Abstract
The hippocampus receives its major cortical input from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC). It is commonly believed that the MEC provides spatial input to the hippocampus, whereas the LEC provides non-spatial input. We review new data which suggest that this simple dichotomy between ‘where’ versus ‘what’ needs revision. We propose a refinement of this model, which is more complex than the simple spatial–non-spatial dichotomy. MEC is proposed to be involved in path integration computations based on a global frame of reference, primarily using internally generated, self-motion cues and external input about environmental boundaries and scenes; it provides the hippocampus with a coordinate system that underlies the spatial context of an experience. LEC is proposed to process information about individual items and locations based on a local frame of reference, primarily using external sensory input; it provides the hippocampus with information about the content of an experience.
Keywords: episodic memory, lateral entorhinal cortex, medial entorhinal cortex, memory, path integration
1. Introduction
It has been known for decades that the hippocampus and the medial temporal lobe are critically involved in the formation of new, declarative memories. However, the precise computations and neural pathways that underlie hippocampal processing are still not well understood. The most striking correlate of hippocampal neuronal firing in rats is the spatial location of the animal [1,2]. The discovery of place cells led O'Keefe & Nadel [1] to propose the cognitive map theory of the hippocampus. According to this theory, ‘the hippocampus is the core of a neural memory system providing an objective spatial framework within which the items and events of an organism's experience are located and interrelated’ [1] (p. 1). The years since the publication of this theory have been marked by often heated debate between groups emphasizing the spatial mapping properties (‘the objective spatial framework’) or the relational learning properties (interrelating ‘the items and events’ of experience) of the hippocampus [3,4]. A resolution to this debate may now be in sight, as investigators in the past decade have increasingly taken a more systems-level approach to studying the hippocampus. Rather than focusing primarily on the CA1 region, which was the target of the overwhelming majority of early physiological studies, investigators are now attempting to understand the differences in functional properties among the hippocampal subfields and among the different structures that send afferents to the hippocampus.
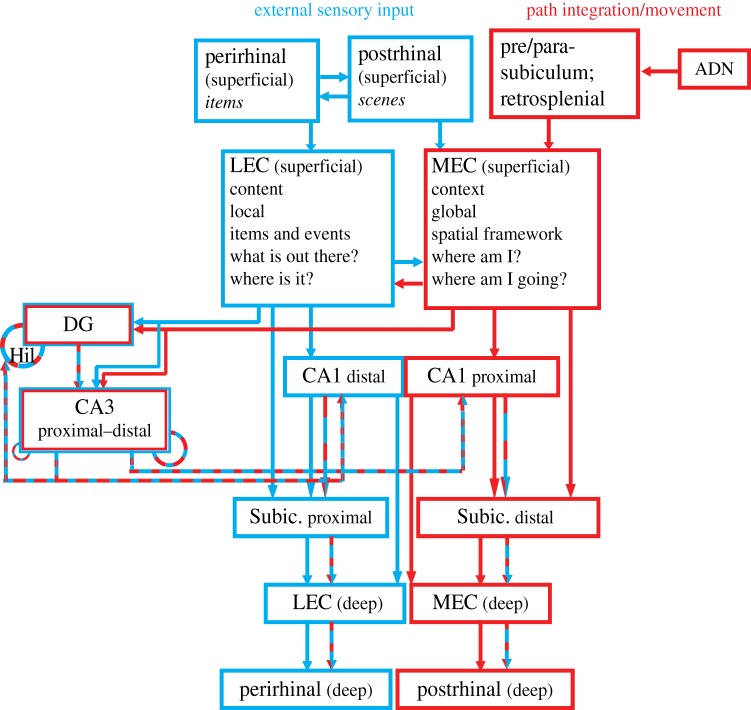
This approach has benefited from a functional anatomical framework (figure 1). The hippocampus receives its primary cortical input from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC) [13]. These two regions, although sharing common middle- and surnames, are very distinct from each other in terms of cytoarchitecture and connectivity with other brain regions. They also have distinct patterns of input to the hippocampus. The LEC innervates the outer third of the molecular layer of the dentate gyrus and the MEC innervates the middle third. There are also differences in their direct projections along the transverse axis of CA1, as the LEC innervates the region of CA1 closer to the subiculum (distal CA1), whereas the MEC innervates the region of CA1 closer to CA2 and CA3 (proximal CA1). This difference in connectivity is mirrored by different properties of place cells along the CA1 transverse axis. Neurons in the MEC-recipient, proximal CA1 have higher spatial specificity and stronger theta modulation than neurons in the LEC-recipient, distal CA1 [18], consistent with known physiological differences between the LEC and MEC [19–21], as discussed below. Based on such anatomical considerations, investigators in the 1990s began to consider that the entorhinal cortex formed two distinct, functional processing streams into the hippocampus [5–8,22–25]. The LEC was considered to be an extension of the ventral (‘what’) stream of processing through the temporal lobe, whereas the MEC was considered to be an extension of the dorsal (‘where’) stream of processing through the parietal lobe. Accordingly, it was proposed that the LEC provided information to the hippocampus about individual items and objects, whereas the MEC provided spatial information. The role of the hippocampus was to combine the ‘what’ and ‘where’ information to form flexible, conjunctive representations of ‘what happened where’, presumably a critical step in the formation of a coherent episodic memory.
