Abstract
Objective:
Nutrients and antioxidants in the medium of immature oocyte have a profound effect on maturation, fertilization and development of resulting embryos. In this study the effects of melatonin as an antioxidant agent on maturation, glutathione level and expression of High mobility group box-1 (HMGB1) gene were evaluated in immature oocytes of mice stained with brilliant cresyl blue (BCB).
Materials and Methods:
In this experimental study, immature oocytes were harvested from ovaries of Naval Medical Research Institute (NMRI) mice. Oocytes were stained with 26 μM BCB for 90 minutes and transferred to in vitro maturation medium containing varying doses of melatonin (10-12, 10-9, 10-6, 10-3 M) and without melatonin, for 22-24 hours. Maturation was monitored using an inverted microscope. Glutathione was assessed by monochlorobimane (MCB) staining and HMGB1 expression in mature oocyte was analyzed using real-time polymerase chain reaction (PCR).
Results:
Melatonin in the concentration of 10-6 M had the most effect on maturation and HMGB1 expression of BCB+ oocytes (p<0.05). Meanwhile melatonin had no effects on glutathione levels. Additionally in immature BCB- oocytes, compared to the control group, melatonin did not affect cytoplasm maturation (p>0.05).
Conclusion:
In vitro treatment with melatonin increases the maturation and HMGB1 expression in BCB+ immature oocytes and has no significant effect on glutathione levels.
Keywords: Melatonin, Glutathione, Oocyte, Brilliant Cresyl Blue Staining, HMGB1
Introduction
Although the quality of in vitro maturation (IVM) is less than in vivo matured oocyte (1), it is a frequent technique in in vitro fertilization (IVF) centres for augmenting the number of mature oocyte for IVF. Maturation is defined in two parts of an oocyte: nuclear maturation visualized by the extrusion of the second polar body and cytoplasm maturation (2). Successful maturation, fertilization and development prior to implantation are dependent on growth and differentiation of immature oocytes and the surrounding cumulus cells. The two major factors affecting embryo production and development are the quality of immature oocyte and the composition of IVM medium (3). The first important step in production of in vitro embryos is selecting high quality oocytes in order to transfer to IVM medium to achieve mature oocytes (4). Generally for such selections few morphological criteria are used including the number of surrounding layers of cumulus cells, cytoplasm homogeneity, follicle and immature oocyte size (5) but they are inconsistent and unreliable (6). Brilliant cresyl blue (BCB) staining is a non-invasive method used for the selection of immature oocyte in animal studies and is related to increased maturation of embryos (7). BCB is a biomarker of glucose-6-phosphate dehydrogenase (G6PD) level in immature oocytes and has the highest expression in good quality oocytes and decreases with maturation (7).
In vitro maturation medium is a vital and effective factor in nuclear maturation, cleavage and embryo maturation, and blastocyst formation (3). In various studies amino acids and antioxidants have been used for oocyte maturation (8). Very low or high concentrations of free radicals in medium affects the maturation and cleavage of embryos in vitro (9). Studies used enzymatic antioxidants to regulate the levels of free radicals, such as catalase, turine and hypoturine (10). Melatonin is a tryptophan derived hormone secreted from pineal gland into oviduct and follicular fluid during ovulation (11). Therefore it exerts an important effect in the reproductive system (12). On the other hand, being an antioxidant, it has a role in scavenging the reactive oxygen species (ROS) in the environment (13). Its positive effects on embryo development through to blastocyst formation have been confirmed (14,15). As noted one of the processes in IVM is cytoplasm maturation encompassing biochemical molecules such as glutathione, phosphorylated proteins and the activation of metabolic pathways. Glutathione production is an important biomarker of cytoplasm maturation in IVM (16). In addition to its antioxidant property, glutathione is important in formation and stabilization of mitotic spindle assembly in mature oocyte and also in the formation of the male pronucleus (17). Studies have shown that higher concentration of glutathione in the cytoplasm is related to higher percentage of IVF success and maturation to blastocyst (18,19).
High mobility group box-1 (HMGB1) gene has various functions including transcription, DNA repair (20) and apoptosis (21). Its product, HMGB1 protein is expressed on the blastomere membrane throughout the maturation process. It has been shown that embryos expressing this protein reach the blastocyst stage in higher numbers (22). Melatonin as an antioxidant could have the potential for inductionor suppression of HMGB1.
