Abstract
Recent interest in the neural bases of spatial navigation stems from the discovery of neuronal populations with strong, specific spatial signals. The regular firing field arrays of medial entorhinal grid cells suggest that they may provide place cells with distance information extracted from the animal's self-motion, a notion we critically review by citing new contrary evidence. Next, we question the idea that grid cells provide a rigid distance metric. We also discuss evidence that normal navigation is possible using only landmarks, without self-motion signals. We then propose a model that supposes that information flow in the navigational system changes between light and dark conditions. We assume that the true map-like representation is hippocampal and argue that grid cells have a crucial navigational role only in the dark. In this view, their activity in the light is predominantly shaped by landmarks rather than self-motion information, and so follows place cell activity; in the dark, their activity is determined by self-motion cues and controls place cell activity. A corollary is that place cell activity in the light depends on non-grid cells in ventral medial entorhinal cortex. We conclude that analysing navigational system changes between landmark and no-landmark conditions will reveal key functional properties.
Keywords: grid cells, place cells, entorhinal cortex, navigation
1. Introduction
The behavioural concept that rats share with people map-like representations of their surroundings [1] was tied to neuroscience by the discovery of hippocampal place cells [2]. In the first statement of a neural theory of navigation, it was proposed that the map was entirely contained within the hippocampus [3]. In the ensuing years, however, it has become clear that portions of the mapping system lie elsewhere, a conclusion drawn from the discovery of a large variety of spatially tuned neuron classes in brain regions connected more or less directly to the hippocampus.
In a currently favoured synthesis of the navigational system [4], its essential elements are place cells, head direction cells [5], boundary cells [6] and grid cells [7]. Central goals of ongoing research are to explain how the location, orientation and spatially periodic signals carried by each of the major cell types arise and how such signals, modified by the activity of additional cell types, permit calculations of paths through the environment. Here, we are interested in aspects of these issues that stem initially from the grid cells of medial entorhinal cortex (MEC); we focus on the information carried by grid cells, their relationship to place cells and the role of grid cells in navigation. Our motivation is to propose several new notions about grid cell function that may complement or even replace the predominant views. After briefly reviewing place cells and grid cells, we address three specific issues:
(i) Are MEC grid cells the precursors of hippocampal place cells? The spatial firing properties of grid cells, when combined according to the right rules, can give rise to the very different properties of place cells. Several ways of accomplishing this transformation have been demonstrated, but the theoretical possibility does not guarantee that it actually happens.
(ii) Is the role of grid cells to provide a rigid spatial metric that adds distance to the orientation information signalled by head direction cells and the topological spatial representation signalled by place cells? In this view, the three main classes of spatially tuned cells correspond to three essential aspects of geometric information, namely, scale, direction and neighbourliness. In brief, the topology of the place cell representation is rotated into the correct angle by the head direction system and properly stretched or compressed to fit into the environment by the grid cell network. We review evidence that calls into question the idea that grid cells function in this way, and that eventually leads to a novel alternative.
(iii) Animals can track their position in a framework provided by landmark stimuli or by using self-motion information. Pure self-motion navigation cannot remain accurate over indefinite distances or times; discrepancies between the computed and true positions will accumulate unless a landmark-based resetting mechanism can put computed position back into register with the true position. Thus, self-motion navigation ultimately requires landmark references. A reverse relationship is assumed in some theoretical descriptions of the overall navigational system [8]: self-motion navigation, referred to as ‘path integration’, gives rise in MEC grid cells to a representation of spatial location that is a required substrate for the hippocampal cognitive map implemented by place cells. We have two disputes with this formulation. Of mainly terminological significance is the idea that self-motion navigation is equivalent to path integration; as discussed below, path integration is just one of several types of self-motion navigation. More importantly, we argue that both landmark-based and self-motion navigation depend primarily on the hippocampal map; both kinds of information are used in essentially the same way in the hippocampus for self-localization, updating of location and selection of efficient, geodesic routes that take the animal from its current position to a goal.
2. Basic place cell properties
Place cells are a subset of CA3 and CA1 hippocampal pyramidal cells characterized by a form of location-specific firing [9]. (Dentate gyrus granule cells have similar properties [10] but are not considered further.) In a small, familiar environment, the discharge of each place cell is rapid only if the rat's head is in a restricted ‘firing field’ [11]. In such circumstances, most place cells have only a single field but in larger environments two or more fields are not uncommon [12]. In open two-dimensional apparatuses, place cells discharge independently of head orientation but on linear tracks they generally fire only when the rat runs in one direction or the other [13]. Place cells are also seen in mice [14–16], bats [17] and humans [18].
Over relatively long recording times the firing field of each cell is stable [11,19], but on shorter time scales they fire sporadically, sometimes more rapidly and sometimes more slowly than expected if their discharge were governed by an inhomogeneous Poisson process whose rate parameter varied with location [20]. This ‘overdispersion’ of discharge may reflect short-term attentional switching and can be reduced by training or by restricting analysis to a single attentional state [21].
Firing field locations are not topographically arranged within the hippocampal pyramidal cell layer so that neighbouring cells can be active in overlapping or widely separated regions [22]. Nevertheless, the place cell subset is conceived as a map of the environment because each location corresponds to a unique constellation of average firing rates across the population, summarized by the vector of the individual rates. Given such a rate vector for a short (less than 0.5 s) time interval, its value can be compared with the values of the average rate vector for each small region (pixel) within the environment [23]. The closest match serves as a reasonably good estimate of the rat's location.
In sufficiently different surroundings, the pyramidal cell population undergoes ‘remapping’ [24] so that a separate map is used to represent each environment. In a remapping between two environments, each cell may participate in one of four ways: it may have a field in both, only in the first, only in the second or in neither; cells with fields in either environment are said to be part of that representation's ‘active subset’, whereas silent cells are part of the complementary inactive subset. To the first approximation, the probability that a cell has a field in either environment is about 0.4 [25,26] and selection from the population appears random, so that the joint probabilities for both environments is given by the binomial distribution [26]. Whether a pair of apparatuses is represented by one or two maps depends on their shape, size and visual appearance, and may also depend on whether recordings are made in a single apparatus put into two different rooms [27].
