Abstract
Prostate cancer is the most frequently diagnosed form of cancer in males in the United States. The disease is androgen driven and the use of orchiectomy or chemical castration, known as androgen deprivation therapy (ADT) has been employed for the treatment of advanced prostate cancer for over 70 years. Agents such as GnRH agonists and non-steroidal androgen receptor antagonists are routinely used in the clinic, but eventually relapse occurs due to the emergence of castration-resistant prostate cancer. With the appreciation that androgen signaling still persists in these patients and the development of new therapies such as abiraterone and enzalutamide that further suppresses androgen synthesis or signaling, there is a renewed need for sensitive and specific methods to quantify androgen precursor and metabolite levels to assess drug efficacy. We describe the development, validation and application of a stable isotope dilution liquid chromatography electrospray ionization selected reaction monitoring mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of serum keto-androgens and their sulfate and glucuronide conjugates using Girard-T oxime derivatives. The method is robust down to 0.2–4 pg on column, depending on the androgen metabolite quantified, and can also quantify dehydroepiandrosterone sulfate (DHEA-S) in only 1 μL of serum. The clinical utility of this method was demonstrated by analyzing serum androgens from patients enrolled in a clinical trial assessing combinations of pharmacological agents to maximally suppress gonadal and adrenal androgens (Targeted Androgen Pathway Suppression, TAPS clinical trial). The method was validated by correlating the results obtained with a hydroxylamine derivatization procedure coupled with tandem mass spectrometry using selected reaction monitoring that was conducted in an independent laboratory.
Keywords: Prostate cancer, Androgen metabolome, Mass spectrometry
1. Introduction
Huggins and Hodges first demonstrated that orchiectomy was an effective treatment for advanced prostate cancer [1]. A number of drugs that suppress the gonadal-pituitary axis have since been approved for the treatment of advanced prostate cancer, e.g. leuprolide and goserelin, which can be combined with androgen receptor (AR) antagonists, e.g. bicalutamide [2]. The evolution of androgen deprivation therapy (ADT) has resulted in significant improvement in the overall survival of patients diagnosed with high risk prostate cancer [3]. However, patients on ADT often relapse and despite castrate levels of serum androgens the tumor recurs as castration resistant prostate cancer (CRPC). The success of new agents in the treatment of CRPC, e.g. abiraterone (Zytiga, a P450 17α-hydroxylase/17,20-lyase (P450 17) inhibitor that blocks androgen synthesis) and MDV3100 or enzalutamide (Xtandi, a potent AR antagonist) indicates that the disease remains hormonally driven [4-6]. Currently, CRPC patients are administered these drugs without assessing the efficacy of ADT on suppressing the androgen metabolites of interest. Resistance to abiraterone and MDV3100 is now being reported, suggesting that adaptation of the androgen axis occurs to surmount the effect of the drug. The clinical challenge is to select the appropriate drug for each patient and to understand mechanisms of drug resistance. To this end, a specific and sensitive method to measure serum and intratumoral androgens is required.