Figure 1.
Parallel processing streams into the hippocampus. This wiring diagram is a simplified version of the real anatomy, leaving out a number of projections. The structure of the diagram emphasizes the dual processing streams that pass through the LEC and MEC. Prior diagrams of these processing streams stressed their origins in the perirhinal-LEC and postrhinal-MEC connections [5–8]. Here, we add the critical connectivity between the MEC and limbic regions involved in movement, location and head direction processing (presubiculum, parasubiculum, retrosplenial cortex and anterior dorsal thalamus). (A different subregion of the parasubiculum projects to the LEC, not shown on this diagram [9].) The LEC and MEC connect to distinct regions of CA1 and subiculum, segregated along the transverse axis of the hippocampus (proximal–distal relative to the DG). CA1 and subiculum send return projections to the deep layers of the entorhinal cortex (EC), completing a processing loop. There is crosstalk along these pathways, both prior to their entry into the hippocampus and especially in the convergent projections to the DG and CA3. Although this article does not discuss DG and CA3 properties in detail, these areas are included in the diagram because they are major components of the classic ‘trisynaptic loop’ circuit of the hippocampus and it is important to place this circuit within the larger context of the MEC–LEC parallel streams. In this illustration, the DG and CA3 is represented as a ‘side loop’ of processing, in which the MEC and LEC streams are merged onto the same CA3 pyramidal cells and DG granule cells and the combined representations are then merged in CA1 with the separate input streams from the direct EC–CA1 projections. Specific mnemonic properties of the DG and CA3 regions are thought to be supported by the recurrent feedback loops represented by the dashed circles. In CA3, the recurrent connections are more prominent in the distal than the proximal regions [10]; moreover, the distal CA3 projects more strongly to proximal CA1 (which receives MEC input), and proximal CA3 projects more strongly to distal CA1 (which receives LEC input). The DG receives feedback from CA3 [11], and a disynaptic recurrent loop via mossy cells of the hilus is also present [12] For a more detailed explanation and references to the primary literature on these anatomical connections, the reader is referred to a number of review articles [5,13–17]. ADN, anterior dorsal nucleus of the thalamus; DG, dentate gyrus; Hil, hilus; Subic., subiculum. (Online version in colour.)
Although the classic dissociation [26,27] between the primate dorsal and ventral visual streams as processing ‘where’ versus ‘what’ remains a viable model, it has undergone refinement since its initial formulation. Current views suggest that the dorsal and ventral streams are each composed of multiple, parallel pathways. Three pathways in the dorsal stream originate in the parietal cortex [28], and four pathways in the ventral stream originate in the occipital cortex [29]. The parieto-medial temporal pathway has a component that leads to the parahippocampal cortex (postrhinal cortex in rats) and appears to encode visual scenes; it has another component that leads to the pre- and parasubiculum and appears to be involved in navigational signals, with such functional cell types as place and head direction cells in rats [28]. The occipitotemporal–medial temporal pathway to the perirhinal cortex appears to be specialized for encoding objects [29]. In rats (where the LEC and MEC are more cleanly segregated than that in primates), the LEC is more strongly connected with the perirhinal cortex and the MEC is more strongly connected with the postrhinal (parahippocampal) cortex and the presubiculum [5,13] (figure 1).
A number of dissociations between the MEC and LEC have appeared in recent years that are consistent with this broad view of spatial versus non-spatial (where versus what) processing. The most fundamental breakthrough was the discovery of grid cells in the MEC [30,31]. Subsequent discoveries of head direction cells, speed-modulated cells and border/boundary cells in this area cemented the idea that the MEC was part of a spatial-processing system [32–34]. In parallel, the LEC was shown to be mostly devoid of spatially tuned neurons, at least in standard tasks in which there were prominent distal landmarks but few prominent, local landmarks [19]. By contrast, when local objects were introduced into the arena, a substantial proportion of superficial LEC neurons were active when the rat investigated the objects, whereas few superficial MEC neurons were affected by the objects [35].
2. Complications with the simple ‘what versus where’ model of lateral entorhinal cortex–medial entorhinal cortex processing
This simple model of LEC = what versus MEC = where has been conceptually valuable in our initial attempts to decipher the information flow through the hippocampus. However, recent results show that (unsurprisingly) reality is more complex than the simple model. Although MEC lesions impair spatial learning [36–38], the primary difficulty with the simple model is the increasing evidence that the LEC subserves both spatial and non-spatial processing. Van Cauter et al. [37] and Wilson et al. [38] have shown that LEC lesions do not affect recognition memory for objects per se, but they have a profound impact when animals have to recognize displaced objects or specific objects placed into specific spatial contexts. Thus, there is clearly a spatial component to LEC function. Furthermore, Hunsaker and colleagues [39] demonstrated that, although MEC lesions primarily caused spatial deficits, there were weaker but significant non-spatial deficits. Similarly, LEC lesions primarily caused non-spatial deficits, but there were weak but significant spatial deficits. Physiologically, one synapse upstream from the entorhinal cortex, Furtak et al. [40] showed that single units in the postrhinal cortex have both spatial and non-spatial correlates. Deshmukh & Knierim [35] demonstrated that a small but significant number of LEC cells (but not perirhinal cells; [41]) showed spatial firing that strongly resembled hippocampal place fields when there were objects present in the environment. Importantly, these putative place fields were at locations where the animal had never experienced objects. Intriguingly, when objects were moved in an environment, a small number of cells fired at locations that the objects previously occupied, showing a memory for the history of object locations in the environment. This property is reminiscent of the misplace activity reported by O'Keefe [2] in his early investigations of place cells. Similar responses have been seen in the hippocampus [42] and anterior cingulate cortex [43]. Such memory responses can last for days to weeks in LEC [44] and anterior cingulate cortex [45], showing that these areas can generate a strong spatial signal related to the remembered locations of objects. Thus, the clean segregation of spatial processing being performed by the postrhinal–MEC stream and non-spatial processing by the perirhinal–LEC stream is untenable.
