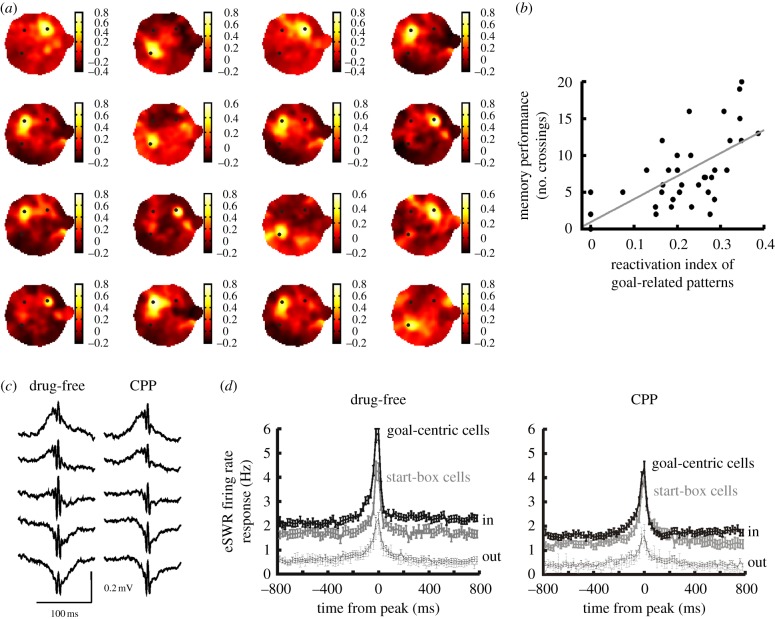
The role of SWRs in spatial goal learning was initially highlighted in experiments performed on multi-arm radial mazes. It was shown that learning the location of rewarded arms could be delayed if SWRs were disrupted, by applying electrical stimulation in subsequent sleep sessions [13,14]. Further evidence came from experiments on the cheeseboard maze, which linked SWRs-related reactivation to consolidation by showing that reactivation of goal locations in sleep predicts future memory performance (figure 2a,b) [39]. Following the learning of reward locations, newly learned locations are reactivated during most SWRs. Importantly, the frequency by which a given goal location is reactivated during sleep predicts the memory performance of the animal, measured in a probe session in which reward was no longer provided. This result is in line with the finding that reward-related hippocampal waking patterns are enhanced during SWR events [42].
Figure 2.
(a) Examples of reactivation maps calculated during sleep SWRs. Reactivation maps quantified the similarity of SWR assembly patterns with the average waking rate patterns expected at different locations. Note that usually one of the reward locations (black dots) is reactivated. (b) Reactivation of goal locations during SWR patterns predicts future memory performance. The number of crossings in probe trials was predicted by the proportions of SWRs in which a goal location was reactivated. (c) Examples of averaged SWRs recorded in learning in drug free and under NMDA blockade (CPP). (d) The averaged in- and out-field SWR responses of pyramidal cells at new goal locations and at the start box (eSWR, exploration-related SWRs). (Adapted from [39].) (Online version in colour.)
Data suggest that waking SWR occurring at goal locations during learning may play a special role in learning [39]. The number of SWRs that occur at the given goal location predicts the future preference of the animal in returning to that location during subsequent probe sessions. Importantly, this correlation was significant only for those SWRs that occurred in brief pauses of exploration, and consequently assembly activity was related to those neurons that code for current location of the animal. Moreover, the strength of SWR network synchronization also predicts future memory performances. Cell firing with the SWRs at the newly learned goal locations are more strongly activated than at the start box where food was also presented as this location was not new to the animal. Therefore, this result is similar to the strengthened SWRs response suggested in novel environments (figure 2c,d) [35]. Moreover, in the experiments in which guideposts marked the goals, the strength of SWRs responses at the goal locations were similar to that at the start box. Collectively, these findings suggest that SWRs responses are facilitated only when new locations are learned. However, data suggest that the strengthened activation of cells at these SWRs is not needed for the animal to learn the task, but it may be needed for the animal to remember these locations later. When NMDA receptors are blocked, animals are able to learn the reward locations but, after a long delay (more than 1 h), they are no longer able to recall these locations during the memory probe trials. In these experiments, as in the local cue-guided condition, cells do not fire stronger during SWRs at the newly learned goal locations (figure 2d, right panel). Therefore, map stabilization might be facilitated by SWR responses at the goal locations.