Positive effects of melatonin on embryo development through to blastocyst formation have been confirmed although its effective dose in oocyte maturation of BCB stained oocyte remains unknown. So the aim of this study was to evaluate the effects of melatonin in IVM medium on glutathione level, HMGB1 expression and maturation of immature oocyte stained with BCB.
Materials and Methods
All experiments and protocols were performed in strict accordance with the guiding principles for the care and use of research animals adopted by the Shahid Beheshti University of Medical Sciences.
All chemicals were purchased from Sigma Chemical Corporation (St. Louis, MO, USA) except where noted otherwise.
Oocyte collection
In this experimental study, oocytes were obtained from female Naval Medical Research Institute (NMRI) mice (Pasteur Institute, Iran) with age 6-8 weeks that were kept under controlled light and temperature conditions with free access to water and food. They had 12 hours light and 12 hours dark conditions. Mice were primed with 10 IU of pregnant mare serum gonadotropin (PMSG). The ovaries were removed 48 hours later and placed in tissue cell culture medium (TCM) 199 Hepes supplemented with 5% foetal bovine serum (FBS). The germinal vesicle (GV) stage oocytes were released by puncturing ovarian follicles with 28G needle.
Brilliant cresyl blue staining
The cumulus-oocyte complexes (COCs) obtained were washed three times in flushingholding medium (FHM) and then incubated in potassium simplex optimized medium (KSOM) supplemented with 4% bovine serum albumin (BSA) containing 26 μM BCB for 90 minutes at 37˚C in humidified air atmosphere. After the incubation time, the oocytes were observed under microscope and classified according to BCB staining as i. dark blue cytoplasm (BCB+) and ii. colourless cytoplasm (BCB–) (23).
In vitro maturation
Each group was placed in 50 μL microdrops of TCM-199 supplemented with 10% FBS, 0.2 mM sodium pyruvate, 2 mM L-Glutamin,10 μg/ mL follicle stimulating hormone (FSH), 10 μg/ mL luteinizing hormone (LH) and 1 μg/mL estradiol -17β with the additional of variety of concentration of melatonin (0, Dimethyl sulfoxide (DMSO), 10-3, 10-6, 10-9, 10-12 M) in a humidified atmosphere with 5% CO2 at 37˚C for 22-24 hours. COCs showing fully expanded cumulus cells after 24 hours maturation period, were assessed by phase contrast inverted microscope (Olympus, Japan) and COCs which were not expanded or showed incomplete expansion were not accounted (24).
Monochlorobimane staining
To estimate the glutathione concentration in oocytes we used a fluorescent indicator of glutathione, monochlorobimane (MCB) (25). Oocytes were incubated with 50 mM MCB in FHM medium for 45 minutes and then fluorescence of MCB was recorded at 390 nm by a digital camera. Intensity of fluorescence was analyzed by Image J software (National Institutes of Health, Bethesda, MD, USA).
Relative expression of high mobility group box-1
Relative amounts of HMGB1 gene transcripts were determined by using a real time PCR (Polymerase chain reaction). At least 10 oocytes were analyzed for each group andtransferred to the bottom of a 0.2 mL Eppendorf tube containing 1.5 μL lysis buffers (26) and processed for reverse transcription-polymerase chain reaction (RT-PCR). All RT-PCR solutions were prepared in Milli-Q Ultrapure water. Two microliters of poly N and 5 μL water were added to embryo and placed in thermocycler for 5 minutes 75˚C. After, the tubes were placed on ice and 9 μL of the following reaction mixture (5x RT Buffer, 200 u RT Enzyme, 10 mM dNTP and 10 u RNase inhibitor) were added to embryo samples. Both RT-PCR and PCR reactions were performed on an applied Bio Rad thermocycler. The amplification program for the reverse transcription step was as follows: 25˚C for 10 minutes, 37˚C for 15 minutes, 42˚C for 45 minutes and 72˚C for 10 minutes.