In a given apparatus, field locations may be controlled by visual, auditory, tactile and olfactory cues from fixed objects [28]. For example, if a uniform cylinder is surrounded by curtains, rotating a single contrasting cue card on its wall to a new position causes firing fields to rotate equally if the rat is out of the apparatus during the rotation [25]; this may be owing to similar control over the discharge of head direction cells [29]. If the rat sees the stimulus rotate, firing fields rotate if the displacement is small (45°) but remain in place if the displacement is large (180°) [30]. The lack of cue control during large rotations suggests that place cells are also affected by self-motion information; with large visible cue rotations, guidance by landmark stimuli can be suppressed if the rat detects the conflict between motion in the environment and a lack of change in its own position [31].
Cue control can also be exerted by distinct objects positioned against the wall of a cylindrical chamber although the same objects are less effective or ineffective if put nearer the cylinder centre [32]. Importantly, when cues with demonstrated rotational control are removed from the apparatus, location-specific firing does not cease nor become disorganized, but instead persists. That place cell discharge does not strictly depend on visual cues clearly distinguishes them from visual system neurons [25,33]. In fact, firing fields look normal in blind rats [34]. In normal rats, fields may remain stable when visual landmark information is totally suppressed, again indicating a role for self-motion cues in updating the rat's location [35]. By contrast, also removing olfactory cues strongly decreases firing field stability, showing that self-motion cues alone are insufficient to stabilize location-specific discharge. Finally, a recent virtual reality investigation of the relative contribution of self-motion and visual information for place cell firing indicates that both input types influence most place cells, although to different extents, even in fully lit conditions [36].
How do the spatial firing properties of place cells arise? Recently, it has become possible to make intracellular recordings from place cells in head-fixed animals in virtual environments or from freely moving rats [37–39]. In either case, as the animal approaches the firing field the cell depolarizes as if owing to summation of excitatory postsynaptic potentials (EPSPs) from afferent inputs; when the depolarization is great enough, the cell discharges and does so at an increasing rate as the animal proceeds through the field. It is therefore presently believed that place cell activity depends on synaptic weights and the discharge of input cells, in contrast to the idea that firing is caused by resonant interactions between an intracellular oscillator and an external theta oscillator with slightly different frequencies (e.g. [40]). The fascinating precession phenomenon [41] may also be explained with recourse to interference between two oscillators. Thus, the tendency of a place cell to fire progressively at earlier phases of the hippocampal theta rhythm as the rat proceeds through its firing field may be owing to summation of theta-frequency membrane voltage oscillations along with the excitatory ‘image’ of the firing field contributed by spatially tuned inputs.
3. Basic grid cell properties
Subsequent to the discoveries of place cells and head direction cells as well as place-like cells in MEC [42], the high density of inputs to the hippocampus from dorsal MEC motivated exploration of single-cell spatial firing properties [43] that culminated in the description of ‘grid cells’ [7]. Grid cells share with place cells a form of location-specific firing but differ in that they are characterized by multiple firing fields arranged in a remarkably regular lattice of equilateral triangles (or regular hexagons). A given grid cell can be described with three parameters, namely, field separation, orientation and spatial phase. Field separation is the distance between two vertices of the smallest triangle. Orientation can be taken as the angle in the range ±30° between horizontal and the closest triangle leg. Spatial phase is the location of a single reference field. Neighbouring grid cells have similar orientations and field separations but their spatial phases are distributed so as to cover the entire apparatus. Similar to place cells, the grid cell discharge is omnidirectional. Grid cells are found in layers 2 and 3 of MEC, which project, respectively, to CA3 and CA1 in the hippocampus. In layers 3, 5 and 6 are found ‘conjunctive cells’, so named because they are directional grid cells. Their activity depends on head direction as well as location and is modulated by the rat's running speed [44].
In a familiar environment, grid cell firing is stable so that firing fields do not drift with time [7]. This is possible only if grid cells receive sensory input about the animal's location, either via a back projection from place cells or from other non-grid MEC cells (e.g. boundary/border cells) [40]. The hexagonal array of grid cell fields appears to be an intrinsic feature of the local network. As the field spacing of individual grid cells is, to the first approximation, independent of the identity of the current environment, it has been inferred that this metric-like quantity is derived from processing of self-motion information; such information might come from vestibular, proprioceptive and other input sources (e.g. optic flow, efference copy) [45–47]. This notion is supported by the finding that grid cell firing patterns do not change drastically in the dark [7]. Subtle field modifications do, however, occur (fig. 5 in [7]) so that part of the stability may be owing to residual environmental information (e.g. olfactory cues) that was not fully eliminated in this experiment. In summary, it seems clear that grid cell firing is controlled by both self-motion and extrinsic sensory input such that the relative strengths of these information sources vary with environmental conditions. The issue of whether the spacing of grid cell fields is properly called a metric is discussed below.
How does grid cell firing change when place cells undergo complete remapping? For grid cells, there is no analogue of the active (or inactive) subset; each grid cell fires in each environment. Nevertheless, when place cells are expected to remap, grid cell spatial phase and orientation change unpredictably although the orientations of neighbouring cells remain in register [48]. As noted above, field separation is constant in familiar environments [49]; its magnitude is believed to be set by intracellular membrane properties (density of different h current subunits). Important exceptions to this constancy are considered later. Different field spacings do not occur haphazardly. To the contrary, there is a gradient of spacing distance along the dorsal-ventral axis of MEC, with larger separations found at more ventral loci [7]. Recent work indicates that field separation distances do not change smoothly but rather fall into groups of nearly the same separation, with intermediate distances not found [50,51].
Interestingly, muscimol inactivation of the hippocampus greatly reduces or eliminates the hexagonal array of grid cell fields [52]. This treatment does not, however, silence grid cells; instead they come to show significant directional tuning. Overall, the average firing rate decreases, as if a net excitatory drive had been removed.
Recent intracellular recordings from grid cells made as head-fixed mice ran along a virtual linear track [53,54] show that their activity is modulated by temporal depolarization gradients whose shape mimics that of the firing field currently being traversed. The timing of spikes within the field is modulated by the concurrent theta rhythm, but here as well as for place cells the fundamental control mechanism has attractor-like features. Nevertheless, the results also show phase-processing sub-threshold membrane potential oscillations [53], which may be consistent with models based on resonant interference between oscillators of nearly equal frequency.
4. A brief aside on ventral MEC place-like cells.
Although our main focus is the role of grid cells in navigation, we first consider another class of spatially tuned MEC cells because we believe that grid cells are ordinarily not the predominant source of location-specific information for place cells, and therefore must provide a plausible alternative. In recordings from more ventral portions of MEC than the grid cell strip, cells in superficial layers (2 and 3) were seen with single firing fields that were larger and noisier than those of hippocampal place cells [42]. More recent work also reports non-grid spatial cells in the grid cell region [55,56].