3. Anatomy supports the interconnectivity of the two processing streams
It is not surprising that MEC and LEC each show some aspects of both spatial and non-spatial processing, as they are connected with each other [5,46] (figure 1). Moreover, as emphasized by Witter et al. [47], there are feedback pathways from the hippocampus to the MEC and LEC and from the MEC and LEC to the neocortex. Because the hippocampus combines the two streams in the dentate gyrus (DG) and CA3, the MEC and LEC may each show spatial and non-spatial processing based on this combined feedback signal. The notion of a hierarchical, one-way processing loop, while convenient for creating conceptual models and organizing complex data into theoretical frameworks, is far removed from how the brain is truly organized. Although the place-like firing [35] and object–location memory responses [35,44] in LEC can be found in the hippocampus-projecting superficial layers, even these layers are presumably subjected to feedback from the hippocampus (similar to that shown for the MEC [48]) owing to cross-laminar connectivity and dendritic arborizations. Does it still make sense, then, to refer to these pathways as separate processing streams? As the anatomy remains persuasive that there are separate processing streams that are nonetheless connected with each other, the question becomes what is the best way to characterize the functions of these streams. The ‘what versus where’ distinction is not the most accurate characterization. Instead, very similar to a hypothesis proposed by Lisman [49], we propose that the MEC is best thought of as part of a global, holistic spatial map that is generated primarily through path integration mechanisms to provide information about where the organism is in its environment, where it is going and how to get there. By contrast, the LEC processes local cues—individual items or conjunctions of items—to provide the content of an experience, including spatial information related to these local objects.
4. Local versus global frameworks in the lateral entorhinal cortex versus medial entorhinal cortex
A recent study by Neunuebel et al. [50] provides strong evidence for the local–global distinction between the LEC and MEC. Superficial MEC and LEC neurons were recorded as rats ran on a circular track with a set of salient local cues on the surface and a set of global landmarks on curtains at the perimeter of the recording environment. After many days of training in this standard environment, LEC and MEC cells were recorded under standard cue configurations and under mismatch cue configurations, in which the set of local cues was rotated anticlockwise by a specific amount and the set of global cues was rotated clockwise by an equal amount. As expected, the MEC cells showed robust spatial firing patterns on the track, often in multiple spots that suggest that these cells were grid cells. The spatial firing of the MEC cells was controlled by the global cues in the cue-mismatch sessions, as firing rate maps rotated clockwise in register with the rotation of the global cues. Because the MEC is associated strongly with the head direction cell system [13,32,51,52], which is known to be primarily controlled by rotation of the global cues in an environment [53–55], this result was expected. The LEC cells did not show strong spatial firing on the track, which was also an expected result based on the known lack of spatial selectivity in these neurons in the absence of discrete, local landmarks [19,20]. However, in the cue-mismatch sessions, a weak but highly statistically significant spatial signal was detected. This signal was controlled by the local framework of the track, as the LEC spatial rate maps rotated anticlockwise on average in register with the rotation of the track. Thus, even though individual cells did not show robust spatial correlates, the population activity clearly showed a representation that was dominated by the local cues. It is not known whether the cells were responding to a specific local cue on the track or whether they were demonstrating a weak but bona fide, cognitive spatial signal. Thus, a precise characterization of the type of information encoded by LEC neural firing remains elusive. Nonetheless, this dissociation between the MEC and LEC reinforces the notion that the MEC is involved in creating a global spatial map, whereas the LEC is more involved in processing local cues.
5. Path integration in medial entorhinal cortex versus local landmarks in lateral entorhinal cortex
Although the LEC may be more involved in object processing than MEC and the MEC may be more involved in spatial processing than LEC, there are enough exceptions to an absolute ‘what versus where’ dichotomy that it is useful to look for a different characterization of how these two regions are functionally and computationally distinct. Largely in agreement with Lisman's hypothesis that the MEC represents information related to ‘self’ and the LEC represents information related to ‘non-self’ [49], we argue here that a better way to characterize these pathways is that (i) the MEC is part of a circuit that performs a path integration computation, using self-motion cues and external input from environmental boundaries and landmarks to bind an internal spatial framework to the external world and (ii) the LEC is part of a circuit that processes information about local landmarks and individual items, used for both spatial and non-spatial computations (figure 1).