After the reverse transcriptase reaction, samples were kept at 4˚C overnight; then to each sample, PCR mixtures were added: 1.25 μL Taq Polymerase, 20.75 μL Master Mix, 2 μL cDNA and 2 μL specific primers. The endogenous control Hprt1 (F: TCCCAGCGTCGTGATTAG, R: CGAGCAAGTCTTTCAGTCC, Accessionno: NM_013556.2) and HMGB1 (F: GAAGTATGAGAAGGATATTGCTG, R: CCAACTTATTCATCATCATCATC, Accession no: NM_010439.3) genes were amplified with the following PCR cycle programme: 94˚C for 3 minutes, 60˚C for 45 seconds, 72˚C for 45 seconds for 40 cycles followed by 72˚C for 7 minutes. Ten microliters of PCR product were mixed with 1 μL loading buffer and were electrophoresed on a 2% agarose gel in Tris-acetate- EDTA (TAE) buffer for 25 minutes. The products were visualised under short-wave length Ultraviolet (UV).
Real-time quantitative PCR was performed to assess the expression of HMGB1 gene using Rotor Gene Q instrument (QIAGEN). Real time PCR reactions were carried out in a total volume of 13 μL according to the manuals for DNA Master SYBR Green I mix (Roche Applied Sciences). The primer concentrations were adjusted to 1 μM for each gene. The cycling parameters were 5 seconds at 95˚C, 3 minutes at 95˚C for denaturation, 15 seconds at 60˚C, 10 seconds at 72˚C for amplification and extension respectively for 40 cycles. The specificity of all individual amplification reactions was confirmed by melting curve analysis. The assays used Hprt1 as the endogenous internal house-keeping gene. Three replications were performed and the mRNA level of each sample was normalized to that of Hprt1 mRNA level. The relative levels of mRNA were analyzed by the REST 2009 Software (QIAGEN).
Statistical analysis
All statistical analysis was performed using Service Provisioning System Software (SPSS) 16 for windows (SPSS, Chicago, IL, USA). The means of metaphase II (MII), cumulus expansion and metaphase I (MI), were compared by non-parametric analysis test (Kruskal-Wallis). Glutathione levels in experimental groups were compared by Analysis of Variance (ANOVA). Data are expressed as means ± SD. A statistically significant difference was accepted at p<0.05.
Results
Oocyte maturation
1238 immature BCB+ oocyte were transferred to IVM media containing varying concentrations of melatonin (10-12, 109, 10-6, 10-3 M, control and DMSO) (Fig 1A). It was observed that compared to the control group and other groups the number of metaphase II oocytes were significantly higher in media supplemented with 10-6 M of melatonin (66 and 76% respectively). However in low concentrations of melatonin (10-12 and 10-9) it had a negative effect on nuclear maturation (41 and 45% respectively). The highest degree of cumulus expansion was seen in the control group (92%) which only had a significant difference with 10-12 M of melatonin group. Metaphase I arrest was seen mostly in oocytes treated with 10-9 and 10-12 M of melatonin (54 and 58% respectively) as compared with the control (31.5%, p<0.05, Table 1).
Fig 1.
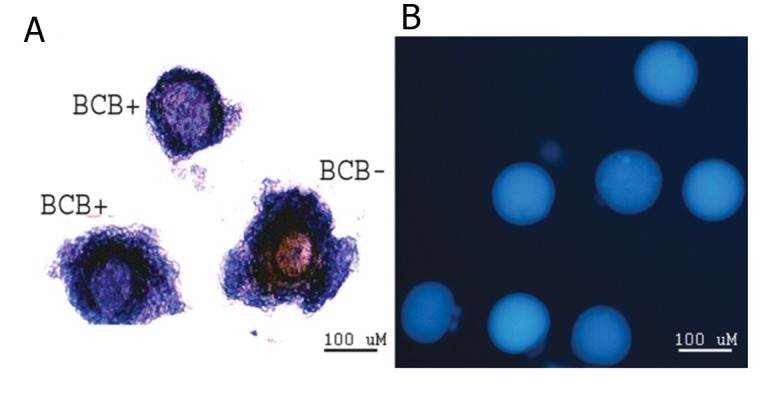
Immature oocytes stained with BCB, oocyte with blue coloration of cytoplasm (BCB+) and without blue cytoplasm (BCB-) (A). Fluorescent intensity of MCB stained mature oocyte (B).
Table 1.