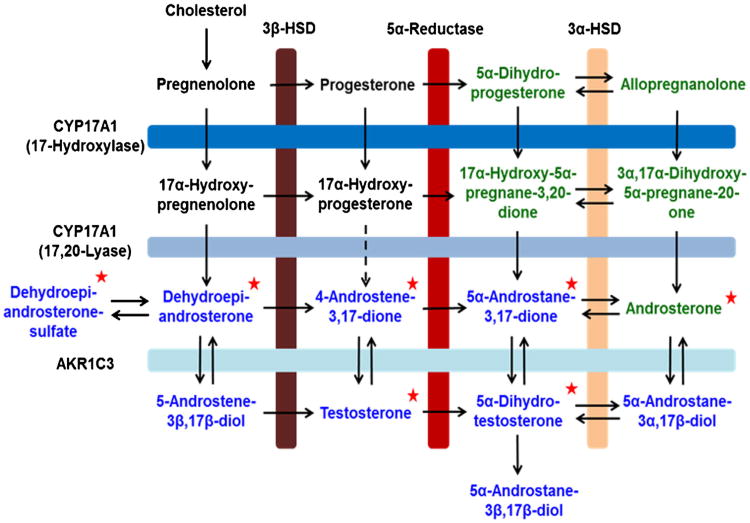
The biosynthesis of the potent AR ligand, 5α-dihydrotestosterone (DHT), can be achieved by several routes and include: the classical pathway via T [dehydroepiandrosterone (DHEA) → Δ4-androstene-3,17-dione (Δ4-AD) →T → DHT]; the backdoor pathway to DHT [5α-pregnane-3,20-dione (DHP)→ allopregnanolone → 3α,17α-dihydroxy-5α-pregnane-20-one→ A→ 3α-androstanediol→ DHT] [7]; and an alternative pathway that by-passes T [DHEA→ Δ4-AD→ 5α-androstane-3,17-dione (Adione)→ DHT] (Scheme 1) [8]. Furthermore, the adrenal gland produces DHEA-S, which is secreted and can be converted to unconjugated DHEA by tissues that possess steroid sulfatase activity. The DHEA can then be converted to T and DHT. DHEA-S may be a ‘precursor pool’ for intratumoral androgen production in CRPC, as these tumors up-regulate enzymes mediating the synthesis of T and DHT from DHEA [9,10]. The androgen precursors, downstream metabolites and androgen conjugates are collectively referred to as the ‘androgen metabolome’. Quantification of the androgen metabolome would provide endpoints for disease surveillance, therapeutic monitoring, and ultimately, personalized treatment of advanced prostate cancer.
Scheme 1.
The androgen metabolome is critical for male development and is therapeutically targeted in advanced prostate cancer. The metabolites in black represent the classical pathway for T and DHT biosynthesis, which act as potent ligands for the androgen receptor. The metabolites in green represent the “backdoor pathway” for T and DHT biosynthesis. The red stars indicate the keto-androgens quantified using the Girard T derivatization, SID-LC-ESI-MS/MS method described in this paper. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Levels of T and DHT are measured in clinical laboratories by competitive radioimmunoassay (RIA) or enzyme-linked immunosorbent assay (ELISA). These assays are quick, economical and relatively high throughput. However, RIAs and ELISAs rely on the specificity of antibodies, which may cross-react with other androgen metabolites or components of the biological matrix, leading to an overestimate of T and DHT levels, particularly under conditions of ADT [11,12]. In addition, antibody based methods only detect one analyte at a time and there is no panel of antibodies to detect all of the androgens of interest. Finally, these methods provide no structural validation of the analyte being measured.
Gas chromatography– and liquid chromatography–mass spectrometry (GC– and LC–MS) have been successfully applied to the quantification of select androgens [13,14]. Derivatization strategies with Girard T reagent [15,16] and picolinic acid [17,18] have been employed to increase sensitivity and afford a reliable MS/MS fragmentation pattern for identification of T and DHT and a few other androgens, but have not been employed in a systematic fashion for all of the androgens of interest. Quantification of the conjugated androgens is critical, but it is complicated by contamination of commercial β-glucuronidase and arylsulfatase with each other and by cholesterol oxidase [19–21]. As such, there remains a need for a comprehensive and reliable methodology for the quantification of both conjugated and unconjugated androgens.