(a). Medial entorhinal cortex
The MEC is connected with a number of brain structures that contain robust spatial signals and movement-related signals, including the parasubiculum, presubiculum and retrosplenial cortex [13]. Grid cells, border cells and head direction cells are the main subtypes of cells in these areas [56]. Place cells have also been reported [19,57–59], but it is not clear from present data whether these cells were a separate class or (i) whether they were low-resolution grid cells that only had a single field in the small apparatus or (ii) whether they were boundary cells. Prominent among the movement-related signals is the theta rhythm, in rats an 8–12 Hz rhythm that is correlated with movement and investigatory behaviours [60]. There are also indications of a modest speed modulation of the firing of cells in the MEC [32], similar to that reported for hippocampal place cells [61,62]. The combination of these factors (spatial signals and movement signals) has propelled the notion that these areas are a major constituent of the path integration circuitry of the brain. The major classes of models of grid cells (the continuous attractor models and the oscillatory interference models) are both explicitly path integration models [63–66]. That is, they both rely on the integration of a velocity vector (speed and direction) over time to produce a periodic position signal. Similar considerations led to earlier models of place cells being driven by a path integration computation [67] and in support of these models, hippocampal lesions can produce deficits in path integration [68].
Confusion about the relationship between path integration and homing behaviour is the source of occasional misunderstanding about why grid cells are thought to be involved in path integration. Often the two concepts are incorrectly regarded as equivalent, because path integration is usually measured behaviourally with a homing task; that is, animals are trained to leave their nest in search of an item (usually food), and then their ability to perform path integration is measured by the accuracy of their return to their starting location, in the absence of external sensory cues. In this case, the homing vector (i.e. the distance and direction from the animal's current location to the start location) is considered to be continuously updated by path integration. The firing properties of grid cells are not obviously related to the calculation of a simple homing vector, however, so why are the grid cells thought to be part of a path integration calculation? To answer this question, one must first recognize that calculation of a homing vector is not equivalent to path integration; rather, it is just a special case of path integration. Mathematically, the integral of velocity over time is position. This position signal can be represented in the brain in a number of ways. It may be a homing vector, a vector to any other fixed reference point or a location on a map. The latter case is precisely analogous to the methods that mariners used for centuries to navigate the seas. They measured their speed and direction at regular intervals and updated their location on a chart, based on the calculation of estimated distance and direction from the last measurement. The path integration models of grid cells compute this type of position signal, with the added feature that the position signal is periodic, leading to the periodic spatial firing patterns of these cells.
Both the speed signal and the head direction signal (thought to be derived from a one-dimensional, angular velocity integration circuit; [69–71]) are derived from the animal's movement through its environment. Any path integration circuit based purely on these self-motion signals will accumulate error over time. It is thus required that the signal be calibrated (intermittently or continuously) with external sensory input in order to keep the spatial representation in the animal's head aligned with the external world. Boundary cells may be involved in keeping the spatial grid aligned with the boundaries of the environment (i.e. they may set the translational phase of the grid) [33,34,72]. Head direction cells are thought to set the orientation of the grid; these cells themselves are known to be reset by prominent visual landmarks when they become misaligned with the allocentric reference frame defined by external landmarks [73]. Furthermore, information about visual scenes that enter the MEC from the parahippocampal/postrhinal cortex [74,75] may play a similar role in keeping the grid bound to the external world, perhaps directly or indirectly through an influence of the boundary cells and head direction cells.
(b). Lateral entorhinal cortex
In contrast to the MEC and its related areas, the LEC contains much weaker spatial and self-motion signals. As mentioned earlier, LEC cells are active when the rat investigates individual objects in an environment [35], and a small fraction of the cells fire at locations from which an object has been moved [35,44]. Other cells fire like place cells in locations where the animal never experienced an object, but apparently only when individual objects are present in the environment [35]. Textures on the surface of a track or salient landmarks outside the apparatus are unable to support spatial firing [20] (although a weak spatial signal can be detected when local and global cues are placed in conflict, as described above [50]). Because LEC cells with spatial properties are rare and few studies of LEC have been performed in freely moving animals, we know very little about the properties of these cells and the cues that drive their firing. However, no head direction cells have ever been reported in LEC, and there is much weaker theta rhythm in the local field potential (LFP) compared with the hippocampus and MEC [21]. (There are hints from published rate maps of a boundary-related signal in the LEC but this has not been studied carefully [19,35]). Thus, the LEC is unlikely to play a major role in the path integration computations of the entorhinal/hippocampal system. Nonetheless, its weak spatial tuning under certain conditions suggests that it does play a role in some types of spatial computations, as well as non-spatial processing.