Development of BCB+ immature oocyte cultured in the maturation medium supplemented with different concentrations of melatonin
| Group | No. oocyte | MI | Cumulus expansion | MII |
|---|---|---|---|---|
| Control | 204 | 64(31.46 ± 3.41) a,b | 187(91.62 ± 1.25)e | 136(66.69 ± 3.26) f,g,h |
| DMSO | 203 | 83(40.76 ± 5.25) | 164(81.04 ± 2.35) | 120(59.24 ± 5.25) |
| Melatonin 10-3M | 198 | 93 (46.56 ± 23.27) | 131(66.24 ± 15.46) | 105(52.73 ± 20.7) |
| Melatonin 10-6M | 188 | 44(23.63 ± 12.29) c,d | 163(87.1 ± 7.22) | 144(76.37 ± 12.29) f,i,k |
| Melatonin 10-9M | 201 | 109(54.13 ± 16.98 )a,c | 161(80.17 ± 14.29) | 92(45.87 ± 16.98)g,i |
| Melatonin 10-12M | 206 | 121(58.90 ± 16.55) b,d | 130(63.15 ± 15.41)e | 85(41.1 ± 16.55) h,k |
Within the same column, values with same letters were significantly different (p <0.05).
Additionally, 334 immature BCB- oocytes were transferred to media containing varying concentrations of melatonin (Fig 1A). The percentage of metaphase II oocytes and cumulus expansion was similar to the concentration of 10-3 M of melatonin (control: 35 and 73%; 10-3 M: 45 and 88% respectively). The majority of metaphase I arrest were seen in 10-12 M (82%) and 10-9 M (83%) which had a significant difference with control and 10-3 M group (60%, Table 2). According to tables 1 and 2 the BCB+ oocytes had a greater expansion and metaphase II oocytes and a lower percentage of metaphase I arrest compared to BCB- counterparts.
Table 2.
Development of BCB- immature oocyte cultured in the maturation medium supplemented with different concentrations of melatonin
| Group | No. 0ocyte | MI | Cumulus Expansion | MII |
|---|---|---|---|---|
| Control | 54 | 32(60.07 ± 11.52)a,b,c | 40(73.41 ± 20.36)d,e,f | 21(38.25 ± 11.65)g,h,i |
| DMSO | 62 | 45(73.18 ± 11.67) | 31(50.9 ± 12.19)d | 17(26.82 ± 11.67) |
| Melatonin 10-3M | 57 | 34(59.95 ± 19.97) | 50(88.28 ± 14.59) | 26(45.82 ± 15.77) |
| Melatonin 10-6M | 64 | 53(82.17 ± 7.50)a | 22(35.20 ± 11.97)e | 11(17.82 ± 7.5)g |
| Melatonin 10-9M | 52 | 43(83.47 ± 11.16)b | 21(40.1 ± 14.3)f | 9 (16.52 ± 11.17)h |
| Melatonin 10-12M | 62 | 54(87.32 ± 12.28 )c | 32(51.81 ± 18.73) | 8(12.68 ± 12.28)i |
Within the same column, values with same letters were significantly different (p <0.05).
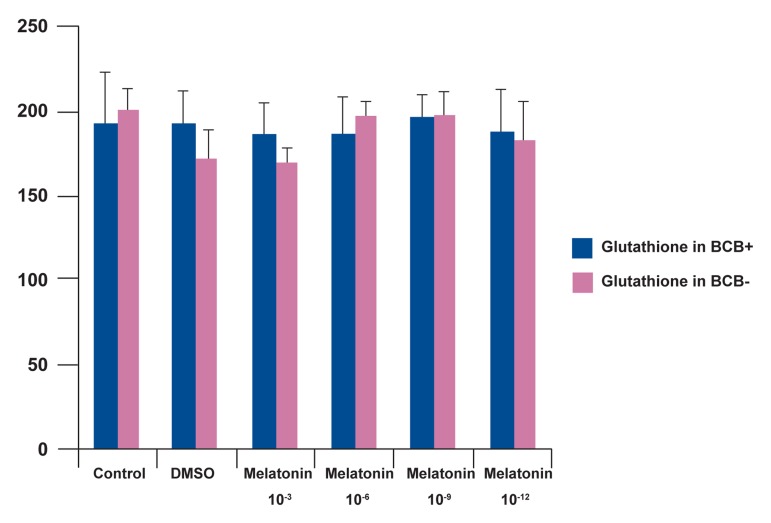
Glutathione level in oocytes
Our results showed that melatonin had no significant effect on the level of glutathione in oocytes (Figs 1B and 2).
Fig 2.
Glutathione level of BCB+ and BCB- oocytes cultured in different concentrations of melatonin. The Glutathione level was evaluated with MCB staining.