Liquid chromatography-electrospray ionization/selected reaction monitoring/mass spectrometry (LC/ESI/SRM/MS) in conjunction with stable isotope dilution methodology is the gold standard for the quantification of small molecule metabolites [22–24]. The use of pre-ionized derivatives such as the Girard oximes provide an extremely sensitive method, which allows for the detection of analytes using a minimal amount of valuable human specimen and is exquisitely specific. Specificity is achieved based on three criteria, retention time on LC, detection of the intact molecule, and product-precursor selected ion transitions specific to each analyte. The inclusion of stable isotope internal standards allows for accurate quantification based on the ratio of unlabeled to labeled analyte and corrects for losses during sample preparation. Herein, we describe the development, validation and application of a SID-LC/ESI/SRM/MS method for the quantification of the keto-androgens as Girard T derivatives and their conjugates in human serum samples. We demonstrate its clinical utility by quantifying conjugated and unconjugated keto-androgens in serum from prostate cancer patients enrolled on the TAPS clinical trial which evaluated combinations of agents to maximally suppress androgen signaling. The agents used in this clinical trial include the GnRH agonist, goserelin acetate, the AR antagonist, bicalutamide and the dual 5α-reductase type 1 and type 2 inhibitor, dutasteride which are all routinely used in ADT for treatment of advanced prostate cancer. Ketoconazole has also found an application in the ADT arena as the efficacy of the anti-fungal agent is attributed to its non-specific P450 17 inhibitory activity.
2. Methods
2.1. Chemicals and reagents
T, Epi-T, Adione, Δ4-AD, androsterone, DHEA, DHEA-S and DHEA-G were from Steraloids (Wilton, NH, USA). DHT, trifluoroacetic acid, Escherichia coli β-glucuronidase Type VII A (Cat. No. G-7646), Helix pomatia arylsulfatase/β-glucuronidase Type H-5 (Cat.No. G-1512), Abalone entrails arylsulfatase/β-glucuronidase (Cat. No. S-9754), p-nitro-catechol sulfate dipotassium salt, Girard's reagent T, sodium acetate, ammonium acetate and phenolphthalein-β-glucuronide were from Sigma-Aldrich (St. Louis, MO, USA). [13C3]-T and [13C3]-DHT were from C/D/N Isotopes (Point-Claire, Quebec, Canada) and Cambridge Isotopes (Andover, MA, USA). Optima grade methanol and anhydrous diethyl ether from Fisher Scientific (Fair Lawn, NJ, USA). Ethanol was from Decon Laboratories (King of Prussia, PA, USA). Charcoal dextran stripped fetal bovine serum was from Atlanta Biologicals (Lawrenceville, GA, USA).
2.2. Extraction and derivatization of keto-androgen standards
Stock solutions were made using commercially available standards for T, DHT, Epi-T, Δ4-AD, Adione, A and DHEA in ethanol for calibration curves and quality control standards. CD-FBS was used as the matrix for calibrators and quality control standards. CD-FBS was aliquoted into glass tubes (200 μL), followed by the addition of 0, 2, 5, 10, 20, 50, 100, 500 and 1000 pg of unlabeled standards along with 200 pg each of [13C3]-T/-DHT. The standards were extracted with 2 mL of anhydrous diethyl ether in glass tubes. Extraction was facilitated by mild agitation on a horizontal shaker for 15 min, followed by centrifugation on a SVC-100H at 1725rpm (Savant, Farmingdale, NY, USA) for 1 h to separate the phases. The organic fraction was transferred to a new set of glass tubes and dried under nitrogen in a VisiPrep 24DL manifold (Supelco, Bellefonte, PA, USA). The dried extract was derivatized with freshly prepared Girard T reagent (20 mM) in 1% trifluoroacetic acid (TFA) in methanol (v/v) for 30 min, dried under vacuo on a vacuum centrifuge SPD121P with vacuum engaged (Thermo Scientific, San Jose, CA, USA) and stored at −80 °C until analysis by LC/ESI/SRM/MS.
