Abstract
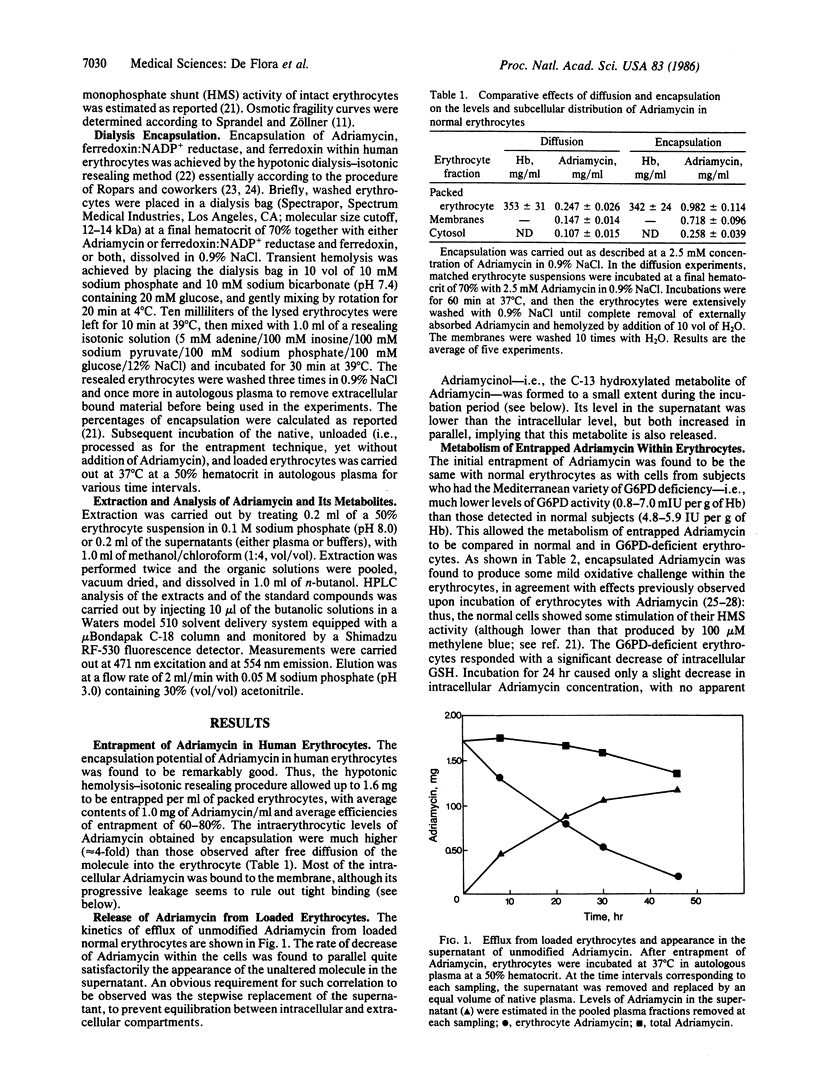
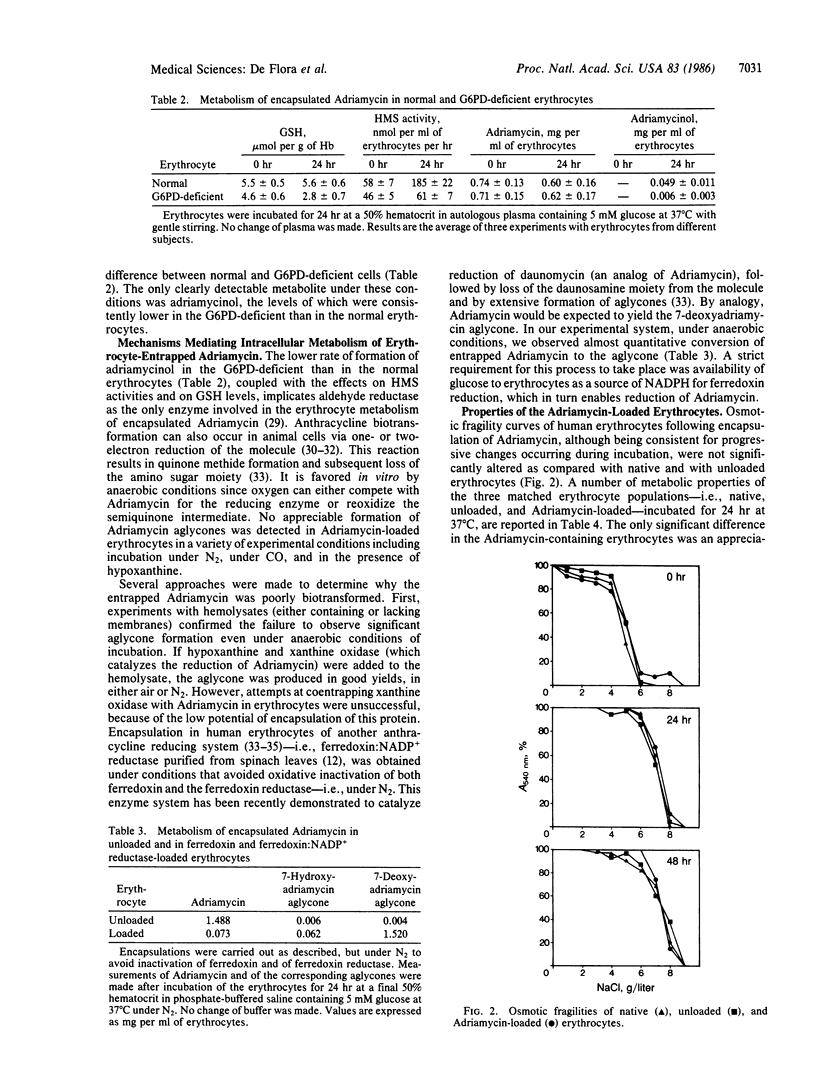
Adriamycin (doxorubicin) was encapsulated in human erythrocytes by means of a dialysis technique involving transient hypotonic hemolysis followed by isotonic resealing. Up to 1.6 mg of the drug was entrapped per ml of packed erythrocytes, with the efficiency of encapsulation being 60-80%. In vitro incubation of the Adriamycin-loaded erythrocytes in autologous plasma was accompanied by progressive release of unaltered Adriamycin in the medium. The efflux was still evident after 50 hr. The metabolism of encapsulated Adriamycin was restricted to limited formation of the C-13 hydroxylated metabolite, adriamycinol, in the normal erythrocytes but not in erythrocytes from individuals deficient in glucose-6-phosphate dehydrogenase (D-glucose-6-phosphate: NADP+ 1-oxidoreductase, EC 1.1.1.49) activity. Reductive bioactivation of encapsulated Adriamycin to yield the corresponding aglycones was not observed in a variety of conditions. However, when NADPH ferredoxin reductase and ferredoxin, both purified from spinach leaves, were co-entrapped within erythrocytes and allowed to catalyze electron transfer to Adriamycin intracellularly under N2, a quantitative conversion to 7-deoxyadriamycin aglycone was obtained. Adriamycin-loaded erythrocytes did not show any significant oxidative damage, except for a variable increase of methemoglobin, suggesting some redox cycling between native Adriamycin and its semiquinone radical. Encapsulation of Adriamycin in autologous human erythrocytes may represent a therapeutic strategy for the slow release in circulation of this antineoplastic drug in order to reduce or prevent its adverse effects and especially the delayed cardiotoxicity that limits its use in patients with neoplastic disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister J. V., Thornalley P. J. The production of hydroxyl radicals by adriamycin in red blood cells. FEBS Lett. 1983 Jun 27;157(1):170–172. doi: 10.1016/0014-5793(83)81139-9. [DOI] [PubMed] [Google Scholar]
- Barr R. D., Davidson A. R., Jung L. K., Mohan Pai K. R. Erythrocytotoxicity induced by cancer chemotherapeutic agents. In vitro studies of osmotic fragility and methaemoglobin generation. Scand J Haematol. 1980 Oct;25(4):363–368. [PubMed] [Google Scholar]
