Abstract
The theta oscillation is a neuroscience enigma. When a rat runs through an environment, large-amplitude theta oscillations (4–10 Hz) reliably appear in the hippocampus's electrical activity. The consistency of this pattern led to theta playing a central role in theories on the neural basis of mammalian spatial navigation and memory. However, in fact, hippocampal oscillations at 4–10 Hz are rare in humans and in some other species. This presents a challenge for theories proposing theta as an essential component of the mammalian brain, including models of place and grid cells. Here, I examine this issue by reviewing recent research on human hippocampal oscillations using direct brain recordings from neurosurgical patients. This work indicates that the human hippocampus does indeed exhibit rhythms that are functionally similar to theta oscillations found in rodents, but that these signals have a slower frequency of approximately 1–4 Hz. I argue that oscillatory models of navigation and memory derived from rodent data are relevant for humans, but that they should be modified to account for the slower frequency of the human theta rhythm.
Keywords: theta oscillations, navigation, memory, electroencephalography, hippocampus
1. Introduction
The hippocampus is a uniquely important brain structure because it has been linked to a broad range of mammalian behaviours. It plays a critical role in various types of brain processes ranging from human episodic memory [1] to learning [2] and spatial navigation [3]. Although some early findings suggested that the hippocampus is primarily involved in spatial processing in rodents [4], a growing body of work has shown that the hippocampus supports general neurocomputational processes in various species that extend far beyond the neural representation of space [5–7].
A central component of many theories of hippocampal function is the theta rhythm. Theta is a large-amplitude neuronal oscillation that appears prominently in electrical recordings from the hippocampus when it is active in supporting the current behaviour [8,9]. Theta oscillations are most extensively studied in rodents, where they reliably appear at approximately 4–8 Hz during voluntary movement [10,11]. However, hippocampal theta oscillations appear during other behaviours besides movement, and in some species besides rodents [12], and thus are thought to support wide-ranging neural processes [6].
Much research has focused on understanding theta's precise physiological role. One set of theories emphasizes the diverse role that theta plays in memory. An example of this is the finding that there is increased efficiency of memory encoding during periods of high theta amplitude [13]. Theta may support memory by serving as a timing signal that causes the groups of neurons that are simultaneously active to spike at similar times and become linked via long-term plasticity [6,14]. Other theories stress the role of theta in computing location during spatial navigation. Specifically, theta may help compute an animal's spatial location during navigation by allowing grid cells in entorhinal cortex [15] to represent location using computations based on theta phase [16]. Owing to the range of different functions that have been ascribed to theta, an emerging view is that theta oscillations are a fundamental signal that are essential for a range of behaviours [7].
Given the prominent role of theta oscillations in theories of mammalian brain function, it might be considered surprising that, in practice, there are many species where hippocampal theta oscillations are rarely observed. In particular, in humans evidence of hippocampal rhythms at 4–8 Hz is weak [17]. Further, a recent study showed that 4–8 Hz theta oscillations are rare in bats [18]. The apparent absence of theta rhythms from bats has been called a ‘potentially cataclysmic’ issue for theta-based theories of spatial navigation and memory [19]. Certainly, theta oscillations cannot be critical for all mammalian memory and navigation if these oscillations are absent from many species! Thus, understanding whether theta is present in all mammals—including humans—has critical implications for understanding the neural basis of memory and navigation.
Here, I review research on human hippocampal oscillations. I focus on direct hippocampal recordings from neurosurgical patients [20] because this is the only method for obtaining human brain data that are comparable with laboratory recordings of rodents. This literature indicates that the human hippocampus does indeed exhibit oscillations that resemble the 4–10 Hz theta oscillations seen in rodents, but that these rhythms instead appear at the slower frequency range of 1–4 Hz. Owing to their slower frequency, these oscillations may have been missed by many previous studies. The existence of human hippocampal oscillations at 1–4 Hz suggests that various theta-driven models of memory and navigation [6,7,16,21–23] are indeed relevant for humans, and perhaps other species, but that they may require modification to account for interspecies variations in frequency, amplitude and duration.
2. Hippocampal theta oscillations in rodents
The vast majority of research on the hippocampal theta rhythm comes from rodent brain recordings. Here, I outline the key findings of this work for comparison with data from humans. There is evidence for two differentiable types of hippocampal theta oscillations in rodents: Type 1 theta, which is related to movement, and Type 2 theta, which appears when the animal is still or anxious [11]. Below, I focus on Type 1 theta because this is the signal most closely linked to navigation and memory.
In rodents, theta appears as a rhythmic fluctuation at 4–10 Hz in recordings from the hippocampus when it is actively engaged in the current behaviour [9]. A wide range of behaviours elicit theta oscillations [6], but theta is most often studied in relation to movement and sensory processing. A challenge in characterizing theta's functional role is that the oscillation is only approximately linked to the timing of specific behavioural events. For example, although theta oscillations often begin when an animal starts to run, these oscillations may persist after running ceases [10,24]. Similarly, although theta oscillations are generally present when an animal sniffs or otherwise perceives the environment, the timing of individual theta cycles does not correspond to timepoints when sensory information enters the brain [25].
These correlational findings strongly suggest that theta oscillations are behaviourally relevant. What is theta's precise physiological function? By comparing the properties of theta oscillations in relation with simultaneous neuronal spiking and behaviour, it demonstrated a range of ways in which theta oscillations play important roles in neural information representation and computation.
One way that theta oscillations support information coding is by supporting a phenomenon called phase coding, in which individual neurons convey information by spiking at particular phases of the theta cycle. An example of phase coding is demonstrated by the hippocampal place cell, which varies the theta phase when it spikes according to the animal's precise location [14,26]. Phase coding appears to be a critical element for allowing behavioural events to be encoded into memory. When two neurons activate to represent related locations or events, phase coding may cause them to activate at theta phases within approximately 50 ms of each other. This coincident spiking could initiate long-term potentiation and cause memory formation by strengthening the synapses that link these cells [6,14].
Theta oscillations also support a different type of neuronal timing, in which they temporally segment different types of neural computations. This segmentation can occur both across and within theta cycles. Across-cycle theta segmentation has been demonstrated in recordings of assemblies of place cells, where remapping between different cognitive maps is paced by the onset of individual theta cycles [27], and in simultaneous recordings of place and head-direction cells, each of which activate on alternate theta cycles [28]. Theta also modulates the nature of neuronal processing through the progression of each individual oscillation. Within a theta cycle, evidence suggests that the peak and trough phases of hippocampal theta are each linked to memory encoding and retrieval processes, respectively [23,29].