2.3. Titration of enzyme preparations for the deconjugation of keto-androgens
The E. coli β-glucuronidase enzyme (25,000 U) and the Abalone entrails sulfatase extract (1000U) were re-suspended separately in 2 mL of 100 mM sodium acetate, pH 7.0 and aliquoted into cryovials for storage at −80 °C until use. The E. coli β-glucuronidase was titrated using phenolphthalein glucuronide where one unit of enzyme activity is defined as the amount that will liberate 1 μg of phenolphthalein/h from phenolphthalein glucuronide at pH 6.8 and 37 °C. Similarly, Abalone entrails sulfatase activity was titrated using a standard p-nitrocatechol-sulfate assay in which one unit of enzyme activity is defined as 1 μmole p-nitrocatechol-sulfate hydrolyzed/h at pH 5.0 and 37 °C. These assays were conducted using reagents and protocols obtained from the manufacturer, Sigma–Aldrich.
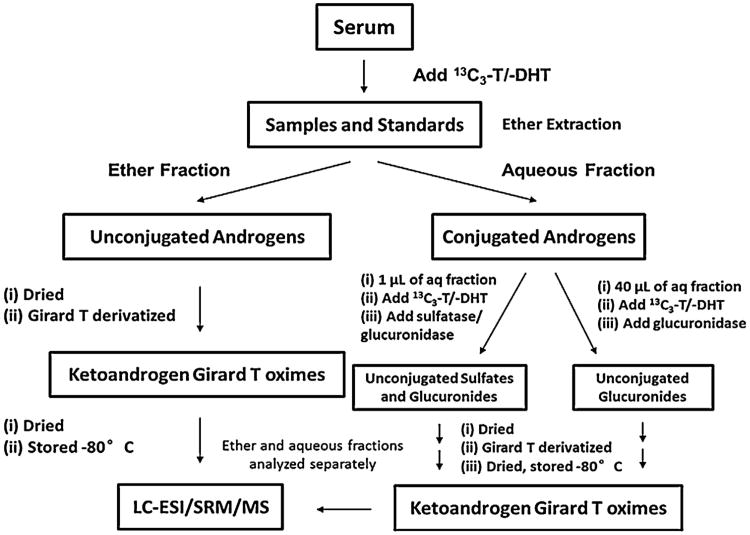
2.4. Extraction and derivatization of conjugated and unconjugated androgens from human patient serum
Human patient serum was shipped on dry ice and stored at −80 °C until extraction, derivatization and analysis were conducted. A 200 μL aliquot of the human serum was spiked with 200pg of [13C3]-T/-DHT and extracted as described above. The organic fraction contained the unconjugated androgens and was derivatized as described above. The aqueous fraction contained the conjugated androgens and was subjected to two separate enzymatic hydrolysis reactions to quantify the glucuronidated and sulfated androgen metabolites. The first reaction was conducted in a glass tube for 24 h at 37 °C and contained 200 mM sodium acetate, pH 5.0, 200pg of [13C3]-T/-DHT, 90 U of E. coli β-glucuronidase and 40 μL of the aqueous fraction. This first reaction affords the quantification of all of the glucuronidated keto-androgens. The second reaction was conducted in a glass tube for 24h at 37 °C and contained 200 mM sodium acetate, pH 5.0, 200 pg of [13C3]-T/-DHT, 90U of E. coli β-glucuronidase, 30U of Abalone entrails sulfatase and 1 μL of the aqueous fraction, a fresh aliquot of 30 U of Abalone entrails sulfatase was added at the 12 h time point. This second reaction affords the quantification of the sulfated and glucuronidated keto-androgens. Following the enzymatic hydrolysis of the conjugated androgens, a second round of diethyl ether extraction and derivatization was conducted as described above and the dried samples stored separately at −80 °C until analyzed by LC/ESI/SRM/MS. The difference between the β-glucuronidase and β-glucuronidase plus sulfatase treatments provided a quantitative estimate of the sulfate conjugates (Scheme 2).
Scheme 2.
The work flow describing the quantification of conjugated and unconjugated keto-androgens using the Girard T derivatization and SID-LC-ESI-MS/MS method.