For many ‘ventral MEC place-like cells’, the discharge rate was greater than zero over the entire apparatus surface, although others had restricted fields. These fields were stable so that longer recordings yielded smoother firing pattern contours. Similar to grid cells but distinct from place cells, each ventral medial entorhinal cortex (vMEC) place-like cell has a field in each environment. Although changing the shape of the apparatus from a cylinder to a square can induce place cell remapping, the spatial firing of vMEC cells is often topologically stretched so that the pattern is similar in both environments.
The spatial firing distributions of some vMEC place-like cells conform to the apparatus boundary [42], a property shared by some hippocampal place cells [6,11,57]; such fields are not circles truncated by the apparatus wall, as can be seen with grid cell fields. In addition to field shape discrepancies between vMEC place-like cells and grid cells, apparatus scaling experiments suggest that vMEC cells are not merely grid cells with very large fields. Specifically, when they were recorded in both 89 and 152 cm cylinders, vMEC cells either scaled or remapped but in no case were new fields seen [58], as would be expected if these units were simply grid cells [7]. In the theory proposed below, we suggest that the vMEC place-like cells supply the essential location-specific information necessary to form place cells.
This idea is not unique: because all MEC cell types project to hippocampal place cells [59], inputs from non-grid cells like boundary/border cells [6] or even non-spatial MEC cells may be important. For example, the boundary vector cell model states that place cells are driven by environmental sensory inputs and provides a detailed mechanistic account of the effects of the environmental manipulations discussed here [57,60,61]. With specific reference to grid cells, O'Keefe & Burgess [40] explicitly state that the environmental sensory inputs, for example those mediated by boundary cells, must drive place cell firing and that this (environmentally driven) place cell firing is used to stabilize grid cell firing relative to the environment, because self-motion information alone cannot specify a stable environmental location.
5. Are grid cells the main precursors for place cells?
The striking triangular array of grid cell firing fields and the strong projection from dorsal MEC to the hippocampus immediately raised two possibilities [7]. First, it was proposed that the varying grid scale permits path integration calculations based on self-motion information obtained from vestibular, proprioceptive and other input sources, an issue we return to below. Second, it was suggested that grid cells are the origin of the spatial signals conveyed to hippocampal place cells.
In the relatively short time since the discovery of grid cells, it has been amply demonstrated with models that their ensemble discharge can be transformed to the discrete firing fields of hippocampal place cells [62,63] (see [64] for a generalized theory and excellent review). The scheme of Solstad et al. [63] takes into account the bias for grid cells with more densely arranged fields in the dorsal part of the strip to preferentially project to the septal hippocampus and for grid cells with more widely spaced fields to project to the temporal hippocampus; the result is the correct prediction that temporal place cells have larger firing fields [65]. Indeed, by allowing for relatively minor changes in grid cell characteristics, it is possible to mimic remapping in the hippocampus [64,66].
It is essential to appreciate, however, that the ability to model the transformation is not a proof that grid cells give rise to place cells. The regular triangular array of grid cell fields in fact allows for a large variety of aesthetically pleasing models that account for how the rat localizes itself [62,67,68] and how, with the inclusion of conjunctive cells, the animal can compute optimal paths to a goal through unexplored space [69]. Nevertheless, the unique spatial firing patterns of grid cells provide what appears to be an overdetermined basis on which to solve navigational problems; given their properties and the right assumptions about how to combine their activity, it is hard to see a geometric question that cannot be answered.
Recent experimental work implies that this caveat is not just a nicety. Two extensive ontological studies suggest that adult-like place cells may precede adult-like grid cells during the early development of exploratory and navigational behaviour, starting around postnatal day 16 in rats. Wills et al. [70] found that the appearance of place cells preceded that of grid cells by 3–4 days. Langston et al. [71] saw that a few grid cells emerged at about the same time as place cells, but their activity was characterized as rudimentary, suggesting rather good agreement with Wills et al. [70]. Even though it can be argued that rudimentary grid cells may provide sufficiently patterned input to the hippocampus to generate location-specific firing [71], the later development of grid cells suggests that they are not necessary to drive place cell firing.
In the second line of investigation, it was shown that inactivating the medial septum by injecting muscimol [55] or local anaesthetic [56] strongly attenuates power in the theta (5–12 Hz) range in medial entorhinal electroencephalogram (EEG) (and the hippocampus). This reduction of theta power is accompanied by a great reduction or abolition of the firing field arrays of grid cells. It was demonstrated that the loss of spatial periodicity was not secondary to the concomitant decrease in grid cell firing rate [56]. Crucially, the location-specific discharge of hippocampal place cells persists after medial septal inactivation by local anaesthetic injection, although the intensity of such discharge decreases [56,72]. Thus, the conclusion that grid cell activity is not necessary for place cell firing is supported.
The dissociation between grid and place cell activity seen in developmental and inactivation studies is supported by the continued presence of hippocampal place cells after massive MEC lesions [73]. Although it is possible that some grid cells were not destroyed by the electrolytic lesions and that only a few grid cells are needed to generate hippocampal place cells, this study also casts doubt on the idea that convergent grid cell (or vMEC place-like cell) discharge gives rise to place cell activity.
An additional, functional reason to doubt that grid cells furnish the key spatial information to place cells comes from simultaneous single-cell and local field potential (LFP) recordings from MEC and the hippocampus. Referred to theta activity from MEC layer 3, the phase interval between the bulk of spike activity in CA3 and in layer 2 of MEC is smaller than that in the reverse direction; similarly, the phase interval between CA1 and layer 3 of MEC is smaller than that in the other direction [74]. It therefore appears unlikely that MEC drives the hippocampus in the simple way expected for the proposed causal connection between grid and place cells. In summary, current evidence suggests that grid cells have at most a supporting role in establishing the hippocampal map and a stronger dissociation is possible.
6. Do grid cells supply a unique metric?
The regular spacings of grid cell fields and initial indications that the spacings are environment-independent have been taken to mean that the lattice provides a scale for the navigational system and specifically for the topologically organized hippocampal map [47,75]. In this view, the intervals between fields with different spacings act as rulers, so that unique combinations of fields are active at each location in the environment. As has become increasingly clear, however, grid cell fields are not invariant. We argue that this critical finding implies that grid cells do not serve to scale the hippocampal map so that it is properly sized for the current environment.