- Bates D. A., Winterbourn C. C. Reactions of Adriamycin with haemoglobin. Superoxide dismutase indirectly inhibits reactions of the Adriamycin semiquinone. Biochem J. 1982 Apr 1;203(1):155–160. doi: 10.1042/bj2030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti U., Guida L., Forteleoni G., Meloni T., De Flora A. Impairment of the calcium pump of human erythrocytes by divicine. Arch Biochem Biophys. 1985 Jun;239(2):334–341. doi: 10.1016/0003-9861(85)90696-4. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bonadonna G., Monfardini S., De Lena M., Fossati-Bellani F. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br Med J. 1969 Aug 30;3(5669):503–506. doi: 10.1136/bmj.3.5669.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G. L., Villacorte D. G., Beutler E. High-yield entrapment of proteins into erythrocytes. Biochem Med. 1977 Oct;18(2):220–225. doi: 10.1016/0006-2944(77)90093-x. [DOI] [PubMed] [Google Scholar]
- Dalmark M. Characteristics of doxorubicin transport in human red blood cells. Scand J Clin Lab Invest. 1981 Nov;41(7):633–639. doi: 10.3109/00365518109090508. [DOI] [PubMed] [Google Scholar]
- Das B., Srivastava S. K. Purification and properties of aldose reductase and aldehyde reductase II from human erythrocyte. Arch Biochem Biophys. 1985 May 1;238(2):670–679. doi: 10.1016/0003-9861(85)90213-9. [DOI] [PubMed] [Google Scholar]
- De Flora A., Benatti U., Guida L., Forteleoni G., Meloni T. Favism: disordered erythrocyte calcium homeostasis. Blood. 1985 Aug;66(2):294–297. [PubMed] [Google Scholar]
- DeLoach J. R. Encapsulation of exogenous agents in erythrocytes and the circulating survival of carrier erythrocytes. J Appl Biochem. 1983 Jun;5(3):149–157. [PubMed] [Google Scholar]
- Di Marco A., Gaetani M., Scarpinato B. Adriamycin (NSC-123,127): a new antibiotic with antitumor activity. Cancer Chemother Rep. 1969 Feb;53(1):33–37. [PubMed] [Google Scholar]
- Fisher J., Abdella B. R., McLane K. E. Anthracycline antibiotic reduction by spinach ferredoxin-NADP+ reductase and ferredoxin. Biochemistry. 1985 Jul 2;24(14):3562–3571. doi: 10.1021/bi00335a026. [DOI] [PubMed] [Google Scholar]
- Henderson C. A., Metz E. N., Balcerzak S. P., Sagone A. L., Jr Adriamycin and daunomycin generate reactive oxygen compounds in erythrocytes. Blood. 1978 Nov;52(5):878–885. [PubMed] [Google Scholar]
- Legha S. S., Benjamin R. S., Mackay B., Ewer M., Wallace S., Valdivieso M., Rasmussen S. L., Blumenschein G. R., Freireich E. J. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982 Feb;96(2):133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Sartiano G. P., Rosenblum S. L., Calkins J. H., Ramsey C. B. The use of erythrocytes for delivery of chemotherapeutic agents to the reticuloendothelial system. Bibl Haematol. 1985;(51):42–49. doi: 10.1159/000410226. [DOI] [PubMed] [Google Scholar]
- Morelli A., Benatti U., Lenzerini L., Sparatore B., Salomino F., Melloni E., Michetti M., Pontremoli S., De Flora A. The interference of leukocytes and platelets with measurement of clucose-6-phosphate dehydrogenase activity of erythrocytes with low activity variants of the enzyme. Blood. 1981 Sep;58(3):642–644. [PubMed] [Google Scholar]