A recent work has suggested an alternative role for waking SWRs contributing to spatial working memory [15]. Experiments that provided support for SWR involvement in spatial working memory were performed on a W-maze. During this task, animals were required to visit all three main arms of the maze sequentially, first in the forward and then in the reverse order. This task has a working memory component when the animal is leaving the middle arms and reference memory component when it is approaching the middle arm from one of the side arms. In this task, the blockade of the waking SWRs at the middle arm causes deficits in selecting the correct side arm. By contrast, the blockade of SWRs at the side arms does not cause deficits in accurately selecting the middle arm. Therefore, these experiments demonstrate a causal link between waking SWRs and spatial working memory requirement. Moreover, it has been also shown that reactivation of waking firing sequences during SWRs in such a maze predicts future choice if the animal is trained in the task [43]. These experiments provide support for a role of waking SWRs in spatial working memory; waking SWRs could provide a role for combining past and future experiences to rapidly update mnemonic neuronal representations to meet task demands in solving spatial tasks. Further support for the role of SWRs to solve ongoing spatial tasks comes from the finding that reactivated firing sequences during waking SWRs often predict the future trajectory of the animal to reach a desired goal in two-dimensional environments [44]. Therefore, waking SWRs may serve diverse functions related to mnemonic task demand: they may assist in the stabilization of spatial memory traces, when these are learned, or by reactivating relevant patterns, SWRs may facilitate proper behavioural choices to dynamically changing task demands, for example those required to perform spatial working memory tasks.
4. Conclusion
As we described above, SWRs trigger transient network synchronization in the hippocampus and beyond in downstream areas, which may serve two independent circuit functions. It may facilitate neuronal plasticity by strengthening connections between cells that are synchronously activated together [27] and, at the same time, enable the transfer of information outside the hippocampus and consequently may trigger the coherent activation of interrelated mnemonic assemblies in different brain areas [9,34,45,46].
Plastic changes facilitated by SWRs are expected to lead to the strengthening of place cell assemblies. This may be a generic role of SWRs, which may take place both during sleep and waking SWRs. During sleep, many different cell assemblies could potentially be strengthened, and sleep may enable interactional changes between different assemblies and ultimately their transformations. By contrast, waking, brief immobility-associated SWRs may emphasize assemblies that code the locations where the SWRs tend to occur, for example reward locations.
SWRs are potentially able to synchronize neurons across multiple downstream brain regions within short time windows. However, so far only limited data are available describing how SWRs in the hippocampus recruit relevant assemblies in extra-hippocampal locations [46,47]. As we suggested above, network synchronization at SWRs could promote map stabilization across interrelated assemblies of the hippocampus and the EC. However, in a wider context, SWRs may initiate interactions with other brain regions to bind place with other behaviourally relevant information—a process that might be needed for the formation of episodic-like memory traces. Therefore, SWRs are well placed to coordinate brain-wide networks involved in systems consolidation. However, SWR synchronization may foster the interactions of brain regions related to other functions as well, for example, spatial working memory may also require the coordinated action of many brain regions [33]. Thus, SWRs may not serve dedicated functions for consolidation, rather their role may be more general in providing a means of coordinating actions of among brain regions.
