Abstract
Emerging evidence suggests that items held in working memory (WM) might not all be in the same representational state. One item might be privileged over others, making it more accessible and thereby recalled with greater precision. Here, using transcranial magnetic stimulation (TMS), we provide causal evidence in human participants that items in WM are differentially susceptible to disruptive TMS, depending on their state, determined either by task relevance or serial position. Across two experiments, we applied TMS to area MT+ during the WM retention of two motion directions. In Experiment 1, we used an “incidental cue” to bring one of the two targets into a privileged state. In Experiment 2, we presented the targets sequentially so that the last item was in a privileged state by virtue of recency. In both experiments, recall precision of motion direction was differentially affected by TMS, depending on the state of the memory target at the time of disruption. Privileged items were recalled with less precision, whereas nonprivileged items were recalled with higher precision. Thus, only the privileged item was susceptible to disruptive TMS over MT+. By contrast, precision of the nonprivileged item improved either directly because of facilitation by TMS or indirectly through reduced interference from the privileged item. Our results provide a unique line of evidence, as revealed by TMS over a posterior sensory brain region, for at least two different states of item representation in WM.
Introduction
Many studies of visual working memory (WM) are motivated by models in which retention of visual information relies on early sensory brain regions, with control over what these regions maintain by prefrontal and parietal brain areas (Postle, 2006; Jonides et al., 2008). Support for this comes from nonhuman primate studies, e.g., single unit recordings from area MT+ have demonstrated that motion-related activity remains across short delays (Bisley and Pasternak, 2000; Pasternak and Greenlee, 2005). In humans, neuroimaging experiments have shown that at the level of early visual cortex, the contents of WM can be decoded during retention (Harrison and Tong, 2009; Serences et al., 2009; Emrich et al., 2013).
Moreover, investigations using transcranial magnetic stimulation (TMS) have demonstrated that moving phosphenes elicited by TMS over MT+ are enhanced when they match a remembered motion direction (Silvanto and Cattaneo, 2010). TMS over early visual cortex also affected the influence of WM on visual search (Soto et al., 2012). TMS improved visual search for matching features held in WM, but disrupted it for nonmatching features. What remains unclear, however, is whether TMS has similar effects on cued features of items to be retrieved from WM.
In most studies that identify regions involved in WM retention, to-be-remembered information is potentially confounded with attention: remembered information is also most relevant to the task, so any neural signature might be a correlate of attention rather than WM (Lewis-Peacock et al., 2012). Indeed, several theoretical cognitive models of WM have proposed that one item (or a subset) might be held in a privileged state over others, with such retention mediated specifically by attention (McElree, 2006; Oberauer, 2009; Cowan, 2011). Items might move in or out of this state, but if privileged, their recall is improved over nonprivileged items (Lepsien and Nobre, 2007; Kuo et al., 2012).
Neural evidence for the existence of different item states in WM has been presented using functional magnetic resonance imaging (fMRI). When task relevance of information was manipulated during retention, only relevant items could be successfully decoded from the BOLD signal, whereas irrelevant ones could not (Lewis-Peacock et al., 2012). Once an irrelevant item became relevant, it could then be successfully decoded, suggesting it had entered the privileged state. [LaRocque et al., 2013; electroencephalography (EEG) analog].
Although there is growing evidence that different representational states exist in WM, the nature and dynamics of changes in retention in sensory areas is unclear. We sought to examine this question using a causal approach, applying TMS to area MT+ during retention of two motion direction memory targets. In our first experiment we used an “incidental cue” to bring one of the targets into a privileged state. In a second experiment, we implicitly manipulated item privilege by presenting the targets serially such that the last item would become privileged by virtue of its recency (Gorgoraptis et al., 2011). Our prediction was that memory precision for items in WM would be differentially affected by TMS over MT+, depending on their representational state.
Materials and Methods
Participants
In Experiment 1, 13 participants took part (7 male; M = 31.6 years; SD = 8.7); 17 participants (10 male; M = 27 years; SD = 5.4) took part in Experiment 2. All participants were right-handed, with no contraindications for TMS and naive to the purpose of the experiment and TMS effects. The study was approved by the local ethics committee.