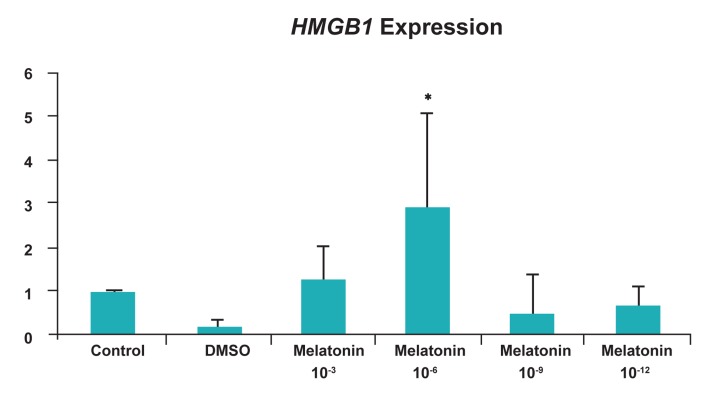
HMGB1 expression level
The expression of HMGB1 gene was analyzed using real time-PCR in BCB+ oocytes. As seen in figure 3, HMGB1 expression was at its highest in 10-6 M of melatonin compared to the control group and also had the lowest level in DMSO treated group (p<0.05) (Fig 3).
Fig 3.
Relative expression levels of HMGB1 gene in BCB+ oocytes cultured with different concentrations of melatonin and without melatonin. The mRNA levels of HMGB1 were analyzed with real-time PCR and mRNA levels were normalized to that of Hprt mRNA level. *; p<0.05.
Discussion
Due to the beneficial uses of BCB staining we used the same protocol for the selection of immature oocytes and results showed that maturation was higher in BCB+ compared to BCB- oocytes which is in accordance with previous studies (7,23). We also compared the effects of the antioxidant melatonin on the maturation of oocytes. Among the most important harmful factors affecting oocyte and embryo are free radicals. They have deteriorating effects on DNA repair, mitotic spindle assembly and maturation of oocyte (9). Studies have utilized various enzymatic antioxidants such as catalase and non-enzymatic antioxidants including thioredoxin pyruvate and glutathione (27). Melatonin (N-acetyl 5-metoxy tryptamin) is a hormone (11) and its role as an antioxidant in the reproductive system was revealed when its levels in the follicular fluid and its receptors on granulosa cells and reproductive organs such as ovary, testis and fallopian tube were discovered. Melatonin stimulates progesterone secretion and suppresses the production of prostaglandins (28). Additionally it has the ability to stimulate the expression a number of antioxidant enzymes (13). Also it has anti-apoptotic potentials on various cells and embryos (24,29). However Takada et al. (30) have reported that melatonin in maturation medium fails to improve oocyte maturation, embryo development rates and DNA damage of bovine embryos. The results of this study showed that the optimum concentration of melatonin in IVM medium is 10-6 M and very low and very high doses have negative effects. Thus as ROS in a controlled concentration is vital for oocyte maturation, very high and very low doses can be detrimental for oocyte during IVM. Therefore the concentration of antioxidants becomes crucial as shown by others (14,24,29). However melatonin had no effect on the maturation of BCB- oocytes in this study.
HMGB1 expression reduces blastocyst apoptosis and subsequently increases survival and development of embryos by suppressing p53 signalling and the expression of apoptosis-related genes Casp3 and Bax (31). HMGB1 expression varies in different tissues in response to ROS and antioxidants for example antioxidants reduce its expression in pancreas and oxidants induce its expression in lymphatic tissues specially monocytes and macrophages (31). By suppressing DNA methyl transferase (DNMT), melatonin increases the expression of genes effective in embryo development (32). In the study of Cui et al. (22) it was observed that the expression of HMGB1 before implantation, was highest inzygote, low in two cell stage and increases inmorulla and blastocyst and also higher levels attribute to maturation, expansion and successive stages of embryo development. Our study, for the first time, revealed that the expression of HMGB1 increases in oocytes treated with 10-6 M concentration of melatonin in IVM medium. It was assumed that melatonin with its antioxidant activity could down regulate the expression of HMGB1, however by suppressing DNA methyl transferase, melatonin increases the transcription of genes involved in early maturation of embryos such as HMGB1. A possible mechanism involved in increased maturation and development of embryos by melatonin, could be the enhanced expression of HMGB1 which in turn increases the transcription and DNA repair processes and oocyte maturation and subsequent development of embryos.