In addition to coordinating the timing of neuronal spiking, a different hypothesized role for theta is signalling broader aspects of behaviour. Here, for example, the frequency of theta is a functionally relevant signal, as it is positively correlated with the animal's running speed [24]. Further, theta's frequency decreases when an animal is in a novel environment [30], which suggests that decreases in theta frequency could be a neural signal of novelty [31].
Beyond the hippocampus, the theta oscillation also is important in coordinating brain-wide activity [6,21]. Neuronal spiking in widespread brain regions is phase locked to hippocampal theta oscillations, including somatosensory [32], prefrontal [33] and entorhinal cortices [34]. This coordination extends beyond individual action potentials, as theta oscillations modulate the timing and amplitude of local and distributed gamma oscillations [22,32,35–37].
The view emerging from this body of work is that, based on studies in rodents, the theta oscillation modulates an extremely wide range of neural patterns, including the timing, rate, spatial distribution and spectral characteristics of neuronal activity in various behaviours. Based on these findings, theta had already been proposed as the central unifying feature for the mammalian memory system, integrating widespread sensory information to support memory and behaviour [6,21]. However, recently, interest in theta oscillations increased even further, owing to the belief that these rhythms were important for the representation of spatial location by grid cells.
3. Theta oscillations and grid cells in bats and rats
The discovery of grid cells in the entorhinal cortex [15,38,39] was a pivotal step in our understanding of how the brain represents spatial information [40]. When a rodent moves through an environment, grid cells encode the animal's spatial location by each spiking at a set of positions that are arranged in a triangular grid across the environment [15]. Following the discovery of grid cells, subsequent research examined how grid patterns were formed and maintained. This work suggested that theta oscillations were critical for these processes [16,41].
Two findings in rodents link theta oscillations to the generation of grid-cell firing patterns. First, there is a correlation between the spacing of the locations in the environment where each grid cell activates and how much the cell's theta-band membrane-potential oscillations accelerate when the cell is active [42]. This suggests that intracellular theta-like patterns help determine how each neuron represents locations in the physical world [16,41]. Second, grid-cell firing patterns are disrupted in intervals when hippocampal theta oscillations are absent [43,44].
These findings suggest that the phase and amplitude of theta oscillations are signals that play an essential role in a properly functioning grid-cell network. Further, it is possible that theta oscillations could be an essential component of widespread neurocomputational processes beyond location, because computational models indicate that other analogous grid-like signals could represent different types of behavioural information [45].
This view was thrown into flux by research suggesting that similar theta oscillations were not existent in species besides rodents. Crawling bats exhibit grid cells without simultaneous theta oscillations [18]. A subsequent study compared the cell-membrane properties of rodent and bat entorhinal neurons and found that, unlike such cells in rats, bat grid cells do not exhibit theta-band resonance patterns [46]. Seemingly, these interspecies variations indicate that bat grid cells are caused by a different, non-theta mechanism that fundamentally differs from the theta-linked grid cells found in rodents [19]. This apparent difference between the bat and rat grid cell systems posed an important challenge for the field [47]. Researchers studying place cells had previously assumed that rats are a meaningful model system for navigation and memory in all mammals. Instead, these findings indicate that the neural basis of spatial processing must be considered separately in different species.
However, a closer examination of these data [46] suggests a different interpretation. It is true that bat entorhinal neurons do not have membrane fluctuations in the traditional 4–8 Hz ‘theta’ band. But, the data actually show that these cells did indeed have significant membrane resonance instead at the slower frequency of approximately 1.5 Hz. Thus, it is possible that bats could indeed exhibit oscillations related to navigation and movement, but that these rhythms occur at a slower frequency of approximately 1.5 Hz compared with the 4–8 Hz of theta oscillations in rodents. The presence of these slower oscillations remained a distinct possibility, because the initial study on theta oscillations in crawling bats [18] could not examine data on frequencies below 4 Hz owing to technical limitations (N. Ulanovsky 2013, personal communication). As I explain below, recordings from humans also show evidence for hippocampal oscillations at slower frequencies, consistent with this view.
4. Hippocampal theta oscillations in humans
For obvious ethical reasons, it is challenging to study the electrical activity of the human hippocampus during behaviour. Although scalp electroencephalography (EEG) recordings can measure aggregate neuronal activity from the outer layers of the neocortex, this technique cannot reliably measure the activity of a deep structure like the hippocampus. However, an emerging approach makes it possible to directly study human brain activity. A standard clinical treatment for patients with severe epilepsy involves having electrodes surgically implanted for several weeks in widespread regions, including deep structures, to precisely map seizure initiation. During free time between clinical procedures, many patients voluntarily participate in research studies in which they perform computerized memory tasks at their bedside [20]. This provides researchers with a unique dataset—direct brain recordings from behaving humans—which provides a critical opportunity for probing the neural basis of cognition.
Epilepsy patients must remain in their hospital bed during testing. However, it is nonetheless possible to study the neural basis of spatial navigation with the use of computerized virtual-reality tasks. In the same way that researchers study navigation in rodents by comparing neuronal activity across different areas of a laboratory environment, they can examine the neural basis of human navigation by comparing brain signals between areas of a video game's virtual environment. In these tasks, patients navigate a three-dimensional spatial environment on a computer monitor and use a handheld joystick to control movement [48–50].
A limitation of studying brain recordings from epilepsy patients is that the precise regions where electrodes are implanted depend on each patient's clinical characteristics. However, approximately 46% of patients have at least one electrode implanted in the hippocampus [51]. I focus here on describing data from these hippocampal electrodes because it allows us to compare neuronal activity in the human hippocampus with analogous recordings conducted in rodents. The clearest pattern emerging from these studies is that the human hippocampus generally exhibits oscillations at 1–4 Hz related to navigation and memory, which suggests these signals are analogous to the 4–8 Hz theta rhythms in rodents.
Beyond raw recordings (figure 1), perhaps the clearest examples of human hippocampal oscillations come from spike-triggered average measurements of local field potentials (figure 2a). By computing the mean hippocampal oscillation across many spikes, this analysis revealed clear examples of spike-linked oscillations, both in the range of approximately 1–4 Hz, as well as faster gamma (35–80 Hz) phase-locked signals [52]. Most notably, in the hippocampus only 1–4 Hz oscillations appeared and no 4–8 Hz signals were present. This is strong evidence that the primary oscillatory signal in the human hippocampus during navigation is at 1–4 Hz rather than at 4–8 Hz as in rodents (figure 2b). Similar 1–4 Hz patterns were also observed in the entorhinal cortex [52], which is the main input to the hippocampus and was shown in rodents to often oscillate synchronously with the hippocampus [34].
Figure 1.