References
- 1.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. 1992. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027. ( 10.1126/science.1589772) [DOI] [PubMed] [Google Scholar]
- 3.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. 1999. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J. Neurosci. 19, RC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsaki G, Leung LW, Vanderwolf CH. 1983. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 287, 139–171. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill J, Senior T, Csicsvari J. 2006. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49, 143–155. ( 10.1016/j.neuron.2005.10.037) [DOI] [PubMed] [Google Scholar]
- 6.Marr D. 1971. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B 262, 23–81. ( 10.1098/rstb.1971.0078) [DOI] [PubMed] [Google Scholar]
- 7.Buzsaki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 8.McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457. ( 10.1037/0033-295X.102.3.419) [DOI] [PubMed] [Google Scholar]
- 9.Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. ( 10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 10.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudrimoti HS, Barnes CA, McNaughton BL. 1999. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. ( 10.1016/S0896-6273(02)01096-6) [DOI] [PubMed] [Google Scholar]
- 13.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223. ( 10.1038/nn.2384) [DOI] [PubMed] [Google Scholar]
- 14.Ego-Stengel V, Wilson MA. 2010. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10. ( 10.1002/hipo.20707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadhav SP, Kemere C, German PW, Frank LM. 2012. Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458. ( 10.1126/science.1217230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisman JE, Otmakhova NA. 2001. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus 11, 551–568. ( 10.1002/hipo.1071) [DOI] [PubMed] [Google Scholar]
- 17.Vinogradova OS. 2001. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11, 578–598. ( 10.1002/hipo.1073) [DOI] [PubMed] [Google Scholar]
- 18.Dere E, Huston JP, Souza Silva MA. 2007. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 31, 673–704. ( 10.1016/j.neubiorev.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 19.Muller R. 1996. A quarter of a century of place cells. Neuron 17, 813–822. ( 10.1016/S0896-6273(00)80214-7) [DOI] [PubMed] [Google Scholar]
- 20.Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. 2004. The long-term stability of new hippocampal place fields requires new protein synthesis. Proc. Natl Acad. Sci. USA 101, 3656–3661. ( 10.1073/pnas.0400385101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. 1998. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280, 2121–2126. ( 10.1126/science.280.5372.2121) [DOI] [PubMed] [Google Scholar]
- 22.Dragoi G, Harris KD, Buzsaki G. 2003. Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39, 843–853. ( 10.1016/S0896-6273(03)00465-3) [DOI] [PubMed] [Google Scholar]
- 23.Stickgold R, Hobson JA, Fosse R, Fosse M. 2001. Sleep, learning, and dreams: off-line memory reprocessing. Science 294, 1052–1057. ( 10.1126/science.1063530) [DOI] [PubMed] [Google Scholar]
- 24.Diekelmann S, Born J. 2010. SLEEP: the memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. ( 10.1038/nrn2762-c2) [DOI] [PubMed] [Google Scholar]
- 25.O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. 2008. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci. 11, 209–215. ( 10.1038/nn2037) [DOI] [PubMed] [Google Scholar]
- 26.Frank LM, Stanley GB, Brown EN. 2004. Hippocampal plasticity across multiple days of exposure to novel environments. J. Neurosci. 24, 7681–7689. ( 10.1523/JNEUROSCI.1958-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King C, Henze DA, Leinekugel X, Buzsaki G. 1999. Hebbian modification of a hippocampal population pattern in the rat. J. Physiol. 521, 159–167. ( 10.1111/j.1469-7793.1999.00159.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J. 2002. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416, 90–94. ( 10.1038/416090a) [DOI] [PubMed] [Google Scholar]
- 29.Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. 2005. Progressive transformation of hippocampal neuronal representations in ‘morphed’ environments. Neuron 48, 345–358. ( 10.1016/j.neuron.2005.09.007) [DOI] [PubMed] [Google Scholar]
- 30.Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397 ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. 2013. Grid cells require excitatory drive from the hippocampus. Nat. Neurosci. 16, 309–317. ( 10.1038/nn.3311) [DOI] [PubMed] [Google Scholar]
- 32.Barry C, Ginzberg LL, O'Keefe J, Burgess N. 2012. Grid cell firing patterns signal environmental novelty by expansion. Proc. Natl Acad. Sci. USA 109, 17 687–17 692. ( 10.1073/pnas.1209918109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr MF, Jadhav SP, Frank LM. 2011. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14, 147–153. ( 10.1038/nn.2732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. 2010. Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–229. ( 10.1016/j.tins.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 35.Cheng S, Frank LM. 2008. New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313. ( 10.1016/j.neuron.2007.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873. ( 10.1126/science.271.5257.1870) [DOI] [PubMed] [Google Scholar]
- 37.Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683. ( 10.1038/nature04587) [DOI] [PubMed] [Google Scholar]
- 38.Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. 2001. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J. Neurosci. 21, 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 13, 995–1002. ( 10.1038/nn.2599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. 2013. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. J. Neurosci. 33, 10 243–10 256. ( 10.1523/JNEUROSCI.0879-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupret D, O'Neill J, Csicsvari J. 2013. Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron 78, 166–180. ( 10.1016/j.neuron.2013.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer AC, Frank LM. 2009. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–921. ( 10.1016/j.neuron.2009.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer AC, Carr MF, Karlsson MP, Frank LM. 2013. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77, 1163–1173. ( 10.1016/j.neuron.2013.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74 ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moscovitch M, et al. 2005. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J. Anat. 207, 35–66. ( 10.1111/j.1469-7580.2005.00421.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji DY, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. ( 10.1038/nn1825) [DOI] [PubMed] [Google Scholar]
- 47.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. 2009. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7, e1000173 ( 10.1371/journal.pbio.1000173) [DOI] [PMC free article] [PubMed] [Google Scholar]