MT+ localization
A standard approach to MT+ localization using fMRI was applied (Huk et al., 2002) using alternating blocks of moving versus static random dot kinematograms (RDKs). Left hemisphere clusters in the vicinity of MT+ (anatomical guidelines as described by Dumoulin et al., 2000) were identified in the native space of each participant and overlaid onto their T1-weighted scan for a Brainsight frameless stereotaxy procedure (Rogue Research). Location of left MT+ was marked on participants scalp for subsequent TMS.
TMS
Stimulation was delivered via a Magtism Rapid2 (Magstim) using a 70 mm figure-eight coil. The coil handle pointed posteriorly rotated ∼45°, inducing a current approximately in the anterior to posterior direction. On each trial, four 20 Hz TMS pulses were applied to left MT+ (or vertex in Experiment 2) either at 60% (“high,” effective intensity) or at 24% (“low,” ineffective intensity) of stimulator output (Silvanto et al., 2005; Pitcher et al., 2009 show similar approaches). In combination with vertex TMS, low intensity trials provide control for nonspecific effects of TMS; e.g., acoustic and tactile artifacts.
Experiment 1: incidental cueing
Stimuli.
On each trial, two RDKs were presented above and below a fixation cross, subtending 10° of visual angle (VA). RDKs consisted of 25 dots (0.1° VA each), displayed within an invisible circular aperture (5.7° VA). The color of the top RDK was randomly chosen on each trial to be either green or red, with the lower RDK assigned the other color.
Dot lifetime and density were constant during RDK presentation. Motion was 100% coherent (constant speed of 4.5°/s) and randomly selected from 0 to 360° for each RDK with no minimum angular separation on each trial. A mask consisting of 5000 dots (50% red, 50% green), covering the entire screen was presented immediately after the offset of the RDKs.
Stimuli were displayed on a 14.1″ CRT display (resolution: 800 × 600 pixels, refresh rate 60 Hz). Participants were seated ∼60 cm from the monitor in a darkened room.
Procedure.
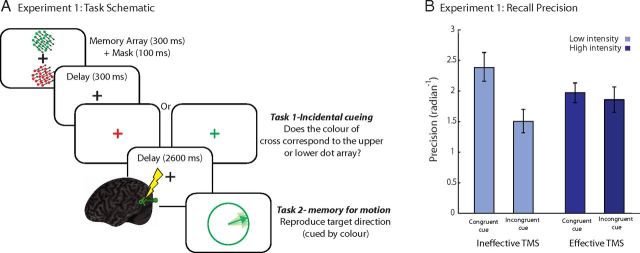
Each trial (Fig. 1A) started with a fixation cross (500 ms), followed by the two RDKs (memory targets, red and green; 200 ms) and the mask (100 ms). This was followed by a 500 ms unfilled delay. The fixation cross color then briefly changed to either red or green (100 ms), the order of which was pseudorandomized across trials. This served as our method of “incidental cueing”. Participants indicated with a key press the location of the RDK with the same color (i.e., above or below the fixation cross; Task 1). This was followed by 2600 ms delay before the administration of TMS. The last pulse in the 250 ms TMS train was followed by a 300 ms interval before memory probe presentation. The probe was of a circle (5.7° VA diameter) presented at the center of the screen with a line from the center positioned at a randomly selected orientation. The probe stimulus was presented in the same color as one of the two RDKs. Using a mouse, participants adjusted the orientation of the line within the circle until it matched the direction of motion of the probed RDK. Accurate reproduction was emphasized over response time.
Figure 1.
A, Schematic of trial events for Experiment 1. Two RDKs were presented simultaneously and participants were asked to keep in mind the direction of both. During a delay, the color of the fixation cross changed and participants were to indicate the location of the RDK of the same color (Task 1). A 20 Hz four-pulse TMS train was then applied to MT+, followed by a delay and then memory probe. Participants had to rotate the probe line to match the direction of motion of the RDK with the same color. The probe matched the color of the Task 1 fixation cross on half the trials (congruent trials) and different on the remaining half (incongruent trials). B, Memory precision for motion directions across conditions. Error bars are SEM.
On half the trials the color of the probe matched that of the incidental cue (congruent probe) whereas the remaining half it did not (incongruent probe). The incidental cue color did not predict which memory target was to be probed and participants were informed of this at the start of the experiment. We used a fully factorial, balanced design with two factors: probe type (congruent, incongruent) and TMS intensity (high, low). Participants completed 200 trials (50 trials/condition) across five blocks.
Experiment 2: serial presentation
Stimuli and procedure.