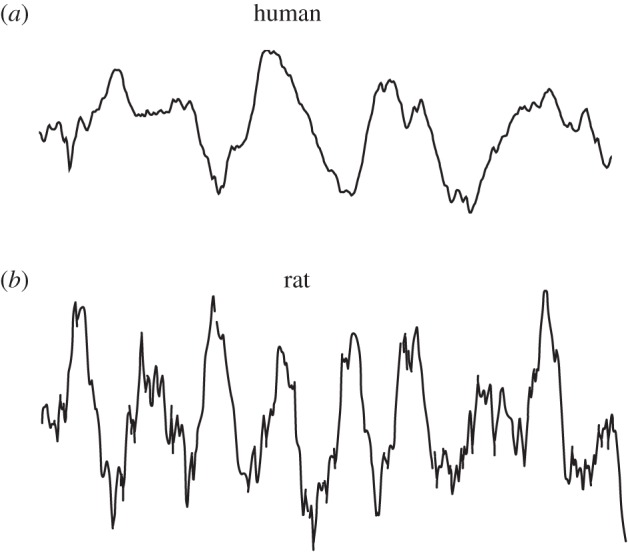
Example hippocampal theta oscillations in rodents and humans. (a) Recording from the human hippocampus (Jacobs Laboratory 2013, unpublished data). (b) Recording from the rodent hippocampus (Reproduced with permission from [33]). Both recordings have a 1 s duration.
Figure 2.
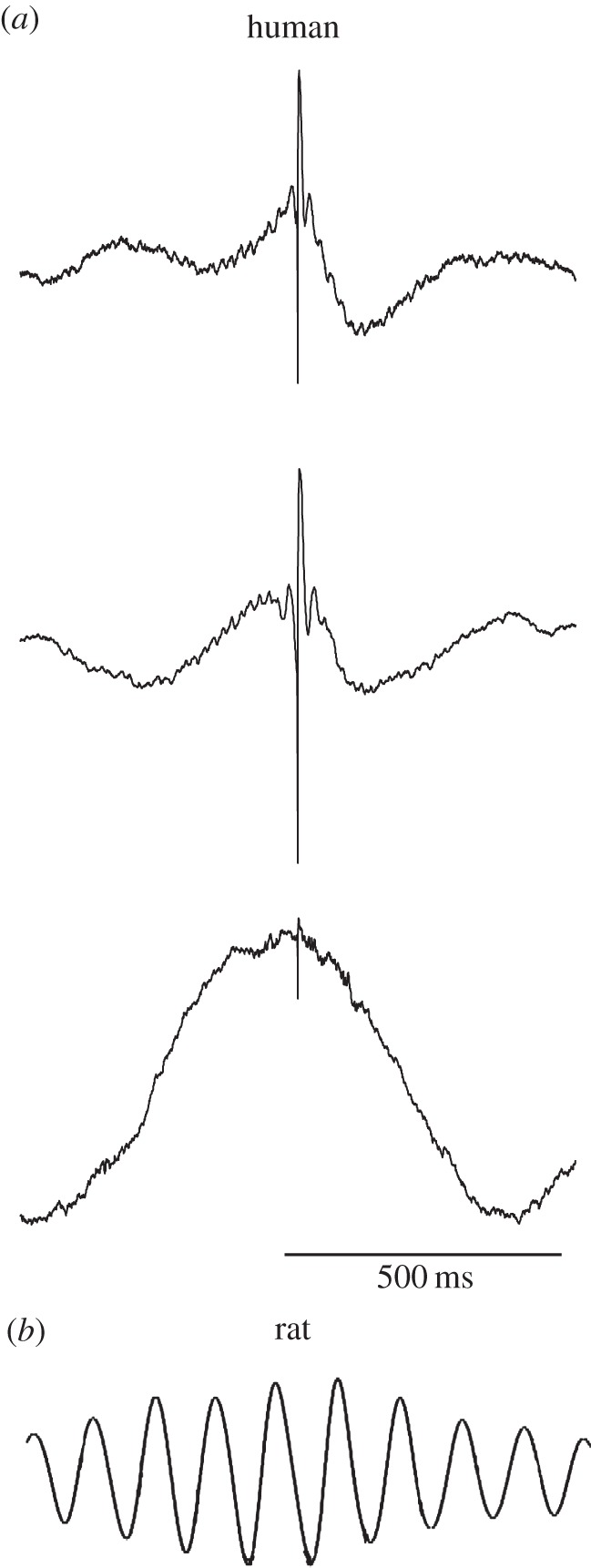
Hippocampal oscillations in rodents and humans. (a) Human hippocampal theta oscillations. Plots show spike-triggered average hippocampal field potentials from three separate patients (Reproduced with permission from [52]). (b) Example of a rodent hippocampal theta oscillation. Plot shows the mean autocorrelation of a theta signal from [53] (Reproduced with permission). Note that plots in (a,b) share the same timescale, as indicated by the scale bar in the bottom panel.
A more direct approach to characterizing the neural oscillations related to navigation in the human hippocampus is to identify signals that vary in power between different types of behaviour. Studies using this approach provide independent evidence for the existence of human hippocampal oscillations at 1–4 Hz that have similar functional properties to the 4–8 Hz theta oscillations found in rodents. Ekstrom et al. [54] compared the amplitude of hippocampal oscillatory activity throughout different types of navigational behaviours (figure 3b). Oscillations at 1–4 Hz most often increased in amplitude during periods of virtual movement compared with stillness ([54], see also [57]). Follow-up work showed that, such as theta in rodents [30], the amplitude of these 1–4 Hz oscillations positively correlated with the speed of movement [55]. Thus, even though they have different absolute frequencies, the functional properties of these human 1–4 Hz hippocampal oscillations during navigation are similar to the theta oscillations observed in rodents.
Figure 3.