Somatic cells and gametes possess high amounts of glutathione which have an important role in oocyte maturation, fertilization and development prior to implantation as its presence in the semen proves its protective role (33). Production of glutathione during oocyte maturation has a profound impact on fertilization and embryo development (17). In oocyte, glutathione stabilises the mitotic spindle against oxidizing agents and is involved in enhancement of metaphase II, normal formation of egg, male pronucleus formation and inhibition of two cell stage arrests (34). The production of glutathione in IVM is influenced by the presence of various thiol amino acids or beta mercaptoethanol (35). In this study melatonin as an antioxidant and DNMT inhibitor was added to oocyte IVM medium to evaluate its effect on cytoplasm maturation. The results revealed that although melatonin increases nuclear maturation it is ineffective on glutathione and cytoplasm maturation. Therefore its effective properties are exerted at the nuclear level by increasing the expression of genes involved in oocyte maturation. Thus for achieving a better maturation it is best that in addition to melatonin other factors with the ability to enhance cytoplasm maturation be considered.
Conclusion
The present study shows that in vitro treatment with melatonin increases the maturation and HMGB1 expression in BCB+ immature oocytes and has no significant effect on glutathione levels.
Acknowledgments
This paper was adapted from the thesis for Master of Science Degree of Maryam Salimi and granted by to financially supported by Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences. There is no conflict of interest in this study.
References
- 1.Moor R, Dai Y. Maturation of pig oocytes in vivo and in vitro. Reprod Suppl. 2001;58:91–104. [PubMed] [Google Scholar]
- 2.Sirard MA. Resumption of meiosis: mechanism involved in meiotic progression and its relation with developmental competence. Theriogenology. 2001;55(6):1241–1254. doi: 10.1016/s0093-691x(01)00480-0. [DOI] [PubMed] [Google Scholar]
- 3.Lanco MR, Demyda S, Moreno MM, Genero E. Developmental competence of in vivo and in vitro matured oocyte. BMBR. 2011;6(7):155–165. [Google Scholar]
- 4.Van Soom A, Vandaele L, Goossens K, de Kruif A, Peelman L. Gamete origin in relation to early embryo development. Theriogenology. 2007;68(Suppl 1):S131–S137. doi: 10.1016/j.theriogenology.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Hendriksen PJ, Vos PL, Steenweg WN, Bevers MM, Dieleman SJ. Bovine follicular development and its effect on the in vitrocompetence of oocytes. Theriogenology. 2000;53(1):11–20. doi: 10.1016/s0093-691x(99)00236-8. [DOI] [PubMed] [Google Scholar]
- 6.Alm H, Torner H, Löhrke B, Viergutz T, Ghoneim IM, Kanitz W. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillantcresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology. 2005;63(8):2194–2205. doi: 10.1016/j.theriogenology.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 7.Bhojwani S, Alm H, Torner H, Kanitz W, Poehland R. Selection of developmentally competent oocytes through brilliant cresyl blue stain enhances blastocyst development rate after bovine nuclear transfer. Theriogenology. 2007;67(2):341–345. doi: 10.1016/j.theriogenology.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Herrick JR, Behboodi E, Memili E, Blash S, Echelard Y, Krisher RL. Effect of macromolecule supplementation during in vitro maturation of goat oocytes on developmental potential. Mol Reprod Dev. 2004;69(3):338–346. doi: 10.1002/mrd.20141. [DOI] [PubMed] [Google Scholar]
- 9.Agawal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stressin assisted reproductive techniquessetting. Fertil Steril. 2006;86(3):503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 10.Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione-containing culture media. Mol Reprod Dev. 1996;43(4):437–443. doi: 10.1002/(SICI)1098-2795(199604)43:4<437::AID-MRD5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Siu AW, Maldonado M, Sanchez-Hidalgo M, Tan DX, Reiter RJ. Protective effects of melatonin in experimental free radical-related ocular diseases. J Pineal Res. 2006;40(2):101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7(6):444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 13.Juknat AA, Méndez Mdel V, Quaglino A, Fameli CI, Mena M, Kotler ML. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005;38(2):84–92. doi: 10.1111/j.1600-079X.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28(1):48–51. doi: 10.1034/j.1600-079x.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- 15.Asgari Z, Ghasemian F, Ramezani M, Bahadori MH. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012;14(3):203–208. [PMC free article] [PubMed] [Google Scholar]