How should we then think about the function of the LEC input to the hippocampus? A major input is from the perirhinal cortex, which appears to encode configurations of cues related to individual objects or items [41,76–79] but may not carry the spatial signals seen in the LEC [41]. Thus, both perirhinal and postrhinal/parahippocampal cortex provide external sensory input to the LEC and MEC, respectively (figure 1), but the perirhinal cortex appears to be selective for small-scale, individual objects, whereas the postrhinal/parahippocampal cortex is selective for large-scale, scene-related information. (In this respect, it is interesting that there appears to be a lateral–medial gradient in the representation of objects of different real-world sizes in the human ventral temporal cortex [80]). Thus, in contrast to the global map of space that the MEC sends the hippocampus, the LEC sends information about individual items that occupy that space. Moreover, the LEC may send information about where those objects are located (or where they used to be found), relative to the current location of the rat [35]. This might explain the place-like activity of the small number of LEC cells. The identification of landmark-vector cells in the hippocampus (cells that fire when the rat is located at a specific distance and direction from an object in the environment) suggests that such an object-based spatial signal does enter the hippocampus [42].
It may be unrealistic to expect that the complex processing of hippocampal inputs can be reduced to simple phrases, for example ‘what versus where’. Just as these concepts have evolved in studies of the dorsal versus ventral processing streams in primate visual cortex [28,29], it appears that these concepts need modification in the medial temporal lobe processing streams (figure 1). Lisman [49] has proposed the alternative hypothesis that the MEC provides information about actions and self-localization, whereas the LEC provides information about external (non-self) cues and their location relative to the organism. This scheme seems to be more accurate than the ‘what versus where’ distinction and it is very similar to our current views. The MEC, in conjunction with other theta-modulated and spatial-processing areas involved in path integration, represents where the animal is in the environment and may provide the hippocampus with a global spatial map or framework that provides the spatial context for an experience. The firing of grid cells and related cells in the MEC appears to be involved in computations related to ‘Where am I?’ and ‘Where am I going?’ By contrast, the LEC may provide the hippocampus with local cue information about discrete items in its environment or discrete sensory cues, such as sounds, smells, etc. These are the individual items experienced during an event that make up the content of an experience to be remembered. Furthermore, the LEC may provide a signal not about where the rat is, but where these individual items are (‘What is out there and where is it?’). There are indications as well that the LEC may act as a filter or gate on the information coming from the perirhinal cortex, allowing only behaviourally relevant or attended information to enter the hippocampus [81,82]. This gating function may explain the variability in the responsiveness to objects across behavioural sessions [35]. That is, in some sessions, an LEC cell may fire when the animal passes an object if the animal attends to the object during that pass. In another session, however, the animal may pass an object but have its attention focused on the next piece of food that it is seeking. In this case, the cell may not fire, because the salience signal was not present to boost the weak sensory signal from the perirhinal cortex. Such a mechanism may be critical for allowing only attended experience to enter the hippocampus, which in the words of Morris & Frey [83], subserves the ‘automatic recording of attended experience’.
6. Summary
We have argued here that the present conceptual dichotomy of the LEC providing a ‘what’ signal to the hippocampus and the MEC providing a ‘where’ signal needs revision, just as the original notion of dorsal–ventral processing streams in primate visual cortex have undergone revision based on an understanding of the more complex underlying anatomy and the functional correlates of these regions. The LEC appears to convey information about individual items, for example objects, but it also conveys information about the remembered locations of objects and about the location of the rat, perhaps in relation to these objects. It appears primarily concerned with local processing, providing information to the hippocampus about the content of an experience, including spatial information. The MEC appears to be part of a path integration circuit that encodes a global spatial framework, a representation of the location of the rat in an allocentric coordinate frame. It provides the hippocampus with the spatial context of an experience. At best, however, this conceptualization is still a rough sketch and is certain to undergo further refinement (or replacement) as more data become available about the anatomical pathways in the circuit and the functional correlates of the components of these pathways.
Funding statement
Work from this laboratory presented in this paper was supported by NIH grants R01 NS039456, R01 MH094146 and T32 NS07467.