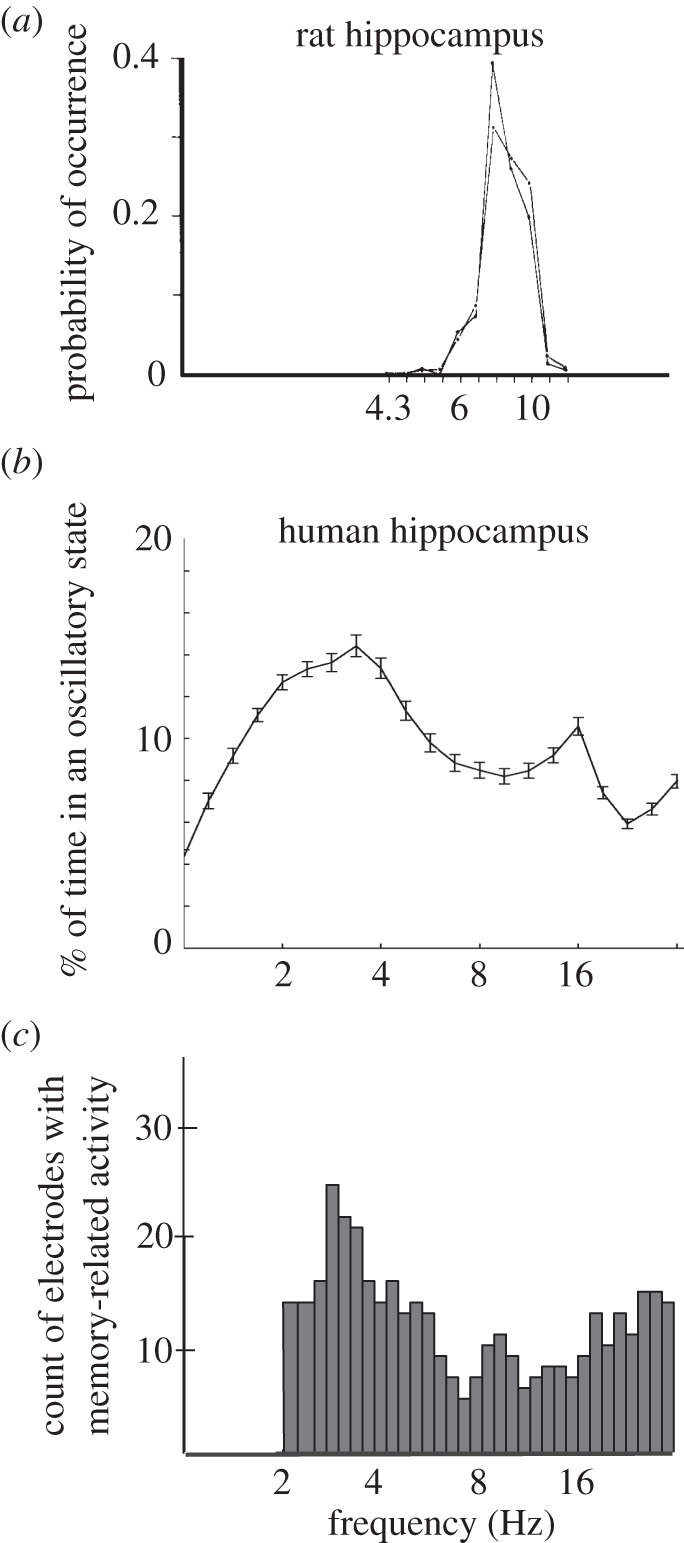
Behaviour-related changes in hippocampal activity reveal human and rodent theta oscillations at different frequencies. (a) Theta oscillations in the rodent hippocampus during movement. Plot depicts the probability of observing a significant oscillation as a function of frequency in a rat during movement. Adapted from [10]. Plots are modified so that their frequency axes are approximately aligned for ease of comparison. (b) Theta oscillations in the human hippocampus during virtual navigation. Plot indicates the probability of observing a significant neuronal oscillation at each frequency. Adapted from [55] with permission. (c) Theta oscillations in the human hippocampus during an episodic memory task. Adapted from [56] with permission.
These studies revealed an additional distinctive feature of human hippocampal 1–4 Hz oscillations, which is that these rhythms generally appeared in short episodes of approximately 2.75 cycles [55]. This short duration [58] is an important difference compared with theta oscillations in rodents, in which it is commonplace for high-amplitude theta oscillations to be sustained for long time intervals [10]. As I describe below, the short duration of these theta episodes is a challenge for theories that grid and place cells code location using theta phase, because they generally rely on the phase being predictable for extended periods of time [59].
Many researchers suggested that the neural structures and signals that support spatial navigation, including oscillations, are also used to support more general memory processes [4,6,7]. This predicts that the same neural signals related to human navigation are recapitulated during non-spatial memory tasks. Consistent with this prediction, there is growing evidence that 1–4 Hz human hippocampal oscillations are also related to task performance in non-spatial behaviours.
A recent study directly examined hippocampal oscillations in patients performing a verbal test of episodic memory [56] and reported two different analyses that showed evidence of significant 3 Hz oscillations. First, mean power spectra from individual hippocampal electrodes showed significant peaks at 3 Hz above the characteristic ‘1/f’ background signal [60]. A second analysis showed that this 3 Hz oscillation was directly linked to memory encoding performance. By comparing the power of oscillatory activity between successful and unsuccessful memory formation, it showed that these 3 Hz oscillations increased in power for successful compared with unsuccessful memory encoding (figure 3c). Critically, these approximately 3 Hz memory-related oscillatory patterns are significantly stronger than patterns at 4–8 Hz, consistent with the view that human memory-related hippocampal oscillations are localized to this slower frequency band.
A different approach to examining hippocampal memory-related oscillations is testing for phase synchrony between the hippocampus and surrounding cortex. It has been suggested on the basis of data in rodents that theta oscillations have a critical role in coordinating hippocampal–neocortical interactions [21,32]. Much research focused specifically on characterizing oscillatory interactions between the hippocampus and the entorhinal cortex, which is its main input and a region where task-related changes in hippocampal theta synchrony clearly appear in rodents [61]. Fell et al. [62] showed that oscillations in the hippocampus and entorhinal cortex exhibit greater phase synchrony at 1–3 Hz during successful compared with unsuccessful memory encoding. (Of note, this synchrony is below the conventional 4–8 Hz ‘theta’ band, even though the term ‘theta’ is used to describe this pattern in this paper's text.) Later studies replicated this pattern by showing that during successful memory encoding there is increased approximately 3 Hz phase synchrony between the hippocampus and a range of neocortical areas [63,64]. Synchronous theta oscillations between the hippocampus and neocortex have been observed previously in animals [32,62] and are an important part of theories that the hippocampus is a central hub in modulating brain-wide neuronal networks [21]. Thus, the existence of human hippocampal–cortical synchrony at approximately 3 Hz is key support for the theory that slower hippocampal oscillations in humans are analogous to rodent theta rhythms.
Although hippocampal theta oscillations in rodents are studied most extensively during navigation and sensorimotor processing, these same oscillations also appear in settings where they are not linked to a particular behaviour, such as during sleep. Thus, an important step in comparing hippocampal oscillations between humans and rodents is assessing whether human 1–4 Hz oscillations are exclusively linked with certain behaviours or whether these signals are a more general phenomenon. Research strongly supports the latter hypothesis, as there is significant evidence for 1–4 Hz hippocampal oscillations in widespread behavioural contexts. Two studies showed that during sleep there are prominent hippocampal oscillations at 1–4 Hz and that no similar signals were present at 4–8 Hz [65,66]. Notably, the amplitude of this 1–4 Hz signal was largest during REM sleep (rapid eye movement sleep), which is the same sleep stage when the largest theta oscillations are observed in rodents. Follow-up work showed that 1–4 Hz human hippocampal sleep oscillations also exhibited phase coupling with simultaneous gamma oscillations [67], which is another property they share with sleep-related 4–8 Hz theta oscillations in rodents [68].