Contrary to the original picture, data now show that the spacing and even arrangement of grid cell fields are elastic. In a study [50], initial exposures to a rectangular box with modified aspect ratio were accompanied by parallel changes in the spacing of fields. For example, in going from a 100 × 100 cm box to a 100 × 70 cm box, field distance was compressed to follow the size reduction. The magnitude of the grid spacing change decreased with repeated entries into the altered box. This outcome implies that grid cell field spacing is not precisely fixed by cell-specific properties but is to some extent modifiable by experience in the surroundings. Importantly, analogous changes in firing field shape and location were reported for place cells after aspect ratio alterations [76].
In the second case, an open square area was transformed by transparent linear barriers into a maze with parallel alleys connected by U-turns at each end [77]; importantly, the transparent barriers did little to alter the overall visual appearance of the arena. The result was that omnidirectional grid cell fields became directional, such that a given cell tended to fire a certain distance from the turn into each alley when running in one direction and at a different distance when running in the other direction. As suggested by the authors, it is possible that a similar transform occurs for place cells, leaving open the possibility that the grid cells still supply a metric. While this is possible, it implies a major change in just what sort of metric is involved.
In the third example [78], grid cells in rats familiar with a 100 cm2 box were recorded in an identically shaped box with altered wall colour, floor composition and scent; recordings were made with the original and novel boxes in the same or different laboratory frames and in the light or the dark. It was seen that field separation increased, an effect accompanied by shifts in grid orientation and phase. As with the shape change experiment [50], the magnitude of the alterations diminished with repeated exposures to the initially novel environment. When place cells were simultaneously recorded in the familiar and novel environments, they underwent complete remapping.
In a recent work [58], grid cells were recorded (simultaneously with vMEC place-like cells and hippocampal place cells) in a cylinder with two distinct cue cards separated by 135° on the wall and after the card separation was changed by ±45°; rats were thoroughly familiarized with each configuration before recordings were made. In agreement with earlier results on place cells [79], the arrangement of grid cell fields was topologically distorted in the fashion described by an empirical vector field equation [80] or by a model in which manipulation of directional cues alter head direction cell firing, which results in a modification of boundary vector cells, and hence the observed changes in place cell firing [61,81,82]. For grid cells, vMEC cells and place cells, the spatial firing patterns stretched but did not remap. In these circumstances, therefore, the modified spatial signal was consistent in different portions of the navigational system. For grid cells, it was as if their field locations are constantly updated to stay in register with those of place cells and in accordance with the visual circumstances. Importantly, the grid cell stretch was seen after many exposures to the altered environment and was not a transient effect. Thus, self-motion information is effectively suppressed, allowing agreement among the three cell types; the end result is that the structure of the grid cell lattice does not remain constant. Together, these findings of altered field geometry imply that grid cells do not function as an invariant, environment-independent metric for properly scaling the environment.
7. Path integration, self-motion-based navigation and cognitive mapping
The original meaning of the term ‘path integration’ was quite specific; with the animal starting at a certain location, often a home box, a sequence of displacement vectors taken by the animal was summed up, such that at any time the inverse of the vector sum could be computed and used to take the animal back to the starting point. Based on behavioural evidence [83], there is no doubt that mammals can use path integration to accurately navigate in the absence of landmark information. This finding firmly established the notion that the mammalian brain has available mechanisms especially devoted to geometric-based locomotion.
Beginning with the idea of path integration, there are two issues we feel are useful to raise. The first is a matter of terminology. By now, path integration has come to refer to any form of landmark-independent navigation in which the animal's location is updated on the basis of self-motion information alone; such information can arise from several sources including vestibular signals, odometry and optic flow. In our opinion, it would be useful to preserve the first, more restrictive meaning of ‘path integration’ and to use a second, more general term to refer to other cases of landmark-independent navigation. For the remainder of this review, we will refer to such updating of spatial information and location as ‘self-motion-based navigation’ to distinguish it from path integration. It should be noted that, regardless of which of these processes is guiding locomotion, the iterative nature of spatial updating must lead to cumulative errors whose magnitude depends on distance travelled or on elapsed time [45]. Thus, a recalibration process must exist that involves gathering of environmental information to correct errors in the rat's calculated position.
The second, more critical issue involves the idea that the ensemble activity of medial entorhinal cells, including grid cells, forms a self-motion-based navigation network whose output is required for the proper operation of the hippocampal map [8,75]. Our difficulty is with the notion that correct navigation always involves a self-motion substrate.
An indication of how the brain honours the distinction between landmark-based and self-motion-based navigation comes from studies of L7PKCI transgenic mice in which long-term depression at synapses between parallel fibres and Purkinje cell synapses is suppressed. At the behavioural level, mutant mice were compared to wild-type litter mates in a simple navigational task. In addition, place cells were recorded from both mouse strains [84].
In the behavioural problem, mice were trained to swim from a fixed starting location to a fixed, marked platform in the light. No performance difference was seen during learning; the transgenic mice acquired the ability to rapidly swim to and climb onto the goal. Subsequently, the mice were tested in the dark, where they had to use self-motion information to take direct, efficient routes. Under these circumstances, the transgenic mice were markedly impaired in escape latency and initial heading when released. Self-motion guidance in the dark was also deficient in the transgenic animals according to the fraction of direct paths to the goal. Interestingly, L7PKCI mice are impaired in a conventional, hidden goal version of the Morris water maze but not on a variant with constraining swimming channels that presumably requires a map-like representation but imposes less burden on the ability to take efficient paths during a trial [85]. Together, these results indicate that transgenic animals are impaired when self-motion information is important for task performance.
Based on these findings, it is possible to understand differences between place cells in transgenic and wild-type mice. In a lighted cylinder that also contains a tall landmark directly in front of a single, contrasting wall card, place cells in both strains are the same according to firing rate, spatial coherence, and within- and across-session stability. By contrast, the L7PKCI mouse place cells were defective in all these measures in the dark, in the absence of the tall landmark. These defects were attributable to the lack of landmarks and not the lack of light or the absence of a fixed visual cue; with the tall object in the cylinder, place cell activity in the dark was the same in L7PKCI and wild-type mice [84].
The reductions induced by landmark removal of firing rate and coherence for L7PKCI place cells are plausibly accounted for by abnormally weak activation of the attractor that stores synaptic weights for the map of the current environment; the weakened activation is taken, in turn, to be the consequence of the impaired signal arising from processing self-motion information. The decreases in within- and across-session stability in the absence of landmarks are taken as direct results of the inability to maintain location-specific firing in the face of degraded self-motion-based positional signalling. This hypothesis is supported by the results of a corroborative experiment in which exposing the mice to a conflict between landmarks and self-motion signals reveals a lack of control by the self-motion inputs [84].
