Abstract
Background
The growing burden of neonatal mortality due to hospital acquired neonatal sepsis in the developing world creates an urgent need for low cost effective infection control measures in low resource settings.
Methods
Using a pre/post comparison design, we measured how rates of staff hand hygiene compliance, colonization with resistant pathogens (defined as ceftazidime- and/or gentamicin-resistant gram-negative rods (GNRs) and resistant gram-positive cocci), bacteremia, and overall mortality changed following the introduction of a simplified package of infection control measures at two neonatal intensive care units (NICUs) in Manila, the Philippines.
Results
Of 1828 NICU neonates admitted, 45.6% became newly colonized with resistant bacteria, 19.6% became bacteremic (78.2% from GNRs), and 33.6% died. 2903 resistant colonizing bacteria were identified of which 85% were resistant GNRs (predominantly Klebsiella spp., Pseudomonas spp., and Acinetobacter spp.) and 14% were Methicillin-resistant Staphylococcus aureus. Contrasting control vs. intervention periods at each NICU, staff hand hygiene compliance improved (At NICU 1 RR=1.3, 95% CI 1.1–1.5; At NICU 2 RR=1.6, 95% CI 1.4–2.0) and overall mortality declined (NICU 1 RR=0.5, 95%CI 0.4–0.6; NICU 2 RR=0.8, 95% CI 0.7–0.9). However, colonization with resistant pathogens and sepsis rates did not change significantly at either NICU.
Discussion
Nosocomial transmission of resistant pathogens was intense at these two Philippines NICUs and dominated by resistant GNRs. Infection control interventions are feasible and possibly effective in resource limited hospital settings.
Keywords: Infection Control, Philippines, NICU, Drug Resistance, Hand Hygiene
INTRODUCTION
Nosocomial infections contribute a growing yet underappreciated share of neonatal mortality in the developing world. [1] Poor hand hygiene practices, [2, 3] reuse of single-use medication vials and devices, [4] and inadequate sterilization of medical equipment [5] are key proximate events that facilitate transmission of nosocomial pathogens. Institutional factors also contribute, notably inadequate resources to fund infection control programs. Overuse of empiric antibiotics is simultaneously a consequence and a contributing factor. [6] A consequence of of these factors has been the emergence of highly pathogenic multi-drug-resistant gram-negative rods (GNRs) and methicillin-resistant Staphylococcus aureus (MRSA) in developing country NICUs, particularly in south and south-east Asia. [7–10] Simple, effective, and affordable infection control interventions appropriate to developing country settings are urgently needed.
In this context, we evaluated the effectiveness of a package of infection control interventions, selected given their proven efficacy in the developed world and perceived feasibility in the developing world, [1] at two NICUs in Manila, the Philippines. Our primary outcome was the rate at which neonates became newly colonized with resistant bacteria. Secondary outcomes included rates of bacteremia, mortality, and staff hand hygiene compliance.
METHODS
Study setting
We enrolled all neonates admitted to either NICU during the study period. The study was conducted at two inner-city level III NICUs in Manila. Key baseline characteristics of the units are summarized in Table 1. NICU 1 resides in the Philippines General Hospital (PGH), the largest public teaching hospital in the Philippines. NICU 2 is housed in the Jose Fabella Memorial Hospital, a busy (~100 deliveries per day) public obstetrical-gynecological charity hospital. The protocol was approved by the PGH and Boston University Medical Center institutional review boards.
Table 1.
Baseline characteristics of the two level III neonatal intensive care units
| Characteristic | NICU 1 | NICU 2 |
|---|---|---|
| Annual deliveries at hospital | 7,000 | 35,000 |
| Annual level III NICU admissions | 825 | 900 |
| Level III NICU mortality in preceding year | 25% | 60% |
| Source of admissions | Inborn patients only | Inborn patients only |
| Organization of unit | Combined level II/III | Combined level II/III |
| Maximum bed capacity | 80 beds (25 level III) | 60 beds |
| Multiple babies per basinet? | No | Yes - up to 3 per basinet |
| Nurse to patient ratios | 1:5 | 1:13 |
| Reuse of single use vials? | Yes | Yes |
| Number of functioning sinks in unit | 7 | 2, with 1 additional sink located outside unit |
| Hand drying system | Cloth towels with irregular replacement of rolls | Cloth towels with irregular replacement of rolls |
| Ethanol hand wash available in hospital? | No | Yes, but seldom used |
| Latex glove supply | Inconsistent | Inconsistent |
| Admixture of intravenous medications | Performed in pharmacy | Performed at bedside |
| First line antibiotic regimen | Piperacillin/tazobactam plus amikacin | Penicillin plus gentamicin |
| Common second line antibiotic regimen | Ciprofloxacin plus meropenem | 2nd and 3rd generation cephalosporins, amikacin, carbapenems |
| Reprocessing of ventilator equipment | On site soap/water then soaked in 2% glutaraldehyde, rinsed in sterile water | On site – method unspecified |
| Neonatal feedings | Formula, mixed under laminar flow hood | Maternal breast milk only |
Study design
The study utilized a quasi-experimental pre/post design. Following a preparatory phase, we introduced the interventions in staggered fashion as shown in Figure 1.