References
- 1.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford, UK: Clarendon Press. [Google Scholar]
- 2.O'Keefe J. 1976. Place units in the hippocampus of the freely moving rat . Exp. Neurol. 51, 78–109. ( 10.1016/0014-4886(76)90055-8) [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J. 1999. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus 9, 352–364. () [DOI] [PubMed] [Google Scholar]
- 4.Shapiro ML, Eichenbaum H. 1999. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons . Hippocampus 9, 365–384. () [DOI] [PubMed] [Google Scholar]
- 5.Burwell RD. 2000. The parahippocampal region: corticocortical connectivity . Ann. NY Acad. Sci. 911, 25–42. ( 10.1111/j.1749-6632.2000.tb06717.x) [DOI] [PubMed] [Google Scholar]
- 6.Manns JR, Eichenbaum H. 2006. Evolution of declarative memory . Hippocampus 16, 795–808. ( 10.1002/hipo.20205) [DOI] [PubMed] [Google Scholar]
- 7.Knierim JJ, Lee I, Hargreaves EL. 2006. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory . Hippocampus 16, 755–764. ( 10.1002/hipo.20203) [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H, Yonelinas AP, Ranganath C. 2007. The medial temporal lobe and recognition memory . Annu. Rev. Neurosci. 30, 123–152. ( 10.1146/annurev.neuro.30.051606.094328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero-Bleda M, Witter MP. 1993. Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat . J. Comp. Neurol. 328, 115–129. ( 10.1002/cne.903280109) [DOI] [PubMed] [Google Scholar]
- 10.Ishizuka N, Weber J, Amaral DG. 1990. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat . J. Comp Neurol. 295, 580–623. ( 10.1002/cne.902950407) [DOI] [PubMed] [Google Scholar]
- 11.Scharfman HE. 2007. The CA3 ‘backprojection’ to the dentate gyrus . Prog. Brain Res. 163, 627–637. ( 10.1016/S0079-6123(07)63034-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. 1996. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo . J. Comp. Neurol. 366, 271–292. () [DOI] [PubMed] [Google Scholar]
- 13.Witter MP, Amaral DG. 2004. Hippocampal formation. In The rat nervous system, vol. 3, 3rd edn (ed. Paxinos G.), pp. 635–704. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 14.Amaral DG, Witter MP. 1989. The three-dimensional organization of the hippocampal formation: a review of anatomical data . Neuroscience 31, 571–591. ( 10.1016/0306-4522(89)90424-7) [DOI] [PubMed] [Google Scholar]
- 15.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. 2000. Anatomical organization of the parahippocampal–hippocampal network . Ann. NY Acad. Sci. 911, 1–24. ( 10.1111/j.1749-6632.2000.tb06716.x) [DOI] [PubMed] [Google Scholar]
- 16.Burwell RD. 2001. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat . J. Comp. Neurol. 437, 17–41. ( 10.1002/cne.1267) [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh SS, Knierim JJ. 2012. Hippocampus . WIREs Cogn. Sci. 3, 231–251. ( 10.1002/wcs.1164) [DOI] [PubMed] [Google Scholar]
- 18.Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser MB, Moser EI. 2010. Spatial representation along the proximodistal axis of CA1 . Neuron 68, 127–137. ( 10.1016/j.neuron.2010.08.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargreaves EL, Rao G, Lee I, Knierim JJ. 2005. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus . Science 308, 1792–1794. ( 10.1126/science.1110449) [DOI] [PubMed] [Google Scholar]
- 20.Yoganarasimha D, Rao G, Knierim JJ. 2011. Lateral entorhinal neurons are not spatially selective in cue-rich environments . Hippocampus 21, 1363–1374. ( 10.1002/hipo.20839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshmukh SS, Yoganarasimha D, Voicu H, Knierim JJ. 2010. Theta modulation in the medial and the lateral entorhinal cortex . J. Neurophysiol. 104, 994–1006. ( 10.1152/jn.01141.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki WA, Miller EK, Desimone R. 1997. Object and place memory in the macaque entorhinal cortex . J. Neurophysiol. 78, 1062–1081. [DOI] [PubMed] [Google Scholar]
- 23.Gaffan D. 1998. Idiothetic input into object–place configuration as the contribution to memory of the monkey and human hippocampus: a review . Exp. Brain Res. 123, 201–209. ( 10.1007/s002210050562) [DOI] [PubMed] [Google Scholar]
- 24.Redish AD, Touretzky DS. 1997. Cognitive maps beyond the hippocampus . Hippocampus 7, 15–35. () [DOI] [PubMed] [Google Scholar]
- 25.Redish AD. 1999. Beyond the cognitive map: from place cells to episodic memory. Cambridge, MA: MIT Press. [Google Scholar]
- 26.Ungerleider LG, Mishkin M. 1982. Two cortical visual systems. In Analysis of visual behavior (eds Ingle DJ, Goodale MA, Mansfield RJW.), pp. 549–586. Cambridge, MA: MIT Press. [Google Scholar]
- 27.Ungerleider LG, Haxby JV. 1994. ‘What’ and ‘where’ in the human brain . Curr. Opin. Neurobiol. 4, 157–165. ( 10.1016/0959-4388(94)90066-3) [DOI] [PubMed] [Google Scholar]
- 28.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing . Nat. Rev. Neurosci. 12, 217–230. ( 10.1038/nrn3008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M. 2013. The ventral visual pathway: an expanded neural framework for the processing of object quality . Trends Cogn. Sci. 17, 26–49. ( 10.1016/j.tics.2012.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. 2004. Spatial representation in the entorhinal cortex . Science 305, 1258–1264. ( 10.1126/science.1099901) [DOI] [PubMed] [Google Scholar]
- 31.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. 2005. Microstructure of a spatial map in the entorhinal cortex . Nature 436, 801–806. ( 10.1038/nature03721) [DOI] [PubMed] [Google Scholar]