In a strong interpretation of the behavioural and single-cell results from L7PKCI mice, the conclusion is that quite normal navigation and cognitive map function are possible without participation from self-motion signals. We infer, in other words, that the two sources of information are dissociable and parallel rather than serial in nature. In the final section, we propose a simple theory of navigation based on the idea that navigation in the light does not require a landmark-independent metric.
8. A two-state theory of navigation
We propose here a limited model of how major cellular components of the navigational system cooperate in representing space and how information flow changes between light and dark conditions. It does not address the origin of the spatial firing properties of any of the cell types and considers only at the descriptive level how the properties of different cell types influence each other. It is useful to say that the terms light and dark are really short-hand for more complex ideas; in lighted conditions we mean that landmark (‘absolute’) information is available, whereas in dark conditions we mean that only self-motion (‘relative’) information is used. Thus, for example, landmark-based navigation is perfectly possible in the absence of illumination if distinguishable objects are present in the environment.
Our theory has two main premises. First, it assumes that the truly map-like representation that calculates and selects routes is based in the hippocampus; the place cells of CA3 and CA1 comprise the machinery by which environmental geometry becomes a determinant of spatial behaviour. Second, the theory proposes that entorhinal grid cells have a crucial navigational role mainly in the dark. In this view, their activity in the light is controlled by visual stimuli so that it stays in register with place cell activity (even though integration of self-motion cues is necessarily involved in generating their regular firing pattern); this control can be exerted by neocortical inputs or by return flow of information from the hippocampus [52]. By contrast, in the dark, grid cell activity is dictated by self-motion cues and controls place cell activity. As a corollary, the location-specific discharge of place cells in the light depends on non-grid cells in vMEC relaying visual information; place cells are not formed by summed-up input from grid cells. Grid cell activity itself may be derived directly from vMEC cells or by return connections from the hippocampus.
Our theory, summarized in figure 1, is based on the idea that the hippocampal map receives two streams of information about the animal's location in its surroundings, one derived from fixed landmarks and the other derived from self-motion signals. These streams are taken to be parallel and independent; information is not processed first by one, and then the other. Thus, for all map-guided navigation, the hippocampus acts as a ‘final common pathway’ for signals arriving along the two streams. We imagine that the encodings in the two streams can be processed as equivalent when they reach the hippocampus.
Figure 1.
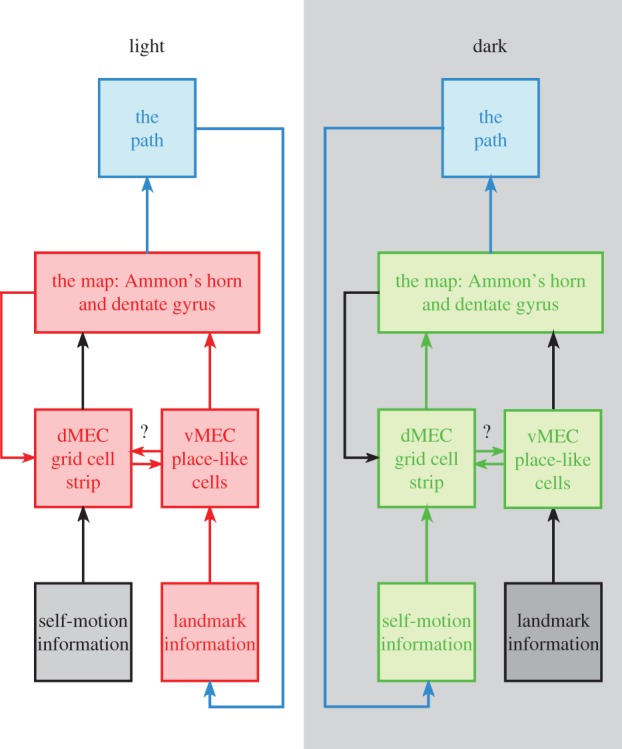
Block diagram summarizing the proposed organization for a two-state model of navigation. The basic notion is that information flow in the navigational system depends on whether the animal is using landmark (as in the light) or self-motion (as in the dark) signals to select its path through space. The short arrows from the Map to the Path stand for the neural machinery required to transform the hippocampal encoding into locomotion. The long arrows from the path stand for changes in sensory information brought about when the path is taken; in the light, this means changes in landmark configuration caused by movement; in the dark it means vestibular and odometry signals that can be used to estimate movement. In the light, the essential information flow to the hippocampus is via ventral MEC place-like cells; in the dark it is via the dorsal MEC grid cell strip. Depending on the state, the navigational loop including the map and the actual path does not depend on network elements linked by black lines. The arrow from the map to the grid cell strip signifies: (a) that hippocampal inactivation reduces the net excitatory drive on grid cells [51], (b) that grid cell fields stay in register with place cell firing fields during the topological distortion induced by small cue conflicts. The bidirectional arrows between the grid cell strip and ventral MEC indicate the possibility of interaction between the landmark and self-motion subsystems. Although a comprehensive review of temporal lobe neuroanatomy [86,87] cites no direct connections between the two portions of MEC, they might be linked via the intermediate zone. dMEC, dorsal medial entorhinal cortex; vMEC, ventral medial entorhinal cortex. (Online version in colour.)
Although we assume that the two input streams are independent with regard to information flow, the landmark stream is preferred for the obvious reason that errors must accumulate if localization depends only on the self-motion stream. In this view, if landmark information is unavailable, self-motion information is used to avoid a complete loss of localization. Navigation using either landmark or self-motion is possible but in many circumstances rapid switching between the two guidance sources occurs; such switching might be the basis for overdispersion of place cell discharge during single passes of the rat through firing fields if drive onto individual cells from the two sources is spatially in register but of different intensity [21]. Such a process might also support the rapid switching between two representations after an animal is moved from one environment to another [88].
Both streams arise in MEC; landmark information is processed by ventral place-like cells whereas, in agreement with virtually all proposals made since their discovery, grid cells (plus conjunctive and head direction cells) process self-motion signals. Central to our theory is the additional idea that throughput in both the ventral and dorsal regions is different depending on whether landmark cues are available or not. The proposed reorganization is summarized in the two parts of figure 1.