Figure 1.
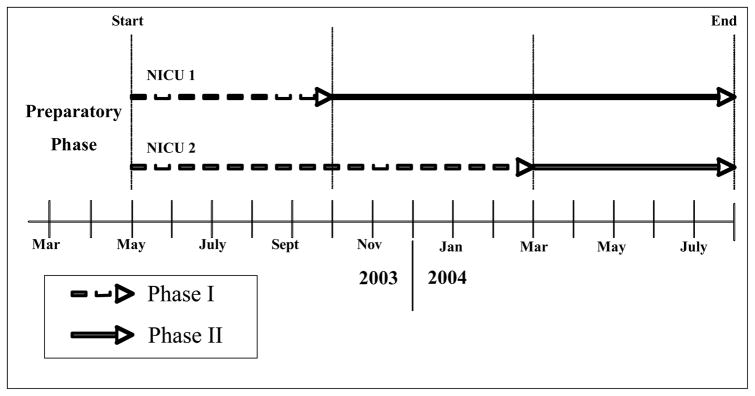
Study Schematic and Timeline
Preparatory phase
Over two months, we standardized indications and procedures for blood cultures, microbiology laboratory procedures, and trained our research assistants. A baseline survey generated site-specific guidance for finalization of the infection control checklist.
Phase I – Baseline surveillance
Our research assistants conducted four forms of surveillance. First, for each neonate, they tracked duration of NICU stay, use of vascular catheters/ventilators, dates and types of infection, and antibiotic use. Second, they conducted regular hand hygiene compliance surveys. Third, they obtained serial surveillance cultures on all neonates. Lastly, they documented all blood culture results.
Phase II – Infection Control Interventions
The Phase II interventions were as follows:
-
Ethanol handrub at each basinet
This was prepared using locally purchased 70% ethanol mixed with glycerin as an emollient.
-
Preparatory workshops for key staff at each unit with the onset of Phase II
These included lectures on hand hygiene and infection contro, interactive case discussions, and a collaborative critique of a video of patient-staff interactions and infection control activities within one of the NICUs.
-
Daily infection control checklists (Table 2a)
Implementing the daily checklist was the responsibility of the NICU teams. Given the large number of checklist items, only one was used per day, selected by listing each item on a note card, and having the team pull a different card each day.
-
Monthly Infection Control Checklist (Table 2b)
The NICU attending physicians were responsible for implementation of the monthly infection control checklist.
Table 2a.
Components of daily infection control checklist
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2b.
Components of monthly infection control checklist
|
|
|
|
|
|
|
Hand Hygiene Assessments
No effort was made to blind NICU staff to the purpose of the observations. One-hour observations were performed intermittently during the day or night shift on a 2:1 ratio. A neonate was chosen and then all hygiene encounters for that patient and the adjacent two neonates were monitored (three neonates per observer period). [11]
Laboratory Methods
Quality control procedures were established using standard American Type Culture Collection (ATCC™, George Mason University Research Laboratory, Manassas, VA) strains. GNRs were categorized into enteric and non-enterics. Enteric bacteria were those whose primary reservoir is the human gut, such as Escherichia coli. We defined non-enterics as bacteria that normally exist in the environment and only occasionally colonize the human gut, such as Pseudomonas aeruginosa.
Surveillance cultures
All neonates underwent screening for resistant GNRs and gram-positive cocci (GPCs) according to a standard schedule. ‘Resistant GNRs’ were those resistant to gentamicin and/or ceftazidime; ‘resistant GPCs’ were MRSA or vancomycin-resistant enterococci (VRE). Surveillance for GNRs and VRE used peri-anal and/or stool swabs, and for MRSA via umbilical and anterior nares swabs (Culturette tubes, Becton Dickinson, Franklin Lakes, NJ). Surveillance for resistant GNRs commenced within 16 hours of admission to a NICU (day 0), and continued on days 2, 7, every 7 days thereafter, and the day of discharge. Screening for resistant MRSA followed the same schedule, omitting the day 0 cultures. The reason for GNR surveillance on day 0 was to determine the proportion of resistant GNR colonization occurring before NICU admission. Screening for resistant GNRs used MacConkey agar plates impregnated with 8 μg/mL gentamicin or 2 μg/mL ceftazidime; screening plates to detect VRE used bile-esculin agar plates with 6 μg/mL of vancomycin; and MRSA screening used Mueller-Hinton agar containing 6 μg/mL of oxacillin. Plates were incubated for 24 hours at 35°C.