2.5. Liquid chromatography separation of keto-androgen derivatives
Chromatography was conducted on a Waters 2690 Alliance HPLC system (Waters Corp., Milford, MA, USA) equipped with a Kinetex XB-C18, 100 mm × 2.1 mm, 2.6 μm, 100 Å column with a matching C18 guard column, a Security Guard Ultra 2.1 mm internal diameter (Phenomenex, Torrance, CA, USA). The mobile phase was comprised of 10 mM ammonium acetate in 0.01% TFA in water (v/v, A) and 0.01% TFA in methanol (v/v, B). The binary gradient was delivered at a flow rate of 0.2 mL/min (0–2 min, 10% B, 2–37 min, 10–80% B, 37–38 min, 80-100% B, 38–45 min 100% B, 45–47 min 100–10% B 47–62 min, 10% B). All samples, calibrators and quality control standards were resuspended in 100 μL of 9:1 (v/v) 10mM ammonium acetate in 0.01% TFA in water: 0.01% TFA in methanol and placed into glass vials with deactivated glass inserts (Waters Corp.).
2.6. Electrospray ionization tandem mass spectrometry of keto-androgen derivatives
MS analysis was performed using a TSQ Quantum triple quadrupole mass spectrometer (Thermo Scientific, San Jose, CA, USA). The injection volume was 20 μL. The Girard T oximes were introduced into the mass spectrometer via the ESI source and analyzed in the positive ion mode. The pre-ionized Girard T oximes (M+) were detected in the SRM mode using the following transitions: m/z 400.4–341.2 for Δ4-AD; m/z 402.4–343.2 for DHEA, T, Adione and EpiT; m/z 404.4–345.2 for DHT, A and epiandrosterone; and m/z 405.4–346.2 for [13C3]-T and m/z 407.4–348.2 for [13C3]-DHT. The ESI-MS/MS conditions were as follows: spray voltage: 4.5 kV; sheath gas: nitrogen, 30 (arbitrary units, gas pressure); auxiliary gas pressure, 10; ion transfer capillary temperature: 270; collision gas: Ar; and capillary offset voltage: 35 V.
2.7. Validation of LC/ESI/SRM/MS quantification of keto-androgen derivatives
Quantification of androgens was validated using quality control standards of low, middle and high levels of quantification. The method was held to the bioanalytical methods standards set forth by the U.S. Food and Drug Administration. Matrix effects were assessed for T and DHT and no significant difference was observed between methanol:water and CD-FBS, in which all calibrators are prepared (Fig. S2). Quality controls (QCs) were run with appropriate linear range of calibrators, 1–100 pg in sample for lower level QCs and 100–10,000 pg in sample for higher level QCs. The lowest limit of quantification (LLOQ) was defined as the level at which quality control standards fell within 20% of the expected value and had a CV (or precision) of 20% or less. Inter- and intra-day accuracy and precision values are shown in Table 1.
Table 1.
Inter-day and intra-day accuracy and precision for keto-androgen quantification.