There are several reasons why it is likely that recordings of 1–4 Hz oscillations in the human hippocampus are not artefacts of recording from epilepsy patients or of particular data analysis techniques. As mentioned above, the phase coupling between gamma oscillations and the 1–4 Hz waves [67] is analogous to findings in rodents of coupling between gamma oscillations and approximately 8 Hz theta waves [32,35]. This indicates that these signals are functionally analogous. When these same analyses were conducted in the human neocortex, there was coupling of gamma amplitude to the phase of 4–8 Hz oscillations [36,69]. The presence of 4–8 Hz oscillations in non-hippocampal areas of epileptic patients suggests that the 1–4 Hz hippocampal oscillations were not an artefact of the data analyses or of brain-wide EEG ‘slowing’.
Furthermore, much of this work has been replicated in healthy subjects. De Araujo et al. [70] used magnetoencephalographic (MEG) recordings to measure oscillatory activity from various brain areas, including both hippocampi, in subjects performing a navigation task. This study confirmed the existence of approximately 3 Hz hippocampal oscillations that were localized to this frequency range and whose amplitude correlated with spatial behaviour. Follow-up MEG work independently confirmed the existence of human hippocampal rhythms during navigation at frequencies slower than where they appear in rodents (approx. 5 and 4 Hz, respectively, in [71,72]) and suggested that these oscillations were localized to the right hemisphere [72]. Beyond navigation, other MEG studies also provided evidence of memory-related hippocampal oscillations at frequencies that are generally slower than where they are found in rodents [73–77].
Overall, there is strong evidence that human hippocampal oscillations related to memory and navigation have a frequency of 1–4 Hz. However, a few recent papers interpret their data as exhibiting human hippocampal oscillations at the traditional band of 4–8 Hz. Rutishauser et al. [78] and Axmacher et al. [79] describe single-neuron and gamma-band phase coupling, respectively, to hippocampal oscillations at approximately 7 Hz. A close inspection of the plots in each of these studies suggests that these patterns also extend to a slower frequency band of approximately 3 Hz. Likewise, Fell et al. [80] and Steinvorth et al. [81] describe memory-related activity with the term ‘theta’. However, visual inspection of their plots indicates signals with a slower frequency than the 4–8 Hz rhythms found in rodents. One possibility is that the human hippocampus exhibits oscillations at multiple frequencies and that the data analyses in these studies blurred together 1–4 and 4–10 Hz oscillations that each occurred at different times. In fact, this was the key point of a recent manuscript by Watrous et al. [82], who reported that the human hippocampus alternately exhibited 2 and 8 Hz oscillations for spatial- and temporal-memory tasks, respectively. This general notion that distinct oscillations underlie different behaviours is supported by the recent finding in rodents that hippocampal neurons are simultaneously modulated by both 7–9 Hz and approximately 4 Hz oscillations, which appear in different brain areas and correlate with movement and memory, respectively [83]. An intriguing area of future research is to investigate the degree to which individual brain regions exhibit multiple oscillations simultaneously [84,85] or vary the frequency of a single oscillation in relation to behaviour [30,53,61].
A study by Cantero et al. [86] appears to be the outlier to this literature, because it reported hippocampal oscillations during sleep only at 4–7 Hz. Unexpectedly, these oscillations did not exhibit the characteristic phase coupling with the amplitude of simultaneous gamma oscillations that was found in every human or rodent study that reported searching for this pattern in the hippocampus. It is possible that this unreplicable result was caused by the orientation of electrodes used in that study, which may not have been positioned ideally to record the 1–4 Hz hippocampal rhythms that exhibit gamma coupling [65,68].
5. Implications of 1–4 Hz human hippocampal theta oscillations
As described above, the discovery of grid cells in bats without theta oscillations [18] attracted controversy [19,47,87] because it seemingly contradicted the notion that theta oscillations were critical for grid cells [16,88]. If confirmed across many species and tasks, this ability for grid-cell representations to exist without theta would seem to undermine theories that had proposed theta rhythms as a central component of the mammalian memory and navigational system. Instead, I believe current data support the view that hippocampal oscillations are a central component of navigation and memory processing in humans—and perhaps in bats and other species—but that they often have a slower frequency than the 4–8 Hz theta oscillations that appear in rodents.
Given its functional similarity to rodent theta, by what name should 1–4 Hz human hippocampal oscillations be referred? Prior studies used an array of names to describe human hippocampal signals with a frequency of 1–4 Hz, including ‘theta’ [62] and ‘delta’ [57], as well as the compromise ‘theta/delta’ [89]. Below, we use the name ‘theta’ for human 1–4 Hz oscillations when they are localized to the hippocampus and behave similarly to rodent theta by activating during memory or navigation tasks. This approach follows the functional naming approach used by Buzsáki [9], who described theta as representing the active state of the hippocampus rather than corresponding to a fixed frequency range. I feel that using the name ‘theta’ is useful because it places emphasis on the important task of understanding the signal's functional relevance to theoretical models derived from data in rodents.
It is possible that the slower frequency of human hippocampal theta oscillations is related to the relatively larger size of the human hippocampus. The human hippocampus has more neurons than in rodents, which could inherently cause oscillations to occur more slowly even without any other physiological changes [53,90]. Nonetheless, if humans have slower hippocampal theta oscillations than in rodents, then it would have several implications for theories on the physiological role of theta in memory processes, as these theories were developed solely from data in rodents. Researchers proposed that theta oscillations supported learning by helping cell assemblies to become linked synaptically. This is thought to occur because active assemblies spike near the trough of each theta cycle, and this interval approximately matches the timescale in which coincident spiking must occur to cause long-term potentiation (LTP) [6,14]. If human theta oscillations were slower than in animals, then they would have a longer period (250–1000 ms) than animal theta. This could allow a broader range of assemblies to be linked synaptically, compared with the shorter approximately 100–250 ms theta cycles in rodents [91]. A slower human theta oscillation would also suggest that this theta-driven LTP phenomenon would be less common because individual cycles would occur less frequently (i.e. approx. 1–4 times per second). Following the notion that theta oscillations support working memory via nested gamma oscillations [22,79,92], the presence of 1–4 Hz human theta oscillations may indicate that humans can simultaneously maintain more items in working memory compared with animals with faster theta.