Blood cultures
One ml of blood was inoculated/blood culture bottle/neonate with suspected bacteremia. Most clinical specimens were initially submitted to the respective microbiology laboratories of the two hospitals and referred to the PGH research microbiology laboratory for confirmation/characterization using BACTEC (Becton Dickinson, Franklin Lakes, NJ).
Antibiotic susceptibility testing
For all screening and clinical isolates, we determined sensitivity via Kirby-Bauer disk diffusion. Susceptibility classifications used National Committee for Clinical Laboratory Standards breakpoints. [12]
Statistical analyses
Analyses were conducted using SAS v. 9.2 and SPSS v. 11. The primary outcome was the proportion of neonates newly colonized with the target bacteria (resistant GNRs or GPCs). We therefore excluded colonizers and bacteremias from NICU days 0–1, reasoning that these reflected pre-NICU exposures. We calculated the incidence density for colonization or confirmed bacteremia (first event per patient-days at risk) for each NICU separately. To prevent double counting identical isolates, we only included the first new isolate from a given neonate, after which that neonate was censored for that isolate only. Subsequent colonizations with a different isolate were still included. Additionally, we calculated incidence density for colonization and sepsis independently of each other. For example, if a neonate became colonized with a given isolate on day 2, and bacteremic with that same isolate on day 4, the bacteremia calculation would not have been adjusted by censoring from the day 2 colonization event.
Secondary outcomes included bacteremia rates (# positive blood cultures/blood culture bottles submitted); incidence density for bacteremia (number of blood stream infections/patient-days); cumulative mortality (NICU deaths/NICU admissions); and pre/post hand hygiene compliance (number of observed contacts between a health care provider and a neonate (appropriate cleansing using soap/water or ethanol handrub)/total number of contacts).
The sample size was for our primary outcome. Assuming a 10% baseline colonization, we estimated that 865 subjects per hospital would be needed to detect a 4% difference in colonization rates.
RESULTS
Patient characteristics
Between May 2003 and July 2004, a total of 925 and 903 (1828) neonates were admitted at NICUs 1 and 2, of whom 83% and 70% respectively came directly from labor and delivery. Mean gestational ages were 34.7 weeks with 62.3% born prior to 36 weeks; mean birth weights were 2085.7 grams, with 68.3% weighing <2500 grams and 27.2% <1500 grams; 96.9% of neonates received antibiotics; 45.8% required central vascular catheters; and 61.6% required some duration of mechanical ventilation.
Colonizations
As shown in Table 3a the isolates found on our colonization surveys were dominated by resistant GNRs. From 8986 colonization swabs, we identified 2903 resistant bacteria: 2476 (85.3%) resistant GNRs; 427 resistant GPCs (14.7%) (Table 3a). Moreover, all 2476 resistant GNRs were, a priori, isolated just from the 4055 rectal/stool swabs, for a crude positivity ratio of 61%. Many neonates (30.7%) had positive stool/rectal cultures for resistant GNRs on the date of admission, implying exposure during labor and delivery. Among neonates not already colonized on NICU days 0–1, 45.6% became newly colonized with a resistant GNR while in the NICUs. We identified 823 new colonizations with a gentamicin-resistant GNR and 771 new colonizations with a ceftazidime-resistant GNR. 763 (92.8%) of the isolates were resistant to both drugs. Only 59 (7.2%) were resistant to gentamicin but not ceftazidime, leaving only 7 isolates (0.9%) that were resistant to ceftazidime but not gentamicin.
Table 3a.