| T | DHT | |||||
|---|---|---|---|---|---|---|
| Theoretical (pg) | 0.2 | 40 | 400 | 1 | 40 | 400 |
| Inter-day | ||||||
| Mean (pg) | 0.22 ± 0.04 | 43.3 ±3.2 | 448 ±16 | 0.96 ±0.18 | 42.7 ± 2.6 | 383 ±16 |
| Accuracy (%) | 109 | 108 | 110 | 96 | 107 | 96 |
| Precision (%) | 20 | 7 | 5 | 18 | 6 | 4 |
| Intra-day | ||||||
| Mean (pg) | 0.12 ±0.01 | 41.2 ±0.5 | 428 ±3 | 0.87 ±0.07 | 41.1 ±0.7 | 392 ±7 |
| Accuracy (%) | 105 | 103 | 107 | 87 | 103 | 98 |
| Precision (%) | 7 | 1 | 1 | 7 | 2 | 2 |
| Δ4-AD | EpiT | |||||
|
| ||||||
| Theoretical (pg) | 1 | 40 | 400 | 1 | 40 | 400 |
| Inter-day | ||||||
| Mean (pg) | 1.05 ±0.18 | 40.1 ± 2.8 | 440 ±21 | 0.98 ± 0.20 | 42.3 ±3.5 | 438 ±18 |
| Accuracy (%) | 105 | 100 | 110 | 98 | 106 | 110 |
| Precision (%) | 17 | 7 | 5 | 20 | 8 | 4 |
| Intra-day | ||||||
| Mean (pg) | 1.00 ±0.06 | 37.7 ±2.0 | 414±8 | 0.99 ± 0.08 | 39.8 ± 1.1 | 421 ±9 |
| Accuracy (%) | 100 | 94 | 103 | 99 | 100 | 105 |
| Precision (%) | 6 | 5 | 2 | 8 | 3 | 2 |
| A | DHEA | |||||
|
| ||||||
| Theoretical (pg) | 1 | 40 | 400 | 4 | 40 | 400 |
| Inter-day | ||||||
| Mean (pg) | 1.09 ±0.20 | 43.4 ± 6.5 | 393 ±31 | 3.86 ±0.62 | 40.2 ± 2.5 | 447 ± 25 |
| Accuracy (%) | 109 | 109 | 98 | 96 | 100 | 112 |
| Precision (%) | 19 | 15 | 8 | 16 | 6 | 6 |
| Intra-day | ||||||
| Mean (pg) | 0.88 ±0.05 | 40.2 ± 0.3 | 432 ±1 | 4.01 ±0.45 | 39.1 ± 1.6 | 428 ± 23 |
| Accuracy (%) | 88 | 101 | 108 | 100 | 98 | 107 |
| Precision (%) | 6 | 1 | 0 | 11 | 4 | 5 |
All data is presented as a mean with standard deviation in pictograms on column. All measurements were done with three replicates per day, three separate days. Δ4-androstene-3,17-dione (Δ4-AD, testosterone (T), dehydroepiandrosterone (DHEA), epitestosterone (EpiT), 5α-dihydrotestosterone (DHT) and androsterone (A) are presented here.
2.8. Validation of enzymatic hydrolysis for quantification of the conjugated keto-androgens
The enzymatic hydrolysis was validated using DHEA-S and DHEA-G standards as substrates followed by quantification of liberated DHEA by mass spectrometry. DHEA-S (1000 pg) was coincubated with 1 μL of CD-FBS and 30 U of H. pomatia sulfatase or Abalone entrails sulfatase in 200 mM sodium acetate, pH 5.0 with 200 pg of [13C3]-T/-DHT at 37 °C for 24 h. DHEA-G (1000 pg) was coincubated with 40 μL of CD-FBS and 90 U of E. coli β-glucuronidase in 200mM sodium acetate, pH 5.0 with 200pg of [13C3]-T/-DHT at 37° C for 24 h. After hydrolysis, samples were extracted, derivatized and analyzed as described above.
A subset of the samples was processed prior to the optimization of the glucuronidase reaction and this is the reason for the smaller ‘n’ for DHEA-G measurements compared to DHEA-S measurements in TAPS Arm 1 (Table 2). Further, androsterone glucuronide was detected in some of the samples, but at lower levels compared to DHEA-G. In future studies, a larger sampling volume of the aqueous fraction for the glucuronidase reaction will yield informative data on the levels of androsterone glucuronide.
Table 2.