In the light (with landmarks), we suggest that the self-motion input to the grid cells is suppressed to the extent that excitatory inputs from the hippocampus can dominate their spatial firing patterns. We argue, in other words, that the ‘return’ signal from the hippocampus to grid cells [52] is not simply facilitatory; in this radical view, the loss of the hexagonal field grid array following temporary hippocampal inactivation indicates a loss of hexagonally organized return information, the same information that keeps grid cell fields in register with hippocampal place cell fields during visual cue conflict [58]. The topological distortion of hippocampal field locations and shapes [58,79] is a consequence of distorted landmark signals via the ventral region. By contrast, the self-motion signal available to the grid cells is overridden by the landmark-based feedback signal from place cells. Other accounts of the distorted grid cell field array during cue conflict are possible; for instance, information might be relayed via the intermediate MEC area.
In the dark (without landmarks), navigation depends on grid cell input to the hippocampus, but now grid cell activity is controlled by self-motion signals. The return pathway may be suppressed (as indicated by a black arrow on the right of figure 1) but in any case, neither place cell fields nor grid cell fields should be distorted if a cue conflict is eliminated by making landmarks invisible. The extent to which ventral MEC place-like cells are affected by the absence of landmarks is an empirical question, but the large changes in their fields induced by removing either one of two salient visual cues [58] implies that major shifts are very likely.
The premises of our theory are subject to several experimental tests. For instance, we expect grid cells to be abnormal or non-existent in L7PKCI mice, despite the presence of place cells that function normally with landmarks; in one type of abnormality, grid cells would be essentially unchanged in the light but would be silent or have only directional selectivity in the dark. As noted above, we expect major changes in the spatial firing characteristics of ventral MEC place-like cells in the dark. It is also possible that the theta-phase timing of hippocampal and MEC cells [74] will shift in the absence of landmarks, so as to make the MEC→hippocampus interval shorter than the reverse interval, as is seen in the light. In a familiar environment, there is no reason to expect a shift in grid cell firing in going from the light to the dark. By contrast, we expect that the distortion of grid cell field locations induced in the light by cue conflict will disappear when the lights are turned off.
Two other predictions arise from the anatomical separation of grid cells and vMEC place-like cells. Thus, lesions of vMEC should preferentially disrupt navigation in the light compared with the dark, whereas lesions of dorsal medial entorhinal cortex (dMEC) should impair self-motion more than landmark-based navigation.
9. Conclusion
In our model, grid cells provide a means by which place cells can track the animal's location using only self-motion information when absolute sensory information is unavailable, degraded or ignored. The grid cell system is seen as a specialized spatial coprocessor that helps the rat to estimate its location when location cannot be precisely determined, but navigation nevertheless takes place on the hippocampal map.
In two regards, this picture is quite different from the more usual ones of how grid cells are related to place cells. First, the grid cells are not obligatory preprocessors for the hippocampal map. Second, the grid cell system does not provide the metric that complements the topological (hippocampal) representation and directional signals. Overall, it is our opinion that analysing extrahippocampal changes in the navigational system between landmark and no-landmark conditions will reveal important aspects of its functioning and may well teach us that the two input streams are independent to an extent not so far appreciated.
Acknowledgement
B.H. received support from the Swartz Foundation and Marie Curie International Outgoing Fellowship within the EU Seventh Framework Programme for Research and Technological Development.
References
- 1.Tolman EC. 1948. Cognitive maps in rats and men. Psychol. Rev. 55, 189–208. ( 10.1037/h0061626) [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely moving rat. Brain Res. 34, 171–175. ( 10.1016/0006-8993(71)90358-1) [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe J, Nadel L. 1978. Hippocampus as a cognitive map. Oxford, UK: Clarendon. [Google Scholar]
- 4.Moser EI, Kropff E, Moser M-B. 2008. Place cells, grid cells, and the brain's spatial representation system. Ann. Rev. Neurosci. 31, 69–89. ( 10.1146/annurev.neuro.31.061307.090723) [DOI] [PubMed] [Google Scholar]
- 5.Taube JS, Muller RU, Ranck JB., Jr 1990. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI. 2008. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. ( 10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 7.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. ( 10.1038/nature03721) [DOI] [PubMed] [Google Scholar]
- 8.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B. 2006. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 7, 663–678. ( 10.1038/nrn1932) [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J. 1979. A review of the hippocampal place cells. Prog. Neurobiol. 13, 419–439. ( 10.1016/0301-0082(79)90005-4) [DOI] [PubMed] [Google Scholar]
- 10.Neunuebel JP, Knierim JJ. 2012. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J. Neurosci. 32, 3848–3858. ( 10.1523/jneurosci.6038-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller RU, Kubie JL, Ranck JB., Jr 1987. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J. Neurosci. 7, 1935–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park E, Dvorak D, Fenton A. 2011. Ensemble place codes in hippocampus: CA1, CA3, and dentate gyrus place cells have multiple place fields in large environments. PLoS ONE 6, e22349 ( 10.1371/journal.pone.0022349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller RU, Bostock EM, Taube JS, Kubie JL. 1994. On the directional firing properties of hippocampal place cells. J. Neurosci. 14, 7235–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. 1996. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349. ( 10.1016/S0092-8674(00)81828-0) [DOI] [PubMed] [Google Scholar]
- 15.Rotenberg A, Mayford M, Hawkins RD, Kandel ER, Muller RU. 1996. Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell 87, 1351–1361. ( 10.1016/S0092-8674(00)81829-2) [DOI] [PubMed] [Google Scholar]
- 16.Cho YH, Giese KP, Tanila HT, Silva AJ, Eichenbaum H. 1998. Abnormal hippocampal spatial representations in aCaMKIIT286A and CREBαΔ-mice. Science 279, 867–869. ( 10.1126/science.279.5352.867) [DOI] [PubMed] [Google Scholar]