A potentially confusing aspect of human electrophysiology is that humans reliably exhibit oscillations in the neocortex at 4–8 Hz [52], which is much faster than the hippocampus's 1–4 Hz rhythms. Previous theories proposed that the hippocampus communicates with the neocortex via theta phase-locked oscillatory loops [21,93]. The fact that human hippocampal and neocortical oscillations occur at such different frequencies suggests that this oscillatory communication occurs via a different means than one-to-one phase synchrony, such as cross-frequency phase coupling [36,37,79,83,94] or dynamic switching between different types of oscillations [82].
A further distinctive feature of human hippocampal theta oscillations is that they appear only for short episodes [95]. These episodes may terminate when the hippocampus alternates between different functional states [82]. This theta intermittency would seem to present a challenge for oscillatory models of grid cells that keep track of the animal's location via quantitative measurements of the theta rhythm's phase variation over time [16,88]. For oscillatory models of grid cells to be relevant for humans, it would seem to require that the path integration system be reset at the beginning of each theta episode [96]. This remains to be demonstrated experimentally. Further, it would be interesting to test for a significant behavioural role for the duration of oscillations in the human hippocampus, as in fact such a pattern was found previously in neocortex [97].
Neuroscientists with various approaches are interested in the fundamental principles of hippocampal function. Identifying the aspects of hippocampal physiology that are conserved across species is important for distinguishing the most important functions of this structure. The issue of whether theta oscillations are a critical aspect of hippocampal computations in all species [19] was highlighted by the finding that oscillations are absent from the bat hippocampus [18]. As mentioned above, the presence of 1–4 Hz hippocampal oscillations in humans, as well as oscillation-like patterns at 1.5 Hz in the bat entorhinal cortex [46], raises the possibility that various species have oscillations at different frequencies with the same functional role as 4–8 Hz theta oscillations in rodents. More research is necessary to confirm that these signals all have the same function. However, at a minimum, the presence of this interspecies diversity helps to emphasize the importance of collecting and analysing brain recordings using general methods at a broad range of frequencies [60] rather than only fixed, pre-specified bands.
Towards the broad goal of understanding the neural basis of human memory and cognition, it is important for future studies to probe the distinctive features of human theta oscillations and to clarify the implications of any interspecies differences. Given the large number of cellular mechanisms that are customized to support theta's timescale in rodents [42,98], the presence of slower theta oscillations in humans and bats suggests that other aspects of neuronal architecture also vary across species. One theory is that slower human brain oscillations, including hippocampal theta, are part of a general trend in which there is an inverse relation between the frequency of a neuronal oscillation and the size of a neuronal network [53,90]. Furthermore, beyond the hippocampus, the presence of slower human theta rhythms may have implications for other neural systems. Fujisawa & Busaki [83] demonstrated that rodent hippocampal spiking was modulated simultaneously by both theta oscillations and a separate 4 Hz rhythm that had half the frequency of theta. Given the slower frequency of human theta, it seems likely that the analog of 4 Hz rodent oscillation also has a slower frequency in humans. This suggests that a broad challenge going forward is to characterize the interrelations between the many different interacting oscillations that appear in the hippocampus and nearby structures [82,83]. Addressing these challenges may reveal distinctive features of human electrophysiology and provide critical insights regarding the neural basis of human cognition.
Acknowledgements
I thank John Burke, Tom Coffey, Mike Kahana, Bradley Lega, Jonathan Miller, Ashwin Ramayya and Stephen Woloszynek for commenting on a draft of this paper.
Funding statement
This study received financial support from the Brain and Behavior Research Foundation.
References
- 1.Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. ( 10.1136/jnnp.20.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry SD, Thompson RF. 1978. Prediction of learning rate from the hippocampal electroenephalogram. Science 200, 1298–1300. ( 10.1126/science.663612) [DOI] [PubMed] [Google Scholar]
- 3.Moser MB, Moser EI. 1998. Functional differentiation in the hippocampus. Hippocampus 8, 608–619. () [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. New York, NY: Oxford University Press. [Google Scholar]
- 5.Eichenbaum H. 2000. A cortical–hippocampal system for declarative memory. Nat. Rev. Neurosci. 1, 41–50. ( 10.1038/35036213) [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G. 2005. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840. ( 10.1002/hipo.20113) [DOI] [PubMed] [Google Scholar]
- 7.Buzsáki G, Moser EI. 2013. Memory, navigation and theta rhythm in the hippocampal–entorhinal system. Nat. Neurosci. 16, 130–138. ( 10.1038/nn.3304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahana MJ, Seelig D, Madsen JR. 2001. Theta returns. Curr. Opin. Neurobiol. 11, 739–744. ( 10.1016/S0959-4388(01)00278-1) [DOI] [PubMed] [Google Scholar]
- 9.Buzsáki G. 2002. Theta oscillations in the hippocampus. Neuron 33, 325–340. ( 10.1016/S0896-6273(02)00586-X) [DOI] [PubMed] [Google Scholar]
- 10.Vanderwolf C. 1969. Hippocampal electrical activity and voluntary movement of the rat. Electroencephalogr. Clin. Neurophysiol. 26, 407–418. ( 10.1016/0013-4694(69)90092-3) [DOI] [PubMed] [Google Scholar]
- 11.Bland BH. 1986. The physiology and pharmacology of hippocampal formation theta rhythms. Prog. Neurobiol. 26, 1–54. ( 10.1016/0301-0082(86)90019-5) [DOI] [PubMed] [Google Scholar]
- 12.Green JD, Arduini AA. 1954. Hippocampal electrical activity in arousal. J. Neurophysiol. 17, 533–557. [DOI] [PubMed] [Google Scholar]
- 13.Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. 2002. Oscillatory brain states and learning: impact of hippocampal theta-contingent training. Proc. Natl Acad. Sci. USA 99, 1616–1620. ( 10.1073/pnas.032662099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. ( 10.1002/hipo.450030307) [DOI] [PubMed] [Google Scholar]
- 15.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. ( 10.1038/nature03721) [DOI] [PubMed] [Google Scholar]
- 16.Burgess N, Barry C, O'Keefe J, London U. 2007. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812. ( 10.1002/hipo.20327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halgren E, Babb TL, Crandall PH. 1978. Human hippocampal formation EEG desynchronizes during attentiveness and movement. Electroencephalogr. Clin. Neurophysiol. 44, 778–781. ( 10.1016/0013-4694(78)90212-2) [DOI] [PubMed] [Google Scholar]
- 18.Yartsev MM, Witter MP, Ulanovsky N. 2011. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107. ( 10.1038/nature10583) [DOI] [PubMed] [Google Scholar]