Results of surveillance for colonization with drug-resistant pathogens
| NICU 1 | NICU 2 | Totals | |||
|---|---|---|---|---|---|
| Phase I | Phase II | Phase I | Phase II | ||
| Neonates admitted | 328 | 597 | 597 | 305 | 1828 |
| Rectal swabs submitted | 638 | 1667 | 1096 | 654 | 4055 |
| Nasal swabs submitted | 433 | 1076 | 609 | 355 | 2473 |
| Umbilical swabs submitted | 429 | 1076 | 599 | 354 | 2458 |
| Total swabs submitted | 1500 | 3819 | 2304 | 1363 | 8986 |
| Total positive isolates (% positive) | 261 (17) | 776 (20) | 1340 (58) | 526 (39) | 2903 (32) |
| Total GPCs found (% of all isolates) | 42 (16) | 116 (15) | 268 (20) | 1 (0) | 427 (15) |
| MRSA | 41 | 111 | 263 | 1 | 416 |
| VRE | 1 | 5 | 5 | 0 | 11 |
| Total GNRs found (% of all positives) | 219 (84) | 660 (85) | 1072 (80) | 525 (100) | 2476 (85) |
| Total enteric GNRs (% total GNRs) | 88 (40) | 595 (90) | 555 (52) | 298 (57) | 1536 (62) |
| Klebsiella pneumoniae ss. pneumoniae | 33 | 216 | 280 | 112 | 641 |
| Klebsiella pneumoniae ss. ozanae | 8 | 265 | 159 | 111 | 543 |
| Escherichia coli | 6 | 45 | 46 | 28 | 125 |
| Citrobacter freundii | 5 | 5 | 7 | 2 | 19 |
| Enterobacter aerogenes | 34 | 44 | 26 | 23 | 127 |
| Enterobacter other | 2 | 20 | 37 | 22 | 81 |
| Total non-enteric GNRs (% total GNRs) | 131 (60) | 65 (10) | 517 (48) | 227 (43) | 940 (38) |
| Pseudomonas aeruginosa | 77 | 10 | 189 | 56 | 332 |
| Acinetobacter baumanii | 16 | 25 | 261 | 117 | 419 |
| Stenotrophomonas maltophilia | 16 | 14 | 42 | 28 | 100 |
| Alcaligenes faecalis | 22 | 16 | 25 | 26 | 89 |
Abbreviations: GPC – gram-positive cocci; GNR – gram-negative rods; MRSA – methicillin-resistant Staphylococcus aureus; VRE – vancomycin resistant enterococci
The five most common resistant colonizers were Klebsiella pneumoniae (including sub-species pneumoniae and ozanae), Acinetobacter baumanii, Pseudomonas aeruginosa, Enterobacter spp., and Escherichia coli. We identified 11 VRE isolates from rectal cultures. MRSA was commonly isolated, particularly at NICU 2 (Table 3a).
Colonization rates did not change significantly at either NICU between Phase I and Phase II (Table 3b), whether considering ceftazidime-resistance, gentamicin-resistance, or both agents. Interestingly, the kinds of GNRs detected changed dramatically at both NICUs, with an increase in enteric GNRs, and a decline of non-enteric GNRs (Table 3a). At NICU 1, the enteric:non-enteric ratio was 40%:60% in Phase I vs. 90%:10% in Phase II (p<0.001). At NICU 2 the enteric:non-enteric ratio was 52%:48% in Phase I vs. 57%:43% in Phase II (p=0.06). MRSA essentially vanished from NICU 2 during Phase II (RR 0.01, 95% CI 0.00–0.05).
Table 3b.
Changes in incidence density for target pathogen colonization, Phase I vs. Phase II
| Incidence density (New colonizations/1000 NICU-days at risk) | ||||||
|---|---|---|---|---|---|---|
| NICU 1 | NICU 2 | |||||
|
| ||||||
| Pathogen category | Phase I | Phase II | P value | Phase I | Phase II | P value |
| Ceftazidime-resistant GNR | 148.2 | 158.9 | 0.59 | 343.9 | 310.8 | 0.31 |
| Gentamicin-resistant GNR | 166.0 | 147.1 | 0.35 | 472.9 | 434.6 | 0.38 |
| GNRs resistant to both ceftazidime and gentamicin | 129.5 | 128.2 | 0.94 | 314.3 | 296.9 | 0.56 |
| MRSA* | 68.3 | 79.3 | 0.54 | 205.8 | 0.0 | <0.001 |
Vancomycin resistant enterococci were too infrequent to calculate meaningful incidence rates.
Abbreviations: GNR – gram-negative rod; MRSA – methicillin resistant Staphylococcus aureus;
Bacteremias
Overall, 358 of 1828 (19.6%) neonates developed at least one bacteremia during their NICU stay, predominantly due to resistant GNRs (78.2% GNRs vs. 11.8% GPCs, p<0.001) (Table 4a). Omitting duplicate isolates taken from the same neonate, 510 of 1262 blood cultures (40.0%) turned positive. The dominant isolates were Klebsiella spp., Enterobacter spp., Pseudomonas spp., and Alcaligenes faecalis and occurred in roughly the same proportions as in the colonization surveys (Table 3a). As with the colonization isolates, there was a shift away from non-enteric GNRs at both NICUs during Phase II (NICU 1, enteric:non-enteric 11%:89% in Phase I vs. 72%:28%, p<0.001; NICU 2, enteric:non-enteric 68%:32% in Phase I, vs. 78%:22% in Phase II, p=0.04). Among the GPCs causing bacteremia, coagulase-negative staphylococci predominated (75%) with only one clinical MRSA isolate, and no VREs. Fungemia was uncommon, chiefly due to non-albicans species of Candida.
Table 4a.