- 17.Yartsev MM, Ulanovsky N. 2013. Representation of three-dimensional space in the hippocampus of flying bats. Science 340, 367–372. ( 10.1126/science.1235338) [DOI] [PubMed] [Google Scholar]
- 18.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. 2003. Cellular networks underlying human spatial navigation. Nature 425, 184–188. ( 10.1038/nature01964) [DOI] [PubMed] [Google Scholar]
- 19.Thompson LT, Best PJ. 1990. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 509, 299–308. ( 10.1016/0006-8993(90)90555-P) [DOI] [PubMed] [Google Scholar]
- 20.Fenton AA, Muller RU. 1998. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc. Natl Acad. Sci. USA 95, 3182–3187. ( 10.1073/pnas.95.6.3182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenton AA, et al. 2010. Attention-like modulation of hippocampal place cell discharge. J. Neurosci. 30, 4613–4625. ( 10.1523/jneurosci.5576-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redish AD, et al. 2001. Independence of firing-correlates of anatomically-proximate hippocampal pyramidal cells. J. Neurosci. 21, RC134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson MA, McNaughton BL. 1993. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058. ( 10.1126/science.8351520) [DOI] [PubMed] [Google Scholar]
- 24.Muller RU, Kubie JL, Bostock EM, Taube JS, Quirk GJ. 1991. Spatial firing correlates of neurons in the hippocampal formation of freely moving rats. In Brain and space (ed. Paillard J.), pp. 296–333. London, UK: Oxford University Press. [Google Scholar]
- 25.Muller RU, Kubie JL. 1987. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. 1999. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124. ( 10.1038/16046) [DOI] [PubMed] [Google Scholar]
- 27.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser M-B. 2005. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309, 619–623. ( 10.1126/science.1114037) [DOI] [PubMed] [Google Scholar]
- 28.O'Keefe J, Conway DH. 1978. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp. Brain Res. 31, 573–590. ( 10.1007/BF00239813) [DOI] [PubMed] [Google Scholar]
- 29.Taube JS, Muller RU, Ranck JB., Jr 1990. Head direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci. 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotenberg A, Muller RU. 1997. Variable place-cell coupling to a continuous viewed stimulus: evidence that the hippocampus acts as a perceptual system. Phil. Trans. R. Soc. Lond. B 352, 1505–1513. ( 10.1098/rstb.1997.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffery KJ, Donnett JG, Burgess N, O'Keefe J. 1997. Directional control of hippocampal place fields. Exp. Brain Res. 117, 131–142. ( 10.1007/s002210050206) [DOI] [PubMed] [Google Scholar]
- 32.Cressant A, Muller RU, Poucet B. 1997. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci. 17, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Keefe J, Speakman A. 1987. Single unit activity in the rat hippocampus during a spatial memory task. Exp. Brain Res. 68, 1–27. ( 10.1007/BF00255230) [DOI] [PubMed] [Google Scholar]
- 34.Save E, Cressant A, Thinus-Blanc C, Poucet B. 1998. Spatial firing of hippocampal place cells in blind rats. J. Neurosci. 18, 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Save E, Nerad L, Poucet B. 2000. Contribution of multiple sensory information to place field stability in hippocampal place cells. Hippocampus 10, 64–76. () [DOI] [PubMed] [Google Scholar]
- 36.Chen GF, King JA, Burgess N, O'Keefe J. 2013. How vision and movement combine in the hippocampal place code. Proc. Natl Acad. Sci. USA 110, 378–383. ( 10.1073/pnas.1215834110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AK, Manns ID, Sakmann B, Brecht M. 2008. Whole-cell recordings in freely moving rats. Neuron 51, 399–407. ( 10.1016/j.neuron.2006.07.004) [DOI] [PubMed] [Google Scholar]
- 38.Harvey CD, Collman F, Dombeck DA, Tank DW. 2009. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946. ( 10.1038/nature08499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epsztein J, Brecht M, Lee AK. 2011. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120. ( 10.1016/j.neuron.2011.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Keefe J, Burgess N. 2005. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus 15, 853–866. ( 10.1002/hipo.20115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. ( 10.1002/hipo.450030307) [DOI] [PubMed] [Google Scholar]
- 42.Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr 1992. The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J. Neurosci. 12, 1945–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B. 2004. Spatial representation in the entorhinal cortex. Science 306, 1258–1264. ( 10.1126/science.1099901) [DOI] [PubMed] [Google Scholar]
- 44.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter M, Moser M-B, Moser EI. 2006. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762. ( 10.1126/science.1125572) [DOI] [PubMed] [Google Scholar]
- 45.Etienne AS, Jeffery KJ. 2004. Path integration in mammals. Hippocampus 14, 180–192. ( 10.1002/hipo.10173) [DOI] [PubMed] [Google Scholar]
- 46.Jeffery KJ, Burgess N. 2006. A metric for the cognitive map: found at last? Trends Cogn. Sci. 10, 1–3. ( 10.1016/j.tics.2005.11.002) [DOI] [PubMed] [Google Scholar]
- 47.Jeffery KJ. 2007. Self-localization and the entorhinal–hippocampal system. Cur. Opin. Neurobiol. 17, 684–691. ( 10.1016/j.conb.2007.11.008) [DOI] [PubMed] [Google Scholar]
- 48.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. 2007. Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446, 190–194. ( 10.1038/nature05601) [DOI] [PubMed] [Google Scholar]
- 49.Giocomo LM, Zilli EA, Fransen E, Hasselmo M. 2007. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science 315, 1719–1722. ( 10.1126/science.1139207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barry C, Hayman R, Burgess N, Jeffery KJ. 2007. Experience-dependent rescaling of entorhinal grids. Nat. Neurosci. 10, 682–684. ( 10.1038/nn1905) [DOI] [PubMed] [Google Scholar]
- 51.Stensola H, Stensola T, Solstad T, Froland K, Moser M-B, Moser EI. 2012. The entorhinal grid map is discretized. Nature 492, 72–78. ( 10.1038/nature11649) [DOI] [PubMed] [Google Scholar]
- 52.Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. 2013. Grid cells require excitatory drive from the hippocampus. Nat. Neurosci. 16, 309–317. ( 10.1038/nn.3311) [DOI] [PubMed] [Google Scholar]
- 53.Domnisoru C, Kinkhabwala AA, Tank DW. 2013. Membrane potential dynamics of grid cells. Nature 495, 199–204. ( 10.1038/nature11973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt-Hieber C, Haeusser M. 2013. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 16, 325–331. ( 10.1038/nn.3340) [DOI] [PubMed] [Google Scholar]