- Morelli A., Benatti U., Salamino F., Sparatore B., Michetti M., Melloni E., Pontremoli S., De Flora A. In vitro correction of erythrocyte glucose 6-phosphate dehydrogenase (G6PD) deficiency. Arch Biochem Biophys. 1979 Oct 15;197(2):543–550. doi: 10.1016/0003-9861(79)90278-9. [DOI] [PubMed] [Google Scholar]
- Rahman A., More N., Schein P. S. Doxorubicin-induced chronic cardiotoxicity and its protection by liposomal administration. Cancer Res. 1982 May;42(5):1817–1825. [PubMed] [Google Scholar]
- Ropars C., Chassaigne M., Villereal M. C., Avenard G., Hurel C., Nicolau C. Resealed red blood cells as a new blood transfusion product. Bibl Haematol. 1985;(51):82–91. doi: 10.1159/000410231. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Burton G. M. The effect of BCNU and adriamycin on normal and G6PD deficient erythrocytes. Am J Hematol. 1979;7(2):97–106. doi: 10.1002/ajh.2830070202. [DOI] [PubMed] [Google Scholar]
- Schwartz H. S. Enhanced antitumor activity of adriamycin in combination with allopurinol. Cancer Lett. 1983 Aug;20(1):69–74. doi: 10.1016/0304-3835(83)90189-1. [DOI] [PubMed] [Google Scholar]
- Schwartz H. S., Parker N. B. Initial biotransformations of daunorubicin to aglycones by rat liver microsomes. Cancer Res. 1981 Jun;41(6):2343–2348. [PubMed] [Google Scholar]
- Shalev O., Leida M. N., Hebbel R. P., Jacob H. S., Eaton J. W. Abnormal erythrocyte calcium homeostasis in oxidant-induced hemolytic disease. Blood. 1981 Dec;58(6):1232–1235. [PubMed] [Google Scholar]
- Shinohara K., Tanaka K. R. The effects of adriamycin (doxorubicin HCl) on human red blood cells. Hemoglobin. 1980;4(5-6):735–745. doi: 10.3109/03630268008997741. [DOI] [PubMed] [Google Scholar]
- Sprandel U., Zöllner N. Osmotic fragility of drug carrier erythrocytes. Res Exp Med (Berl) 1985;185(1):77–85. doi: 10.1007/BF01851531. [DOI] [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971 Jan;20(1):95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Turrini F., Naitana A., Mannuzzu L., Pescarmona G., Arese P. Increased red cell calcium, decreased calcium adenosine triphosphatase, and altered membrane proteins during fava bean hemolysis in glucose-6-phosphate dehydrogenase-deficient (Mediterranean variant) individuals. Blood. 1985 Aug;66(2):302–305. [PubMed] [Google Scholar]
- Winterbourn C. C., Gutteridge J. M., Halliwell B. Doxorubicin-dependent lipid peroxidation at low partial pressures of O2. J Free Radic Biol Med. 1985;1(1):43–49. doi: 10.1016/0748-5514(85)90028-5. [DOI] [PubMed] [Google Scholar]