Blood stream isolates, by NICU and study period
| NICU 1 | NICU 2 | Total | |||
|---|---|---|---|---|---|
| Phase I | Phase II | Phase I | Phase II | ||
| Total # of blood cultures submitted | 197 | 504 | 289 | 272 | 1262 |
| Total # of positive isolates (% positive) | 32 (16) | 99 (20) | 216 (76) | 163 (63) | 536 (43) |
| Total GPCs identified (% all positives) | 0 (0) | 25 (5) | 16 (6) | 19 (7) | 60 (11) |
| Methicillin-sensitive S. aureus | 0 | 4 | 5 | 3 | 12 |
| MRSA | 0 | 1 | 0 | 0 | 1 |
| Vancomycin-sensitive Enterococcus | 0 | 2 | 0 | 0 | 2 |
| VRE | 0 | 0 | 0 | 0 | 0 |
| Coagulase-negative staphylococci | 0 | 18 | 11 | 16 | 45 |
| Total GNRs # (% of all positives) | 27 (84) | 32 (32) | 198 (92) | 142 (87) | 417 (89) |
| Total enteric GNRs # (% total GNRs) | 3 (11) | 23 (72) | 135 (68) | 111 (78) | 287 (69) |
| Klebsiella spp. | 0 | 9 | 76 | 78 | 163 |
| Escherichia coli | 0 | 0 | 16 | 8 | 24 |
| Citrobacter spp. | 0 | 0 | 2 | 2 | 4 |
| Enterobacter spp. | 3 | 14 | 44 | 34 | 95 |
| Proteus rettgeri | 0 | 1 | 0 | 0 | 1 |
| Total non-enteric GNR # (% total GNRs) | 24 (89) | 9 (28) | 63 (32) | 31 (22) | 130 (31) |
| Pseudomonas spp. | 0 | 5 | 52 | 15 | 72 |
| Acinetobacter baumanii | 0 | 1 | 3 | 4 | 8 |
| Stenotrophomonas maltophilia | 0 | 3 | 4 | 4 | 11 |
| Alcaligenes faecalis | 22 | 0 | 2 | 6 | 30 |
| Burkholderia cepacia | 0 | 0 | 3 | 3 | 6 |
| Flavobacterium spp. | 2 | 0 | 0 | 0 | 2 |
| Achromobacter spp. | 1 | 0 | 0 | 0 | 1 |
| Other pathogens (% all positives) | 5 (3) | 42 (8) | 2 (1) | 2 (1) | 59 (1) |
Abbreviations: GPC – gram-positive cocci; GNR – gram-negative rod; MRSA – methicillin-resistant S. aureus; VRE – vancomycin-resistant Enterococcus
Bacteremia rates at NICU 2 far exceeded those at NICU 1. However, adjusting for patient-time at risk, overall bacteremia rates did not change at either unit from Phase I to Phase II (Table 4b). However, this concealed a decline in non-enteric bacteremias, particularly at NICU 1, with an absolute and relative increase in enteric bacteremias at both NICUs. In addition, NICU 1 experienced a small but significant increase in the incidence of bacteremia with coagulase negative staphylococci and non-albicans Candida spp.
Table 4b.
Changes in bacteremia rates, Phase I vs. Phase II
| NICU 1 Incidence density (Bacteremias/1000 days at risk) | NICU 2 Incidence density (Bacteremias/1000 days at risk) | |||||
|---|---|---|---|---|---|---|
| Pathogen category | Phase I | Phase II | P value | Phase I | Phase II | P value |
| All bacteremias | 21.4 | 28.1 | 0.23 | 163.3 | 166.9 | 0.85 |
| GNR bacteremias | 16.8 | 8.3 | 0.02 | 141.5 | 143.0 | 0.93 |
| Enteric | 2.1 | 6.1 | 0.07 | 90.5 | 112.6 | 0.12 |
| Non-enteric | 13.7 | 2.5 | <0.01 | 36.6 | 27.0 | 0.12 |
| GPC bacteremias | 0.0 | 6.0 | <0.01 | 9.0 | 14.7 | 0.19 |
| Candida spp. | 3.5 | 14.6 | <0.01 | 0.6 | 1.7 | 0.40 |
Abbreviations: NICU – Neonatal intensive care unit
NICU Mortality
Mortality rates were high at both NICUs, but declined during Phase II. Of 1828 neonates admitted, 615 (33.6%) died. At NICU 1, the risk of death declined during Phase II (290 deaths/1000 admissions in Phase I vs. 144 deaths/1000 admissions in Phase II, RR=0.50, 95% CI 0.38–0.64), for a 15% absolute risk reduction (95% CI 9%–20%). Mortality also declined at NICU 2 during Phase II (598 deaths/1000 admission in Phase I vs. 481 deaths/1000 admissions in Phase II, RR 0.81, 95% CI 0.71–0.91) for a 12% absolute risk reduction (95% CI 6%–18%).