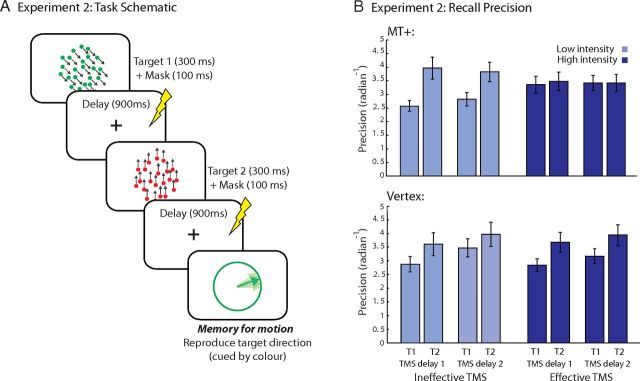
On each trial, a sequence of two RDKs was centrally presented (Fig. 2A; identical properties to Experiment 1). Following a 500 ms fixation cross, each RDK was presented for 300 ms (+100 ms mask). Between RDKs 1 and 2, there was a 900 ms unfilled delay. After RDK 2 there was a 900 ms delay, followed by the memory probe. High- or low-intensity TMS was applied during either the first or second delay, 300 ms after its onset, the order of which was pseudorandomized across trials. In this way, we attempted to account for temporal differences in applying TMS relative to target encoding. Probe procedure was identical to Experiment 1. The intertrial interval was varied to ensure a minimum of 5000 ms between TMS trains.
Figure 2.
A, Schematic of trial events for Experiment 2. A sequence of two RDKs moving in different directions and colors were presented. TMS was applied 300 ms after the start of the delay interval either in the first delay or after presentation of both RDKs. At the end of each trial, a probe stimulus was presented requiring participants to reproduce the direction of one of the memory targets with the same color as probe. B, Memory precision for motion across conditions for MT+ and vertex TMS conditions. Error bars are SEM.
In this experiment, we included a second, separate session in which TMS was applied to vertex. This was to further demonstrate that TMS effects on behavior were specific to MT+ stimulation. Vertex was identified for each participant as Cz according to the 10–20 EEG electrode localization system. The order of stimulation site (MT+, vertex) was counterbalanced across participants. All experimental procedures were identical across the two sessions.
Precision calculation.
Recall error was defined as the difference in response angle from target angle. We calculated recall precision as the reciprocal of the circular SD of response error (Berens, 2009). Precision is a measure of response variability; less variability corresponds to more precise memory. Trials in which response times exceeded 10 s were excluded from analyses.
Results
Experiment 1
Participants performed the incidental cueing task well, with mean accuracy of 95% (SD = 5.7); mean response times of 833 ms (SD = 210). Trials with an incorrect response on the incidental cueing task were excluded from further analyses.
For motion direction recall, we investigated the behavioral effect of congruent versus incongruent probes on memory precision for the low-TMS condition. Precision was significantly higher on congruent trials (t(1,12) = 4.13; p = 0.001; Fig. 1B), indicating successful incidental cueing. Thus participants were more accurate in their responses if during the retention interval they had attended to the RDK of the same color.
Next we assessed whether high- versus low-TMS affected recall precision. A two-way ANOVA with factors TMS intensity and trial type yielded a significant interaction (F(1,12) = 11.79, p = 0.005). This was due to a significant decrease in memory precision after high-TMS on congruent trials (t(12) = 2.72, p = 0.019; Fig. 1B) and a marginally significant increase on incongruent trials (t(12) = 2.07, p = 0.06). After high-TMS, behavioral advantage of incidental cueing disappeared, removing the significant difference between congruent versus incongruent probe responses (t(12) = 0.05, n.s.). Thus the privileged precision of recall for congruent items was abolished.
Experiment 2
Memory precision was calculated for each target position (Item 1 or 2 in the sequence), TMS intensity (high or low), TMS position (first or second delay), and TMS region (MT+, vertex). Under low-TMS, precision was significantly higher for Item 2 compared with Item 1 (t(33) = 4.38, p < 0.001); i.e., a recency effect, our index of privileged state.
Next, we assessed the effect of MT+ (versus vertex) TMS on recall precision for the privileged, last item compared with the earlier item in the sequence. A four-way omnibus ANOVA with the factors of TMS intensity, target position in the sequence, TMS position, and region yielded a significant three-way interaction between TMS intensity, target position and region (F(1,16) = 11.63, p = 0.004).