- 32.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. 2006. Conjunctive representation of position, direction, and velocity in entorhinal cortex . Science 312, 758–762. ( 10.1126/science.1125572) [DOI] [PubMed] [Google Scholar]
- 33.Savelli F, Yoganarasimha D, Knierim JJ. 2008. Influence of boundary removal on the spatial representations of the medial entorhinal cortex . Hippocampus 18, 1270–1282. ( 10.1002/hipo.20511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. 2008. Representation of geometric borders in the entorhinal cortex . Science 322, 1865–1868. ( 10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 35.Deshmukh SS, Knierim JJ. 2011. Representation of non-spatial and spatial information in the lateral entorhinal cortex . Front. Behav. Neurosci. 5, 69 ( 10.3389/fnbeh.2011.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferbinteanu J, Holsinger RM, McDonald RJ. 1999. Lesions of the medial or lateral perforant path have different effects on hippocampal contributions to place learning and on fear conditioning to context . Behav. Brain Res. 101, 65–84. ( 10.1016/S0166-4328(98)00144-2) [DOI] [PubMed] [Google Scholar]
- 37.Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. 2013. Distinct roles of medial and lateral entorhinal cortex in spatial cognition . Cereb. Cortex 23, 451–459. ( 10.1093/cercor/bhs033) [DOI] [PubMed] [Google Scholar]
- 38.Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. 2013. Lateral entorhinal cortex is critical for novel object-context recognition . Hippocampus 23, 352–366. ( 10.1002/hipo.22095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunsaker MR, Chen V, Tran GT, Kesner RP. 2013. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model . Hippocampus 23, 380–391. ( 10.1002/hipo.22097) [DOI] [PubMed] [Google Scholar]
- 40.Furtak SC, Ahmed OJ, Burwell RD. 2012. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination . Neuron 76, 976–988. ( 10.1016/j.neuron.2012.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshmukh SS, Johnson JL, Knierim JJ. 2012. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: comparison with lateral entorhinal cortex . Hippocampus 22, 2045–2058. ( 10.1002/hipo.22046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deshmukh SS, Knierim JJ. 2013. Influence of local objects on hippocampal representations: landmark vectors and memory . Hippocampus 23, 253–267. ( 10.1002/hipo.22101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weible AP, Rowland DC, Pang R, Kentros C. 2009. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex . J. Neurophysiol. 102, 2055–2068. ( 10.1152/jn.00214.2009) [DOI] [PubMed] [Google Scholar]
- 44.Tsao A, Moser MB, Moser EI. 2013. Traces of experience in the lateral entorhinal cortex . Curr. Biol. 23, 399–405. ( 10.1016/j.cub.2013.01.036) [DOI] [PubMed] [Google Scholar]
- 45.Weible AP, Rowland DC, Monaghan CK, Wolfgang NT, Kentros CG. 2012. Neural correlates of long-term object memory in the mouse anterior cingulate cortex . J. Neurosci. 32, 5598–5608. ( 10.1523/JNEUROSCI.5265-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolorfo CL, Amaral DG. 1998. Entorhinal cortex of the rat: organization of intrinsic connections . J. Comp. Neurol. 398, 49–82. () [DOI] [PubMed] [Google Scholar]
- 47.Witter MP, Canto CB, Couey JJ, Koganezawa N, O'Reilly KC. 2014. Architecture of spatial circuits in the hippocampal region. Phil. Trans. R. Soc. B 369, 20120515 ( 10.1098/rstb.2012.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kloosterman F, Van Haeften T, Witter MP, Lopes DSF. 2003. Electrophysiological characterization of interlaminar entorhinal connections: an essential link for re-entrance in the hippocampal–entorhinal system . Eur. J. Neurosci. 18, 3037–3052. ( 10.1111/j.1460-9568.2003.03046.x) [DOI] [PubMed] [Google Scholar]
- 49.Lisman JE. 2007. Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets . Prog. Brain Res. 163, 615–625. ( 10.1016/S0079-6123(07)63033-7) [DOI] [PubMed] [Google Scholar]
- 50.Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ. 2013. Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex . J. Neurosci. 33, 9246–9258. ( 10.1523/JNEUROSCI.0946-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witter MP, Moser EI. 2006. Spatial representation and the architecture of the entorhinal cortex . Trends Neurosci. 29, 671–678. ( 10.1016/j.tins.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 52.Hargreaves EL, Yoganarasimha D, Knierim JJ. 2007. Cohesiveness of spatial and directional representations recorded from neural ensembles in the anterior thalamus, parasubiculum, medial entorhinal cortex, and hippocampus . Hippocampus 17, 826–841. ( 10.1002/hipo.20316) [DOI] [PubMed] [Google Scholar]
- 53.Taube JS, Muller RU, Ranck JB., Jr 1990. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci. 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zugaro MB, Berthoz A, Wiener SI. 2001. Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. J. Neurosci. 21, RC154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoganarasimha D, Yu X, Knierim JJ. 2006. Head direction cell representations maintain internal coherence during conflicting proximal and distal cue rotations: comparison with hippocampal place cells . J. Neurosci. 26, 622–631. ( 10.1523/JNEUROSCI.3885-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. 2010. Grid cells in pre- and parasubiculum . Nat. Neurosci. 13, 987–994. ( 10.1038/nn.2602) [DOI] [PubMed] [Google Scholar]