Serum keto-androgen levels from patients enrolled in the Targeted Androgen Pathway Suppression (TAPS) clinical trial.
| Goserelin + bicalutamide | Goserelin + dutasteride | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before (n = 5) | After(n = 6) | p-Valuea | Before (n = 7) | After (n = 7) | p-Valuea | |
| Analyte (ng/dL) | ||||||
| A | 11 ± 4 | 11 ± 8 | NS | 23 ± 16 | 9 ± 8 | <0.05 |
| DHEA | 169 ± 86 | 201 ± 147 | NS | 211 ± 100 | 242 ± 203 | NS |
| Δ4-AD | 52 ± 13 | 72 ± 50 | NS | 93 ± 51 | 123 ± 124 | NS |
| T | 349 ± 138 | 17 ± 16 | <0.005 | 511 ± 186 | 21 ± 28 | <0.0005 |
| DHT | 22 ± 13 | 13 ± 11 | NS | 44 ± 25 | 4 ± 4 | <0.005 |
| DHEA-G | 507, 348 | ND, ND, 357 | 507 ± 143 | 557 ± 200 | NS | |
| DHEA-S | 127,410 ± 39,507 | 87,389 ± 48,433 | 291,494 ± 112,489 | 236,205 ± 61,637 | NS | |
| Goserelin + bicalutamide + dutasteride | Goserelin + dutasteride + ketoconazole | |||||
|
|
|
|||||
| Before (n = 10) | After (n= 10) | p-Valuea | Before (n= 11) | After (n= 13) | p-Valuea | |
|
| ||||||
| Analyte (ng/dL) | ||||||
| A | 24 ± 19 | 11 ± 9 | NS | 16 ± 6 | 6 ± 4 | <0.005 |
| DHEA | 126 ± 90 | 102 ± 65 | NS | 182 ± 79 | 181 ± 238 | NS |
| Δ4-AD | 76 ± 40 | 60 ± 27 | NS | 91 ± 43 | 72 ± 71 | NS |
| T | 508 ± 261 | 9 ± 3 | <0.0001 | 634 ± 520 | 8 ± 7 | <0.0001 |
| DHT | 49 ± 28 | 11 ± 6 | <0.005 | 54 ± 45 | 8 ± 6 | <0.0001 |
| DHEA-G | 5353 ± 12,985 | 2082 ± 3422 | NS | 2398 ± 3737 | 478 ± 136 | <0.05 |
| DHEA-S | 97,679 ± 81,579 | 68,398 ± 66,636 | NS | 169,826 ± 161,716 | 49,166 ± 53,517 | <0.05 |
All values represent the mean and standard deviation of the sample size denoted, except for the DHEA-G in the goserelin and bicalutamide arm where the individual values are listed. Patients enrolled in the clinical trial had localized advanced prostate cancer Gleason grade 3/4. Before and after 12 weeks of therapy serum samples were taken for androgen measurement. Men who would have met eligibility, but were already initiated on combined androgen blockade with an LHRH agonist and bicalutamide were accrued into arm 1 if they agreed to undergo prostatectomy within 3 months. Doses of drugs were as follows: (1) Men in arm 1 received 10.8 mg goserelin once and bicalutamide (50 mg daily). (2) Men in arm 2 received goserelin 10.8 mg once with high dose dutasteride 3.5 mg, once daily until prostatectomy. (3) Men in arm 3 received one dose of goserelin (10.8 mg), dutasteride (3.5 mg, once daily) and bicalutamide (50 mg, daily), which was initiated 7 days prior to goserelin and continued until prostatectomy. (4) Men in arm 4 received one dose of goserelin (10.8 mg), dutasteride (3.5 mg, once daily), bicalutamide (50 mg, daily) ketoconazole (200 mg, three times daily) and prednisone (5 mg, daily) until prostatectomy.
One-tailed Mann-Whitney with 95% confidence intervals was used to derive p-values comparing analyte levels before and after treatment.
2.9. Human subjects research
All serum samples from human prostate cancer patients were recruited to the TAPS clinical trial of neo-adjuvant androgen deprivation therapy (NCT00298155). Informed consent for the use of these specimens was obtained from the donor and all samples were de-identified to protect patient privacy. The human subject protocol was approved by the respective IRB at the participating institutions.
2.10. Statistical analysis
The Mann–Whitney test, one-tailed, with 95% confidence intervals was used to determine the p-values for TAPS data (Table 2), when comparing analyte concentrations before and after treatment. The null hypothesis is that therapy does not lower androgen levels. Statistics were done using the software, Prism (GraphPad, La Jolla, CA, USA).