- 19.Giocomo LM, Moser EI. 2011. Spatial representation: maps in a temporal void. Curr. Biol. 21, R962–R964. ( 10.1016/j.cub.2011.10.024) [DOI] [PubMed] [Google Scholar]
- 20.Jacobs J, Kahana MJ. 2010. Direct brain recordings fuel advances in cognitive electrophysiology. Trends Cogn. Sci. 14, 162–171. ( 10.1016/j.tics.2010.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller R. 1991. Cortico-hippocampal interplay and the representation of contexts in the brain. Berlin, Germany: Springer. [Google Scholar]
- 22.Lisman J, Idiart MA. 1995. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science 267, 1512–1515. ( 10.1126/science.7878473) [DOI] [PubMed] [Google Scholar]
- 23.Hasselmo ME, Bodelon C, Wyble BP. 2002. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817. ( 10.1162/089976602317318965) [DOI] [PubMed] [Google Scholar]
- 24.McFarland WL, Teitelbaum H, Hedges EK. 1975. Relationship between hippocampal theta activity and running speed in the rat. J. Comp. Physiol. Psychol. 88, 324–328. ( 10.1037/h0076177) [DOI] [PubMed] [Google Scholar]
- 25.Berg RW, Whitmer D, Kleinfeld D. 2006. Exploratory whisking by rat is not phase locked to the hippocampal theta rhythm. J. Neurosci. 26, 6518–6522. ( 10.1523/JNEUROSCI.0190-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huxter J, Burgess N, O'Keefe J. 2003. Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425, 828–832. ( 10.1038/nature02058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. 2011. Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246–249. ( 10.1038/nature10439) [DOI] [PubMed] [Google Scholar]
- 28.Brandon MP, Bogaard AR, Schultheiss NW, Hasselmo ME. 2013. Segregation of cortical head direction cell assemblies on alternating theta cycles. Nat. Neurosci. 16, 739–748. ( 10.1038/nn.3383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douchamps V, Jeewajee A, Blundell P, Burgess N, Lever C. 2013. Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. J. Neurosci. 33, 8689–8704. ( 10.1523/JNEUROSCI.4483-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. 2008. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 18, 340–348. ( 10.1002/hipo.20394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry C, Ginzberg LL, O'Keefe J, Burgess N. 2012. Grid cell firing patterns signal environmental novelty by expansion. Proc. Natl Acad. Sci. USA 109, 17 687–17 692. ( 10.1073/pnas.1209918109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. 2008. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697. ( 10.1016/j.neuron.2008.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siapas A, Lubenov E, Wilson MA. 2005. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151. ( 10.1016/j.neuron.2005.02.028) [DOI] [PubMed] [Google Scholar]
- 34.Chrobak JJ, Buzsáki G. 1998. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci. 18, 388–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsáki G. 1995. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 15, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. 2006. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628. ( 10.1126/science.1128115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. 2012. Cross-frequency phase–phase coupling between theta and gamma oscillations in the hippocampus. J. Neurosci. 32, 423–435. ( 10.1523/JNEUROSCI.4122-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doeller CF, Barry C, Burgess N. 2010. Evidence for grid cells in a human memory network. Nature 463, 657–661. ( 10.1038/nature08704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs J, et al. 2013. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci. 16, 1188–1190. ( 10.1038/nn.3466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser E, Kropff E, Moser M. 2008. Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 31, 69–89. ( 10.1146/annurev.neuro.31.061307.090723) [DOI] [PubMed] [Google Scholar]
- 41.Jeewajee A, Barry C, O'Keefe J, Burgess N. 2008. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus 18, 1175–1185. ( 10.1002/hipo.20510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. 2007. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722. ( 10.1126/science.1139207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. 2011. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599. ( 10.1126/science.1201652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. 2011. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595. ( 10.1126/science.1201685) [DOI] [PubMed] [Google Scholar]
- 45.Sreenivasan S, Fiete I. 2011. Grid cells generate an analog error-correcting code for singularly precise neural computation. Nat. Neurosci. 14, 1330–1337. ( 10.1038/nn.2901) [DOI] [PubMed] [Google Scholar]
- 46.Heys JG, MacLeod KM, Moss CF, Hasselmo ME. 2013. Bat and rat neurons differ in theta-frequency resonance despite similar coding of space. Science 340, 363–367. ( 10.1126/science.1233831) [DOI] [PubMed] [Google Scholar]
- 47.Barry C, Doeller CF. 2013. 3D mapping in the brain. Science 340, 279–280. ( 10.1126/science.1237569) [DOI] [PubMed] [Google Scholar]
- 48.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. 2003. Cellular networks underlying human spatial navigation. Nature 425, 184–187. ( 10.1038/nature01964) [DOI] [PubMed] [Google Scholar]
- 49.Jacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I. 2010. A sense of direction in human entorhinal cortex. Proc. Natl Acad. Sci. USA 107, 6487–6482. ( 10.1073/pnas.0911213107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs J, et al. 2010. Right-lateralized brain oscillations in human spatial navigation. J. Cogn. Neurosci. 22, 824–836. ( 10.1162/jocn.2009.21240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. 2007. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb. Cortex 17, 1190–1196. ( 10.1093/cercor/bhl030) [DOI] [PubMed] [Google Scholar]
- 52.Jacobs J, Kahana MJ, Ekstrom AD, Fried I. 2007. Brain oscillations control timing of single-neuron activity in humans. J. Neurosci. 27, 3839–3844. ( 10.1523/JNEUROSCI.4636-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304, 1926–1929. ( 10.1126/science.1099745) [DOI] [PubMed] [Google Scholar]
- 54.Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. 2005. Human hippocampal theta activity during virtual navigation. Hippocampus 15, 881–889. ( 10.1002/hipo.20109) [DOI] [PubMed] [Google Scholar]
- 55.Watrous AJ, Fried I, Ekstrom AD. 2011. Behavioral correlates of human hippocampal delta and theta oscillations during navigation. J. Neurophysiol. 105, 1747–1755. ( 10.1152/jn.00921.2010) [DOI] [PubMed] [Google Scholar]
- 56.Lega BC, Jacobs J, Kahana MJ. 2011. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22, 748–761. ( 10.1002/hipo.20937) [DOI] [PubMed] [Google Scholar]
- 57.Clemens Z, et al. 2013. Increased mesiotemporal delta activity characterizes virtual navigation in humans. Neurosci. Res. 76, 67–75. ( 10.1016/j.neures.2013.03.004) [DOI] [PubMed] [Google Scholar]
- 58.van Vugt MK, Sederberg PB, Kahana MJ. 2007. Comparison of spectral analysis methods for characterizing brain oscillations. J. Neurosci. Methods 162, 49–63. ( 10.1016/j.jneumeth.2006.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgess N. 2008. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18, 1157–1174. ( 10.1002/hipo.20518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manning JR, Jacobs J, Fried I, Kahana MJ. 2009. Broadband shifts in LFP power spectra are correlated with single-neuron spiking in humans. J. Neurosci. 29, 13 613–13 620. ( 10.1523/JNEUROSCI.2041-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M-B, Moser EI. 2009. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. ( 10.1038/nature08573) [DOI] [PubMed] [Google Scholar]