Hand Hygiene Compliance
Hand hygiene compliance improved at both NICUs during Phase II. Compliance was evaluated an average of 18 hours per NICU per month, for 513 one-hour observations and 5423 patient contacts (Table 5). Comparing Phase II vs. Phase I, the likelihood of pre-contact hygiene compliance improved at both units (NICU 1, RR 1.3, 95% CI 1.15–1.49; NICU 2, RR 1.61, 95% CI 1.40–1.86). The Phase II interventions had no impact on the overall likelihood of post-contact hygiene compliance at NICU 1 (RR 0.94, 95% CI 0.79–1.11) though there was a preferential shift towards ethanol handrub over soap/water (p<0.001). At NICU 2, the likelihood of post-contact hand hygiene compliance increased during Phase II (RR 1.6, 95% CI 1.31–1.98), again with preferential use of ethanol handrub (p=0.01).
Table 5.
Hand hygiene compliance rates by NICU and study period
| NICU 1 | NICU 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of observations | None % | EtOH % | Soap/water % | No. of observations | None % | EtOH % | Soap/water % | ||
| Pre-contact | Phase I | 440 | 67.1 | 2.1 | 30.9 | 852 | 76.5 | 16.3 | 7.2 |
| Phase II | 952 | 56.8 | 24.6 | 18.6 | 476 | 62.2 | 28.4 | 9.5 | |
| Post-ontact | Phase I | 440 | 75.9 | 1.4 | 22.5 | 851 | 86.9 | 8.3 | 4.6 |
| Phase II | 946 | 74.1 | 9.7 | 16.0 | 475 | 79 | 12.6 | 7.8 | |
Abbreviations: EtOH – Ethanol based handrub; NICU – neonatal intensive care unit.
DISCUSSION
This project tested the effectiveness of a package of infection control interventions among two of the largest NICUs in the Philippines. The interventions were associated with increased hand hygiene compliance in general and alcohol-based handrubs in particular. However, the overall incidence of colonization with resistant bacteria and of bacteremias was unchanged. Thus, while mortality rates declined substantially, we were unable to conclude that the interventions were responsible.
Our data provide some bleak insights into the epidemiology of drug-resistant bacteria at these NICUs: colonization pressure with resistant pathogens was intense, with correspondingly high rates of bacteremia and mortality. Moreover, the spectrum of pathogens causing sepsis was remarkable for its dissimilarity to NICUs in the developed world. Of the most common pathogens, four were non-enterics: A. baumanii, Ps. aeruginosa, St. maltophilia, and Al. faecalis, an uncommon pathogen and rarely described in the developing world. [13–15] The high frequency of non-enterics GNRs, and high rates of drug resistance strongly imply that nosocomial transmission, rather than mother to child transmission during delivery, was responsible. MRSA colonization was common (though sepsis rare), and VRE appeared to be an emerging threat – worrisome given the lack of prior reports of VRE in the Philippines.
The most common agents of early neonatal sepsis in the developed world are group B streptococci (GBS) and Escherichia coli. Yet here GBS sepsis was conspicuous for its absence, echoing results from the international infections in pregnancy study group, which found GBS colonization rates to be one half of those found in Dublin, Ireland, and one third that found in Philadelphia, the United States. [16, 17] Zaidi’s recent review of hospital-acquired neonatal sepsis in developing nations also found GBS in only 2.3% of sepsis episodes. [18] This is problematic given that current guidelines for neonatal sepsis – such as the WHO’s pocket manual for the management of hospital illnesses in resource-poor countries [19] – assume that GBS and fully sensitive enteric GNRs are the primary agents of early neonatal sepsis.
Thirty percent of neonates arrived at the NICUs already colonized with resistant GNRs. While not a planned part of the study, this prompted us to investigate what conditions/practices in the labor and delivery ward might be contributing. Several possibilities emerged. One was that latex gloves, frequently in short supply, were often rinsed and reused after air-drying on a rack. We also noted that it was standard practice for obstetricians to swab each mother’s perineum with povidone-iodine prior to delivery. These solutions were decanted from open large-necked containers into multiuse bottles. Ps. aeruginosa and other environmental GNRs can be resistant to povidone-iodine. Therefore, contamination of the stock supplies could systematically expose the neonates to iodine-resistant pathogens during delivery. [20–22] Further environmental studies would be helpful to test these observations and identify other potential exposure sources.
We observed a shift from non-enteric GNRs isolates during Phase II, particularly at NICU 1. Because this shift occurred among the invasive isolates and the colonizing isolates, this probably represents a true shift in the epidemiology of these nosocomial pathogens. Whether this was due to our interventions, or factors external to our study, is unclear.