We followed up the significant interaction with contrasts evaluating effects of TMS on MT+ and vertex separately. There was a significant interaction between TMS intensity and target position when TMS was applied to MT+ but not to vertex (F(1,16) = 11.03, p = 0.011 and F(1,16) = 0.26, n.s., respectively).
Examination of trials in which TMS was applied in Delay2 to MT+, a two-way ANOVA with factors TMS intensity and target position yielded a significant interaction (F(1,16) = 7.55, p = 0.014: Fig. 2B, MT+). Paired t tests on precision of the last item showed that high-TMS abolished the recency effect compared with low-TMS (t(16) = 0.01, n.s. and t(16) = 2.80, p < 0.013, respectively). Conversely, precision for Item 1 was significantly higher with high- compared with low-TMS (t(16) = 2.12, p = 0.05). Thus, recall of the first item improved whereas for the second item it became worse with high-TMS over MT+. There was no effect of TMS intensity on recall precision when applied to vertex in the delay period (F(1,16) = 0.5, n.s.).
On trials in which TMS was applied in Delay 1 to MT+, a two-way ANOVA with factors TMS intensity and target position yielded a significant interaction (F(1,16) = 5.61, p = 0.031; Fig. 2B, MT+). Memory precision increased on high- versus low-TMS trials when Item 1 was probed (t(16) = 2.27, p = 0.037). Compared with low-TMS trials (t(16) = 3.31, p = 0.004), the recency effect disappeared on high intensity trials (t(16) = 0.26, n.s.). Thus high-TMS in Delay1 had similar effects to high-TMS in Delay2. There was no effect of TMS intensity on recall precision when applied to vertex in Delay1 (F(1,16) = 0.08, n.s.).
Finally, paired t tests showed that precision of the last item was not statistically different across TMS intensities for vertex applied during Delay1 (t(16) = 0.2, n.s.) and Delay2 (t(16) = 0.06, n.s.) indicating that TMS effects on precision were specific to MT+ stimulation.
Discussion
Our study sought to provide causal evidence for different representational states of items maintained in WM in early visual cortex. We explicitly and implicitly manipulated the state of two items in WM to place one in a privileged state relative to the other. In our first experiment, we used an incidental cueing manipulation during the retention period and found that participants subsequently reported the direction of RDKs with the same (congruent) color with greater precision. When disruptive TMS was applied to MT+, this precision advantage was abolished, whereas the other item's recall precision instead increased (Fig. 1). In our second experiment, presenting the items sequentially induced a privileged state for the last item in the sequence so that it was later recalled with higher precision. TMS over MT+ abolished this recall advantage for the last item and instead increased precision of memory for the earlier item in the sequence.
These findings provide direct evidence for the existence of at least two different states of representations in WM. Further the results provide the most direct evidence yet in humans for short-term retention of visual information relying on brain areas traditionally considered to subserve a perceptual function. However, as suggested by our findings, the information maintained in sensory regions that are susceptible to disruptive TMS, may only reflect the privileged item rather than the full content of WM.
Our findings for distinct representational states are supported by several theoretical models of WM, in which at least one item is held in a focus of internal attention (McElree, 2006; Oberauer, 2009; Cowan, 2011; Olivers et al., 2011). Evidence for different states in memory come from retro-cueing paradigms in which explicitly directing attention to already encoded items can place them in a privileged state, with the result being a behavioral advantage for these items (Griffin and Nobre, 2003; Pertzov et al., 2012), and also increased neural activity relative to nonattended items (Lepsien et al., 2005, 2011). In a fMRI experiment (Lewis-Peacock et al., 2012) which varied the task relevance of items in WM within a trial, only the relevant item (i.e., the privileged item) could be decoded from involved brain areas. This was in contrast to irrelevant items that were unable to be decoded, until, that is, they became task relevant.
Physiologically, privileged items might be maintained by sustained neuronal firing, rendering them “detectable” by methods such as fMRI. TMS can differentially affect neurons at different levels of activation (Silvanto et al., 2008); a privileged item may be in a more active state, and therefore more susceptible to TMS-induced neuronal depolarization (i.e., “neural noise”) or suppression of the signal coded by active neurons (Ruzzoli et al., 2011).