- 55.Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. 2011. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599. ( 10.1126/science.1201652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. 2011. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595. ( 10.1126/science.1201685) [DOI] [PubMed] [Google Scholar]
- 57.Lever C, Burton S, Jeewajee A, O'Keefe J, Burgess N. 2009. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 29, 9771–9777. ( 10.1523/jneurosci.1319-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song E, Fox SE, Rivard B, Muller RU. 2012. Neuronal representations of two visual stimuli modifications on the network level of the spatial cognition in rat brain. Program No. 293.05. Neuroscience Meeting Planner New Orleans, LA: Society for Neuroscience. [Google Scholar]
- 59.Zhang S-J, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser M-B, Moser EI. 2013. Optogenetic dissection of entorhinal–hippocampal functional connectivity. Science 340 ( 10.1126/science.1232627) [DOI] [PubMed] [Google Scholar]
- 60.Hartley T, Burgess N, Lever C, Cacucci F, O'Keefe J. 2000. Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379. () [DOI] [PubMed] [Google Scholar]
- 61.Barry C, Burgess N. 2007. Learning in a geometric model of place cell firing. Hippocampus 17, 786–800. ( 10.1002/hipo.20324) [DOI] [PubMed] [Google Scholar]
- 62.Fuhs MC, Touretzky DS. 2006. A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276. ( 10.1523/jneurosci.1353-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solstad T, Moser EI, Einevoll GT. 2006. From grid cells to place cells: a mathematical model. Hippocampus 16, 1026–1031. ( 10.1002/hipo.20244) [DOI] [PubMed] [Google Scholar]
- 64.Cheng S, Frank LM. 2011. The structure of networks that produce the transformation from grid cells to place cells. Neuroscience 197, 293–306. ( 10.1016/j.neuroscience.2011.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung MW, Wiener SI, McNaughton BL. 1994. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 14, 7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monaco JD, Abbott LF. 2011. Modular realignment of entorhinal grid cell activity as a basis for hippocampal remapping. J. Neurosci. 31, 9414–9425. ( 10.1523/jneurosci.1433-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiete IR, Burak Y, Brookings T. 2008. What grid cells convey about rat location. J. Neurosci. 28, 6858–6871. ( 10.1523/jneurosci.5684-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sreenivasan S, Fiete I. 2011. Grid cells generate an analog error-correcting code for singularly precise neural computation. Nat. Neurosci. 14, 1330–1337. ( 10.1038/nn.2901) [DOI] [PubMed] [Google Scholar]
- 69.Kubie JL, Fenton AA. 2012. Linear look-ahead in conjunctive cells: an entorhinal mechanism for vector-based navigation. Front. Neural Circuits 6, 20 ( 10.3389/fncir.2012.00020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wills TJ, Cacucci F, Burgess N, O'Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328, 1573–1576. ( 10.1126/science.1188224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. 2010. Development of the spatial representation system in the rat. Science 18, 1576–1580. ( 10.1126/science.1188210) [DOI] [PubMed] [Google Scholar]
- 72.Mizumori SJY, McNaughton BL, Barnes CA, Fox K. 1989. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: Evidence for pattern completion in hippocampus. J. Neurosci. 9, 3915–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Cauter T, Poucet B, Save E. 2008. Unstable CA1 place cell representations in rats with entorhinal cortex lesions. Eur. J. Neurosci. 27, 1933–1946. ( 10.1111/j.1460-9568.2008.06158.x) [DOI] [PubMed] [Google Scholar]
- 74.Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. 2009. Theta oscillations provide temporal windows for local circuit computation in the entorhinal–hippocampal loop. Neuron 64, 267–280. ( 10.1016/j.neuron.2009.08.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moser EI, Moser MB. 2008. A metric for space. Hippocampus 18, 1142–1156. ( 10.1002/hipo.20483) [DOI] [PubMed] [Google Scholar]
- 76.O'Keefe J, Burgess N. 1996. Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. ( 10.1038/381425a0) [DOI] [PubMed] [Google Scholar]
- 77.Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser M-B, Moser EI. 2009. Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci. 12, 1325–1332. ( 10.1038/nn.2396) [DOI] [PubMed] [Google Scholar]
- 78.Barry C, Ginzberg LL, O'Keefe J, Burgess N. 2012. Grid cell firing patterns signal environmental novelty by expansion. Proc. Natl Acad. Sci. USA 109, 17 687–17 692. ( 10.1073/pnas.1209918109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fenton AA, Csizmadia G, Muller RU. 2000. Conjoint control of hippocampal place cell firing by two visual stimuli. I. The effects of moving the stimuli on firing field positions. J. Gen. Physiol. 116, 191–209. ( 10.1085/jgp.116.2.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fenton AA, Csizmadia G, Muller RU. 2000. Conjoint control of hippocampal place cell firing by two visual stimuli. II. A vector-field theory that predicts modifications of the representation of the environment. J. Gen. Physiol. 116, 211–221. ( 10.1085/jgp.116.2.211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgess N, Hartley T. 2002. Orientational and geometric determinants of place and head-direction. In Advances in neural information processing systems (eds Dietterich TG, Becker S, Ghahramani Z.), 14th edn, pp. 165–172. Cambridge, MA: MIT Press. [Google Scholar]
- 82.Touretzky DS, Weisman WE, Fuhs MC, Skaggs WE, Fenton AA, Muller RU. 2005. Deforming the hippocampal map. Hippocampus 15, 41–55. ( 10.1002/hipo.20029) [DOI] [PubMed] [Google Scholar]
- 83.Mittelstaedt ML, Mittelstaedt H. 1980. Homing by path integration in a mammal. Naturwissenschaften 67, 566–567. ( 10.1007/BF00450672) [DOI] [Google Scholar]
- 84.Rochefort C, Arabo A, André M, Poucet B, Save E, Rondi-Reig l. 2011. Cerebellum shapes hippocampal spatial code. Science 334, 385–389. ( 10.1126/science.1207403) [DOI] [PubMed] [Google Scholar]
- 85.Burguière E, Arleo A, Hojjati MR, Elgersma Y, De Zeeuw CI, Berthoz A, Rondi-Reig L. 2005. Spatial navigation impairment in mice lacking cerebellar LTD: a motor adaptation deficit? Nat. Neurosci. 8, 1292–1294. ( 10.1038/nn1532) [DOI] [PubMed] [Google Scholar]
- 86.Van Strien NM, Cappaert NL, Witter MP. 2009. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 10, 272–282. ( 10.1038/nrn2614) [DOI] [PubMed] [Google Scholar]
- 87.Sugar J, Witter MP, van Strien NM, Cappaert NLM. 2011. The retrosplenial cortex: intrinsic connectivity and connections with the (para) hippocampal region in the rat. An interactive connectome. Front. Neuroinform. 5, 1–13. ( 10.3389/fninf.2011.00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser MB. 2011. Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246–249. ( 10.1038/nature10439) [DOI] [PubMed] [Google Scholar]