The rate of hand hygiene compliance at the start of the project was low, but improved at both NICUs. Moreover, the modest absolute increase in hygiene compliance rates concealed a much larger proportional increase in ethanol-based handrub use, a vastly superior modality to soap and water. [23, 24] While discouraging that hand hygiene compliance rates did not rise more, such results are actually quite typical. [11] In a trial at Children’s Hospital Boston, Harbarth et al saw hand hygiene compliance rise following their intervention, but the effect was transient, declining from 42.5% to 35.1% within months. [25] Other studies documented post-intervention compliance rates as low as 14% [26], though typical post-intervention compliance rates are between 40–60%. [11] Such findings emphasize the variability in responses to behavioral interventions, and the enormous challenges inherent in effecting sustained behavioral change.
Ultimately, we were unable to conclude definitively that our interventions were effective. On the one hand, improvements in hand hygiene compliance demonstrate the feasibility of infection control through behavioral change in a resource-limited setting. Furthermore, mortality declined significantly at both NICUs during Phase II – a positive, though surprising, outcome. On the other hand, our conceptual model assumes that improved hand hygiene reduces colonization pressure, with lowered bacteremia and mortality rates being down-stream effects. The fact that colonization and bacteremia rates remained stable during Phase II conflicts with this model, and calls into question whether our interventions vs. external factors led mortality rates to drop – a result reminiscent of a recent publication by Rupp and colleagues, where hygiene compliance improved without changing transmission rates. [27] The one notable exception was the disappearance of MRSA colonization from NICU 2 during Phase II. This was surprising and hard to reconcile given the very high rates of MRSA colonization during Phase I at NICU 2 and stable MRSA colonization rates at NICU 1.
Our study had two main limitations. First is that pre/post comparisons are inherently vulnerable to confounding by intercurrent seasonal or secular events. However, randomization would only have been realistic in the setting of a large multicenter trial. Another limitation is that resource constraints did not allow us to collect process indicators to monitor whether other aspects of our intervention, such as the daily and monthly checklists, were being implemented as intended.
In conclusion, this study at two of the largest NICUs in the Philippines demonstrates that enhanced infection control interventions are feasible in developing settings and possibly effective. The epidemiology of neonatal sepsis at these NICUs is characterized by intense colonization pressure with multi drug-resistant GNRs, with correspondingly high rates of bacteremia and mortality. MRSA and VRE appear to be emerging threats. By contrast, the absence of GBS suggests that this pathogen is unimportant in this context. Reducing the burden of resistant nosocomial infections in developing hospital settings must be a priority for regional and global public health agencies. Further research is needed to understand barriers to improving hand hygiene compliance, to identify reservoirs for environmental pathogens as a key step in interrupting their transmission, and to explore the cost implications of implementing vs. not implementing hospital infection control programs in resource-poor settings.
Acknowledgments
We particularly wish to thank our research assistants: J. Estrada, R. Canseco, E. Aduan, and A. Geraldez. This work was supported by a cooperative agreement between Boston University and the Office of Health and Nutrition of the United States Agency for International Development: GHS-A-00-03-00020-00. Dr. Gill’s effort was supported by NIH/NIAIDS K23 AI 62208. The funders played no role in the study design, or the analysis, interpretation, and presentation of the study results.
Footnotes
Conflict of Interest: None of the authors has a financial conflict of interest.
References
- 1.Goldmann DA, Huskins WC. Control of nosocomial antimicrobial-resistant bacteria: a strategic priority for hospitals worldwide. Clin Infect Dis. 1997 Jan;24( Suppl 1):S139–45. doi: 10.1093/clinids/24.supplement_1.s139. [DOI] [PubMed] [Google Scholar]
- 2.Parry MF, Hutchinson JH, Brown NA, Wu CH, Estreller L. Gram-negative sepsis in neonates: a nursery outbreak due to hand carriage of Citrobacter diversus. Pediatrics. 1980 Jun;65(6):1105–9. [PubMed] [Google Scholar]
- 3.Pawa AK, Ramji S, Prakash K, Thirupuram S. Neonatal nosocomial infection: profile and risk factors. Indian Pediatr. 1997 Apr;34(4):297–302. [PubMed] [Google Scholar]