But why does TMS lead to improvement of memory for the nonprivileged item? There are two possibilities. First, behavioral findings suggest that a privileged item directly interferes with nonprivileged items in WM (Pertzov et al., 2012). With TMS over early visual cortex, the strength of the privileged item representation was weakened, effectively reducing interference from the privileged item on the nonprivileged item, resulting in higher precision for the latter. In this case, TMS did not have a direct effect. Nonprivileged items may instead be maintained by modulation in temporary synaptic weight changes of neuronal populations (Mongillo et al., 2008; Buonomano and Maass, 2009; Stokes et al., 2013) or by sustained neuronal firing in other nonsensory regions (Fuster and Alexander, 1971; Goldman-Rakic, 1995), both of which could render such items less susceptible to the disruptive effects of MT+ TMS.
Alternatively, it is possible that the nonprivileged items were directly facilitated by TMS. Studies have shown that when neuronal representations are suppressed or inhibited, TMS can have a facilitatory effect (Guzman-Lopez et al., 2011; Silvanto and Soto, 2012; Soto et al., 2012). This could explain improvements for nonprivileged items, which might have been suppressed or inhibited state. Note that in both experiments, both items were always relevant to the task, and never as distractors (Silvanto and Soto, 2012), making the active suppression/inhibition of nonprivileged items, in our view, less likely. Importantly, both accounts of the differential effects of TMS are consistent with the existence of different representational states in WM.
In Experiment 2, TMS delivered before the second item yielded similar results to TMS delivered after its presentation. This might be due to TMS decreasing effective encoding of the upcoming item. Several studies have shown that TMS applied to MT+ before the onset of a motion stimulus will affect subsequent perception (Maus et al., 2013), which would account for the effects of TMS before presentation of Item 2 (Théoret et al., 2002; Sack et al., 2006).
These findings have important implications for neural computational models of WM. Many models are specified by “attractor networks” in which self-sustaining spatiotemporal patterns of neuronal activity retain information over time. The majority of models assume static representations during the retention interval in which the activity of individual cells encode the same items over the entirety of a retention period (Seung and Sompolinsky, 1993; Lisman et al., 1998; Compte et al., 2000; Durstewitz et al., 2000; Mongillo et al., 2008; Dempere-Marco et al., 2012; Wei et al., 2012). Several models have, however, proposed dynamic item representation during retention (Fujisawa et al., 2008; Barak et al., 2010; Pascanu and Jaeger, 2011; Stokes et al., 2013). Our results provide empirical support for the latter class of models. Given the compelling evidence we have provided, we suggest that computational models, and experimental studies, should therefore take into account dynamic representations in WM.
Footnotes
This work was supported by the Wellcome Trust to Jon Driver and M.H., and the Brain Research Trust to N.Z. We thank Paul Bays and Yoni Pertzov for helpful discussion.
The authors declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Barak O, Tsodyks M, Romo R. Neuronal population coding of parametric working memory. J Neurosci. 2010;30:9424–9430. doi: 10.1523/JNEUROSCI.1875-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Soft. 2009;31:1–21. [Google Scholar]
- Bisley JW, Pasternak T. The multiple roles of visual cortical areas MT/MST in remembering the direction of visual motion. Cereb Cortex. 2000;10:1053–1065. doi: 10.1093/cercor/10.11.1053. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Maass W. State-dependent computations: spatiotemporal processing in cortical networks. Nat Rev Neurosci. 2009;10:113–125. doi: 10.1038/nrn2558. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Cowan N. The focus of attention as observed in visual working memory tasks: making sense of competing claims. Neuropsychologia. 2011;49:1401–1406. doi: 10.1016/j.neuropsychologia.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempere-Marco L, Melcher DP, Deco G. Effective visual working memory capacity: an emergent effect from the neural dynamics in an attractor network. PloS One. 2012;7:e42719. doi: 10.1371/journal.pone.0042719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Emrich SM, Riggall AC, Larocque JJ, Postle BR. Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short-term memory. J Neurosci. 2013;33:6516–6523. doi: 10.1523/JNEUROSCI.5732-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gorgoraptis N, Catalao RF, Bays PM, Husain M. Dynamic updating of working memory resources for visual objects. J Neurosci. 2011;31:8502–8511. doi: 10.1523/JNEUROSCI.0208-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin IC, Nobre AC. Orienting attention to locations in internal representations. J Cogn Neurosci. 