- 16.Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. Use of low-salt culture medium for in vitro maturation of porcine oocytes is associated with elevated oocyte glutathione levels and enhanced male pronuclear formation after in vitro fertilization. Biol Reprod. 1994;51(4):633–639. doi: 10.1095/biolreprod51.4.633. [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8(4):485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 18.Boquest AC, Abeydeera LR, Wang WH, Day BN. Effect of adding reduced glutathione during insemination on the development of porcine embryos in vitro. Theriogenology. 1999;51(7):1311–1319. doi: 10.1016/S0093-691X(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim IH, Van Langendonckt A, Van Soom A, Vanroose G, Casi AL, Hendriksen PJ, et al. Effect of exogenous glutathione on the in vitro fertilization of bovine oocytes. Theriogenology. 1999;52(3):537–547. doi: 10.1016/S0093-691X(99)00150-8. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi ME, Beltrame M. Upwardly mobile proteins.Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep. 2000;1(2):109–114. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brezniceanu ML, Völp K, Bösser S, Solbach C, Lichter P, Joos S, et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17(10):1295–1297. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 22.Cui XS, Shen XH, Kim NH. High mobility group box 1 (HMGB1) is implicated in preimplantation embryo development in the mouse. Mol Reprod Dev. 2008;75(8):1290–1299. doi: 10.1002/mrd.20694. [DOI] [PubMed] [Google Scholar]
- 23.Wu YG, Liu Y, Zhou P, Lan GC, Han D, Miao DQ, et al. Selection of oocytes for in vitro maturation by brilliant cresyl blue staining: a study using the mouse model. Cell Res. 2007;17(8):722–731. doi: 10.1038/cr.2007.66. [DOI] [PubMed] [Google Scholar]
- 24.Farahavar A, Shahne AZ. Effect of melatonin on in vitro maturation of bovine oocytes. Afr J Biotechnol. 2010;9(17):2579–2583. [Google Scholar]
- 25.Ajduk A, Ciemerych MA, Nixon V, Swann K, Maleszewski M. Fertilization differently affects the levels of cyclin B1 and M-phase promoting factor activity in maturing and metaphase II mouse oocytes. Reproduction. 2008;136(6):741–752. doi: 10.1530/REP-08-0271. [DOI] [PubMed] [Google Scholar]
- 26.Zuccotti M, Boiani M, Ponce R, Guizzardi S, Scandroglio R, Garagna S, et al. Mouse Xist expression begins at zygotic genome activation and is timed by a zygotic clock. Mol Reprod Dev. 2002;61(1):14–20. doi: 10.1002/mrd.1126. [DOI] [PubMed] [Google Scholar]
- 27.Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione-containing culture media. Mol Reprod Dev. 1996;43(4):437–443. doi: 10.1002/(SICI)1098-2795(199604)43:4<437::AID-MRD5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi J, Park SM, Lee E, Kim JH, Jeong YI, Lee JY, et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol Reprod Dev. 2008;75(7):1127–1135. doi: 10.1002/mrd.20861. [DOI] [PubMed] [Google Scholar]
- 30.Takada L, Junior AM, Mingoti GZ, Balieiro JC, Coelho LA. Melatonin in maturation media fails to improve oocyte maturation, embryo development rates and DNA damage of bovine embryos. Sci Agric. 2010;67(4):393–398. [Google Scholar]
- 31.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81(3):741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korkmaz A, Reiter RJ. Epigenetic regulation: a new research area for melatonin? J Pineal Res. 2008;44(1):41–44. doi: 10.1111/j.1600-079X.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 33.Aitken RJ. The Amoroso lecture.The human spermatozoon-- a cell in crisis? J Reprod Fertil. 1999;115(1):1–7. doi: 10.1530/jrf.0.1150001. [DOI] [PubMed] [Google Scholar]
- 34.Nasr-Esfahani MH, Johnson MH. Quantitative analysis of cellular glutathione in early preimplantation mouse embryos developing in vivo and in vitro. Hum Reprod. 1992;7(9):1281–1290. doi: 10.1093/oxfordjournals.humrep.a137843. [DOI] [PubMed] [Google Scholar]
- 35.Luberda Z. The role of glutathione in mammalian gametes. Rep Biol. 2005;5(1):5–17. [PubMed] [Google Scholar]