- 62.Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. 2003. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur. J. Neurosci. 17, 1082–1088. ( 10.1046/j.1460-9568.2003.02522.x) [DOI] [PubMed] [Google Scholar]
- 63.Babiloni C, et al. 2010. Cortical sources of resting EEG rhythms in mild cognitive impairment and subjective memory complaint. Neurobiol. Aging 31, 1787–1798. ( 10.1016/j.neurobiolaging.2008.09.020) [DOI] [PubMed] [Google Scholar]
- 64.Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J. 2013. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. J. Neurosci. 33, 10 439–10 446. ( 10.1523/JNEUROSCI.0513-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bódizs R, Kántor S, Szabó G, Szûcs A, Erõss L, Halász P. 2001. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus 11, 747–753. ( 10.1002/hipo.1090) [DOI] [PubMed] [Google Scholar]
- 66.Moroni F, et al. 2012. Slow EEG rhythms and inter-hemispheric synchronization across sleep and wakefulness in the human hippocampus. Neuroimage 60, 497–504. ( 10.1016/j.neuroimage.2011.11.093) [DOI] [PubMed] [Google Scholar]
- 67.Clemens Z, Weiss B, Szücs A, Eröss L, Rásonyi G, Halász P. 2009. Phase coupling between rhythmic slow activity and gamma characterizes mesiotemporal rapid-eye-movement sleep in humans. Neuroscience 163, 388–396. ( 10.1016/j.neuroscience.2009.06.044) [DOI] [PubMed] [Google Scholar]
- 68.Ferrara M, Moroni F, De Gennaro L, Nobili L. 2012. Hippocampal sleep features: relations to human memory function. Front. Neurol. 3, 57 ( 10.3389/fneur.2012.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs J, Kahana MJ. 2009. Neural representations of individual stimuli in humans revealed by gamma-band ECoG activity. J. Neurosci. 29, 10 203–10 214. ( 10.1523/JNEUROSCI.2187-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Araujo DB, Baffa O, Wakai RT. 2002. Theta oscillations and human navigation: a magnetoencephalography study. J. Cogn. Neurosci. 14, 70–78. ( 10.1162/089892902317205339) [DOI] [PubMed] [Google Scholar]
- 71.Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. 2008. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 28, 5983–5990. ( 10.1523/JNEUROSCI.5001-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaplan R, Doeller CF, Barnes GR, Litvak V, Düzel E, Bandettini PA, Burgess N, Eichenbaum HB. 2012. Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biol. 10, e1001267 ( 10.1371/journal.pbio.1001267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guderian S, Düzel E. 2005. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15, 901–912. ( 10.1002/hipo.20125) [DOI] [PubMed] [Google Scholar]
- 74.Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. 2009. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc. Natl Acad. Sci. USA 106, 5365–5370. ( 10.1073/pnas.0900289106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poch C, Fuentemilla L, Barnes GR, Düzel E. 2011. Hippocampal theta-phase modulation of replay correlates with configural-relational short-term memory performance. J. Neurosci. 31, 7038–7042. ( 10.1523/JNEUROSCI.6305-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guitart-Masip M, Barnes GR, Horner A, Bauer M, Dolan RJ, Duzel E. 2013. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J. Neurosci. 33, 442–451. ( 10.1523/JNEUROSCI.2573-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staudigl T, Hanslmayr S. 2013. Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr. Biol. 23, 1101–1106. ( 10.1016/j.cub.2013.04.074) [DOI] [PubMed] [Google Scholar]
- 78.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. 2010. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907. ( 10.1038/nature08860) [DOI] [PubMed] [Google Scholar]
- 79.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl Acad. Sci. USA 107, 3228–3233. ( 10.1073/pnas.0911531107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N. 2011. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J. Neurosci. 31, 5392–5397. ( 10.1523/JNEUROSCI.3668-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinvorth S, Wang C, Ulbert I, Schomer D, Halgren E. 2009. Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus 20, 166–173. ( 10.1002/hipo.20597) [DOI] [PubMed] [Google Scholar]
- 82.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. 2013. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat. Neurosci. 16, 349–356. ( 10.1038/nn.3315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujisawa S, Buzsáki G. 2011. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 72, 153–165. ( 10.1016/j.neuron.2011.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. 2006. Theta oscillations in human cortex during a working memory task: evidence for local generators. J. Neurophysiol. 95, 1630–1638. ( 10.1152/jn.00409.2005) [DOI] [PubMed] [Google Scholar]
- 85.Montgomery SM, Betancur MI, Buzsaki G. 2009. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J. Neurosci. 29, 1381–1394. ( 10.1523/JNEUROSCI.4339-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. 2003. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J. Neurosci. 23, 10 897–10 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barry C, Bush D, O'Keefe J, Burgess N. 2012. Models of grid cells and theta oscillations. Nature 488, E1 ( 10.1038/nature11276) [DOI] [PubMed] [Google Scholar]
- 88.Hasselmo ME, Giocomo LM, Zilli EA. 2007. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252–1271. ( 10.1002/hipo.20374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mormann F, Osterhage H, Andrzejak RG, Weber B, Fernández G, Fell J, Elger CE, Lehnertz K. 2008. Independent delta/theta rhythms in the human hippocampus and entorhinal cortex. Front. Hum. Neurosci. 2, 3 ( 10.3389/neuro.09.003.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steriade M. 2001. Impact of network activities on neuronal properties in corticothalamic systems. J. Neurophysiol. 86, 1–39. [DOI] [PubMed] [Google Scholar]
- 91.Diba K, Buzsáki G. 2008. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J. Neurosci. 28, 13 448–13 456. ( 10.1523/JNEUROSCI.3824-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lisman J, Buzsaki G. 2008. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 34, 974–980. ( 10.1093/schbul/sbn060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buzsáki G. 2006. Rhythms of the brain. New York, NY: Oxford University Press. [Google Scholar]
- 94.Palva JM, Palva S, Kaila K. 2005. Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 25, 3962–3972. ( 10.1523/JNEUROSCI.4250-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. 2013. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 23, 656–661. ( 10.1002/hipo.22124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasselmo ME. 2008. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus 18, 1213–1229. ( 10.1002/hipo.20512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. 1999. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784. ( 10.1038/21645) [DOI] [PubMed] [Google Scholar]
- 98.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. 2003. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. ( 10.1038/nature01374) [DOI] [PubMed] [Google Scholar]