- 4.Archibald LK, Ramos M, Arduino MJ, et al. Enterobacter cloacae and Pseudomonas aeruginosa polymicrobial bloodstream infections traced to extrinsic contamination of a dextrose multidose vial. J Pediatr. 1998 Nov;133(5):640–4. doi: 10.1016/s0022-3476(98)70104-0. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi M, Angulo M, Sifuentes-Osornio J. Disinfection and sterilization practices in Mexico. J Hosp Infect. 1995 Sep;31(1):25–32. doi: 10.1016/0195-6701(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 6.Isturiz RE, Carbon C. Antibiotic use in developing countries. Infect Control Hosp Epidemiol. 2000 Jun;21(6):394–7. doi: 10.1086/501780. [DOI] [PubMed] [Google Scholar]
- 7.Babay HA, Twum-Danso K, Kambal AM, Al-Otaibi FE. Bloodstream infections in pediatric patients. Saudi Med J. 2005 Oct;26(10):1555–61. [PubMed] [Google Scholar]
- 8.Tseng YC, Chiu YC, Wang JH, Lin HC, Su BH, Chiu HH. Nosocomial bloodstream infection in a neonatal intensive care unit of a medical center: a three-year review. J Microbiol Immunol Infect. 2002 Sep;35(3):168–72. [PubMed] [Google Scholar]
- 9.Chandrashekar MR, Rathish KC, Nagesha CN. Reservoirs of nosocomial pathogens in neonatal intensive care unit. J Indian Med Assoc. 1997 Mar;95(3):72–4. 7. [PubMed] [Google Scholar]
- 10.Espino Hernandez M, Couto Ramos MJ, Fiol Ferrer N, Rojas Hernandez N. Resistance to antimicrobials and combination therapy assessment in neonatal sepsis. Rev Panam Salud Publica. 2003 Apr;13(4):214–21. doi: 10.1590/s1020-49892003000300003. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. MMWR. 2002;51(RR16):1–56. [Google Scholar]
- 12.NCCLS. Performance standards for antimicrobial susceptibility testing: 9th informational supplement. Villanova, PA: National Committee for Clinical Laboratory Standards; 1991. [Google Scholar]
- 13.Berry AM. B. alcaligenes faecalis septicemia and meningitis in the newborn. Report of an unusual case. Indian J Pediatr. 1967 Jul;34(234):242–4. doi: 10.1007/BF02756292. [DOI] [PubMed] [Google Scholar]
- 14.Khetarpal SK. B. Alcaligenes Faecalis Septicemia in the Newborn. Clin Pediatr (Phila) 1964 Feb;3:108–10. doi: 10.1177/000992286400300211. [DOI] [PubMed] [Google Scholar]
- 15.Kumhar GD, Ramachandran VG, Gupta P. Bacteriological analysis of blood culture isolates from neonates in a tertiary care hospital in India. J Health Popul Nutr. 2002 Dec;20(4):343–7. [PubMed] [Google Scholar]
- 16.Whitney CG, Daly S, Limpongsanurak S, et al. The international infections in pregnancy study: group B streptococcal colonization in pregnant women. J Matern Fetal Neonatal Med. 2004 Apr;15(4):267–74. doi: 10.1080/14767050410001668617. [DOI] [PubMed] [Google Scholar]
- 17.Thinkhamrop J, Limpongsanurak S, Festin MR, et al. Infections in international pregnancy study: performance of the optical immunoassay test for detection of group B streptococcus. J Clin Microbiol. 2003 Nov;41(11):5288–90. doi: 10.1128/JCM.41.11.5288-5290.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005 Mar-Apr;365(9465):1175–88. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Pocket book of hospital care for children: Guidelines for the management of common illnesses with limited resources. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 20.Panlilio AL, Beck-Sague CM, Siegel JD, et al. Infections and pseudoinfections due to povidone-iodine solution contaminated with Pseudomonas cepacia. Clin Infect Dis. 1992 May;14(5):1078–83. doi: 10.1093/clinids/14.5.1078. [DOI] [PubMed] [Google Scholar]
- 21.Wisplinghoff H, Schmitt R, Wohrmann A, Stefanik D, Seifert H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J Hosp Infect. 2007 Jun;66(2):174–81. doi: 10.1016/j.jhin.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Berkelman RL, Anderson RL, Davis BJ, et al. Intrinsic Bacterial Contamination of a Commercial Iodophor Solution: Investigation of the Implicated Manufacturing Plant. Appl Environ Microbiol. 1984 Apr;47(4):752–6. doi: 10.1128/aem.47.4.752-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilhermetti M, Hernandes SE, Fukushigue Y, Garcia LB, Cardoso CL. Effectiveness of hand-cleansing agents for removing methicillin-resistant Staphylococcus aureus from contaminated hands. Infect Control Hosp Epidemiol. 2001 Feb;22(2):105–8. doi: 10.1086/501872. [DOI] [PubMed] [Google Scholar]
- 24.Kjolen H, Andersen BM. Handwashing and disinfection of heavily contaminated hands--effective or ineffective? J Hosp Infect. 1992 May;21(1):61–71. doi: 10.1016/0195-6701(92)90154-e. [DOI] [PubMed] [Google Scholar]
- 25.Harbarth S, Pittet D, Grady L, et al. Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance. Pediatr Infect Dis J. 2002 Jun;21(6):489–95. doi: 10.1097/00006454-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: The impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med. 2000 Apr 10;160(7):1017–21. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
- 27.Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol. 2008;29(1):8–15. doi: 10.1086/524333. [DOI] [PubMed] [Google Scholar]