2003;15:1176–1194. doi: 10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Guzman-Lopez J, Silvanto J, Seemungal BM. Visual motion adaptation increases the susceptibility of area V5/MT to phosphene induction by transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1951–1955. doi: 10.1016/j.clinph.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo BC, Stokes MG, Nobre AC. Attention modulates maintenance of representations in visual short-term memory. J Cogn Neurosci. 2012;24:51–60. doi: 10.1162/jocn_a_00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque JJ, Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Decoding attended information in short-term memory: an EEG study. J Cogn Neurosci. 2013;25:127–142. doi: 10.1162/jocn_a_00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cereb Cortex. 2007;17:2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: interactions between attentional orienting and working-memory load. Neuroimage. 2005;26:733–743. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Thornton I, Nobre AC. Modulation of working-memory maintenance by directed attention. Neuropsychologia. 2011;49:1569–1577. doi: 10.1016/j.neuropsychologia.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Neural evidence for a distinction between short-term memory and the focus of attention. J Cogn Neurosci. 2012;24:61–79. doi: 10.1162/jocn_a_00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- Maus GW, Ward J, Nijhawan R, Whitney D. The perceived position of moving objects: transcranial magnetic stimulation of area MT+ reduces the flash-lag effect. Cereb Cortex. 2013;23:241–247. doi: 10.1093/cercor/bhs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElree B. Accessing recent events. In: Ross BH, editor. Psychology of learning and motivation. Vol. 46. San Diego: Academic; 2006. pp. 155–200. [Google Scholar]
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Design for a working memory. In: Ross B.H., editor. Psychology of learning and motivation. Vol. 51. Oxford: Academic; 2009. pp. 45–100. [DOI] [Google Scholar]
- Olivers CN, Peters J, Houtkamp R, Roelfsema PR. Different states in visual working memory: when it guides attention and when it does not. Trends Cogn Sci. 2011;15:327–334. doi: 10.1016/j.tics.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Pascanu R, Jaeger H. A neurodynamical model for working memory. Neural Netw. 2011;24:199–207. doi: 10.1016/j.neunet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Pertzov Y, Bays PM, Joseph S, Husain M. Rapid forgetting prevented by retrospective attention cues. J Exp Psychol Hum Percept Perform. 2013;39:1224–1231. doi: 10.1037/a0030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Charles L, Devlin JT, Walsh V, Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzoli M, Abrahamyan A, Clifford CW, Marzi CA, Miniussi C, Harris JA. The effect of TMS on visual motion sensitivity: an increase in neural noise or a decrease in signal strength? J Neurophysiol. 2011;106:138–143. doi: 10.1152/jn.00746.2010. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Linden DE, Goebel R, Muckli L. The temporal characteristics of motion processing in hMT/V5+: combining fMRI and neuronavigated TMS. Neuroimage. 2006;29:1326–1335. doi: 10.1016/j.neuroimage.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychol Sci. 2009;20:207–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS, Sompolinsky H. Simple models for reading neuronal population codes. Proc Natl Acad Sci U S A. 1993;90:10749–10753. doi: 10.1073/pnas.90.22.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Cattaneo Z. Transcranial magnetic stimulation reveals the content of visual short-term memory in the visual cortex. Neuroimage. 2010;50:1683–1689. doi: 10.1016/j.neuroimage.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Soto D. Causal evidence for subliminal percept-to-memory interference in early visual cortex. Neuroimage. 2012;59:840–845. doi: 10.1016/j.neuroimage.2011.07.062. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex. 2005;15:1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Soto D, Llewelyn D, Silvanto J. Distinct causal mechanisms of attentional guidance by working memory and repetition priming in early visual cortex. J Neurosci. 2012;32:3447–3452. doi: 10.1523/JNEUROSCI.6243-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. Dynamic coding for cognitive control in prefrontal cortex. Neuron. 2013;78:364–375. doi: 10.1016/j.neuron.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théoret H, Kobayashi M, Ganis G, Di Capua P, Pascual-Leone A. Repetitive transcranial magnetic stimulation of human area MT/V5 disrupts perception and storage of the motion aftereffect. Neuropsychologia. 2002;40:2280–2287. doi: 10.1016/S0028-3932(02)00112-4. [DOI] [PubMed] [Google Scholar]
- Wei Z, Wang XJ, Wang DH. From distributed resources to limited slots in multiple-item working memory: a spiking network model with normalization. J Neurosci. 2012;32:11228–11240. doi: 10.1523/JNEUROSCI.0735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]