- 57.Taube JS. 1995. Place cells recorded in the parasubiculum of freely moving rats . Hippocampus 5, 569–583. ( 10.1002/hipo.450050608) [DOI] [PubMed] [Google Scholar]
- 58.Cho J, Sharp PE. 2001. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex . Behav. Neurosci. 115, 3–25. ( 10.1037/0735-7044.115.1.3) [DOI] [PubMed] [Google Scholar]
- 59.Cacucci F, Lever C, Wills TJ, Burgess N, O'Keefe J. 2004. Theta-modulated place-by-direction cells in the hippocampal formation in the rat . J. Neurosci. 24, 8265–8277. ( 10.1523/JNEUROSCI.2635-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderwolf CH. 1969. Hippocampal electrical activity and voluntary movement in the rat . Electroencephalogr. Clin. Neurophysiol. 26, 407–418. ( 10.1016/0013-4694(69)90092-3) [DOI] [PubMed] [Google Scholar]
- 61.McNaughton BL, Barnes CA, O'Keefe J. 1983. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats . Exp. Brain Res. 52, 41–49. ( 10.1007/BF00237147) [DOI] [PubMed] [Google Scholar]
- 62.Wiener SI, Paul CA, Eichenbaum H. 1989. Spatial and behavioral correlates of hippocampal neuronal activity . J. Neurosci. 9, 2737–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasselmo ME, Giocomo LM, Zilli EA. 2007. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons . Hippocampus 17, 1252–1271. ( 10.1002/hipo.20374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuhs MC, Touretzky DS. 2006. A spin glass model of path integration in rat medial entorhinal cortex . J. Neurosci. 26, 4266–4276. ( 10.1523/JNEUROSCI.4353-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burgess N, Barry C, O'Keefe J. 2007. An oscillatory interference model of grid cell firing . Hippocampus 17, 801–812. ( 10.1002/hipo.20327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair HT, Gupta K, Zhang K. 2008. Conversion of a phase- to a rate-coded position signal by a three-stage model of theta cells, grid cells, and place cells . Hippocampus 18, 1239–1255. ( 10.1002/hipo.20509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samsonovich A, McNaughton BL. 1997. Path integration and cognitive mapping in a continuous attractor neural network model . J. Neurosci. 17, 5900–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whishaw IQ. 1998. Place learning in hippocampal rats and the path integration hypothesis . Neurosci. Biobehav. Rev. 22, 209–220. ( 10.1016/S0149-7634(97)00002-X) [DOI] [PubMed] [Google Scholar]
- 69.McNaughton BL, Chen LL, Markus EJ. 1991. ‘Dead reckoning’, landmark learning, and the sense of direction: a neurophysiological and computational hypothesis . J. Cogn. Neurosci. 3, 190–202. ( 10.1162/jocn.1991.3.2.190) [DOI] [PubMed] [Google Scholar]
- 70.Skaggs WE, Knierim JJ, Kudrimoti HS, McNaughton BL. 1995. A model of the neural basis of the rat's sense of direction . Adv. Neural Inf. Process Syst. 7, 173–180. [PubMed] [Google Scholar]
- 71.Zhang K. 1996. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory . J. Neurosci. 16, 2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knierim JJ, Hamilton DA. 2011. Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation . Physiol. Rev. 91, 1245–1279. ( 10.1152/physrev.00021.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taube JS. 2007. The head direction signal: origins and sensory-motor integration . Annu. Rev. Neurosci. 30, 181–207. ( 10.1146/annurev.neuro.29.051605.112854) [DOI] [PubMed] [Google Scholar]
- 74.Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment . Nature 392, 598–601. ( 10.1038/33402) [DOI] [PubMed] [Google Scholar]
- 75.Epstein RA, Parker WE, Feiler AM. 2007. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition . J. Neurosci. 27, 6141–6149. ( 10.1523/JNEUROSCI.0799-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu XO, Brown MW, Aggleton JP. 1995. Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices . Eur. J. Neurosci. 7, 753–765. ( 10.1111/j.1460-9568.1995.tb00679.x) [DOI] [PubMed] [Google Scholar]
- 77.Norman G, Eacott MJ. 2005. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats . Behav. Neurosci. 119, 557–566. ( 10.1037/0735-7044.119.2.557) [DOI] [PubMed] [Google Scholar]
- 78.Bussey TJ, Saksida LM, Murray EA. 2005. The perceptual–mnemonic/feature conjunction model of perirhinal cortex function . Q. J. Exp. Psychol. B. 58, 269–282. ( 10.1080/02724990544000004) [DOI] [PubMed] [Google Scholar]
- 79.Burke SN, Maurer AP, Hartzell AL, Nematollahi S, Uprety A, Wallace JL, Barnes CA. 2012. Representation of three-dimensional objects by the rat perirhinal cortex . Hippocampus 22, 2032–2044. ( 10.1002/hipo.22060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konkle T, Oliva A. 2012. A real-world size organization of object responses in occipitotemporal cortex . Neuron 74, 1114–1124. ( 10.1016/j.neuron.2012.04.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. 2003. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal–hippocampal circuit . J. Neurophysiol. 89, 2176–2184. ( 10.1152/jn.01033.2002) [DOI] [PubMed] [Google Scholar]
- 82.de Curtis M, Pare D. 2004. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus . Prog. Neurobiol. 74, 101–110. ( 10.1016/j.pneurobio.2004.08.005) [DOI] [PubMed] [Google Scholar]
- 83.Morris RG, Frey U. 1997. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Phil. Trans. R. Soc. Lond. B 352, 1489–1503. ( 10.1098/rstb.1997.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]