3. Results
3.1. Separation and detection of unconjugated keto-androgens as Girard-T oximes
The detection and quantification of key keto-androgens that comprise the androgen metabolome poses several technical challenges. First, many of the androgens of interest are regio- or stereoisomers and have exactly the same molecular weight. For example, T, Epi-T, Adione and DHEA all have the same molecular weight. Similarly, DHT and A have the same molecular weight. Thus, it is critical that these androgens are chromatographically separated. Second, androgen metabolites do not ionize uniformly, necessitating their derivatization with Girard T to produce pre-ionized oxime derivatives. The LC conditions employed achieved baseline resolution of all androgen metabolites (Fig. 1). However, in some instances, namely T, a mixture of both syn- and anti-Girard T oxime derivatives was observed and separated, in which case the major product peak was used as the reference ion. The ratio of the major to minor product peaks is consistent from run to run and between samples. ESI mass spectra were produced on the Girard T oxime derivatives in the positive ion mode and subjected to collision induced dissociation. They gave intense positive ions corresponding to the intact pre-ionized molecule and a major product ion resulting from the characteristic neutral loss of 59, which was employed in the SRM transition as described above.
Fig. 1.
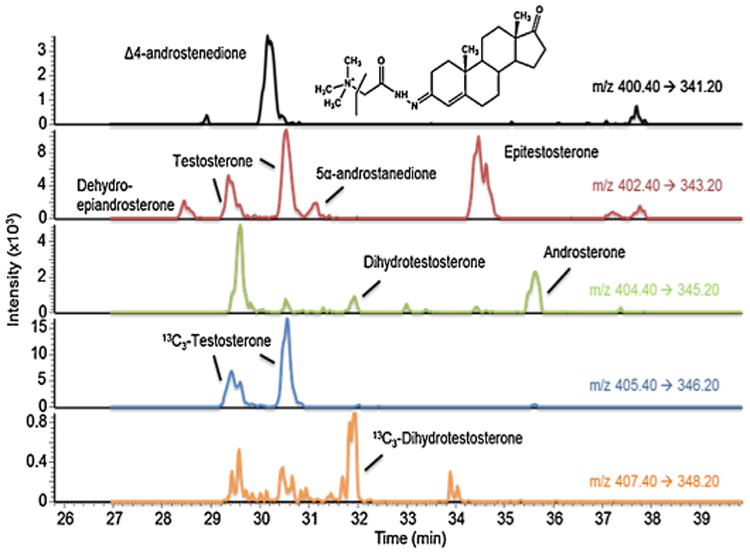
Representative ion-chromatograms of androgen standards 100 pg on column. Channels (from top to bottom) correspond to mass transition 400.4–341.2 for Δ4-AD Girard T derivative (the chemical structure of which is depicted in the figure); mass transition 402.4–343.2 for DHEA, T, Adione and Epi-T Girard T derivatives; mass transition 404.4–345.2 for DHT and A Girard T derivatives; mass transition 405.4-346.2 for 13C3-T and mass transition 407.4-348.2 for 13C3-DHT.
3.2. Calibration curves and quantification of unconjugated keto-androgens
Commercially available androgen standards were spiked with the stable isotope internal standards [13C3]-T (for T, Epi-T, Δ4-AD and DHEA, since they all contain either a Δ4- or Δ5-ene) or with [13C3]-DHT (for DHT, Adione and A, since they are 5α-reduced androgens). Linear regression analysis from 2 to 1000 pg in sample yielded regression coefficients of 0.999 for all analytes except for A, which gave a regression coefficient of 0.991 (Fig. 2). The signal to noise ratio of the peaks for the lowest level standard on the calibration curve was greater than 3 to 1. At the LLOQ, accuracy and precision values fell within 20%, Table 1.
Fig. 2.
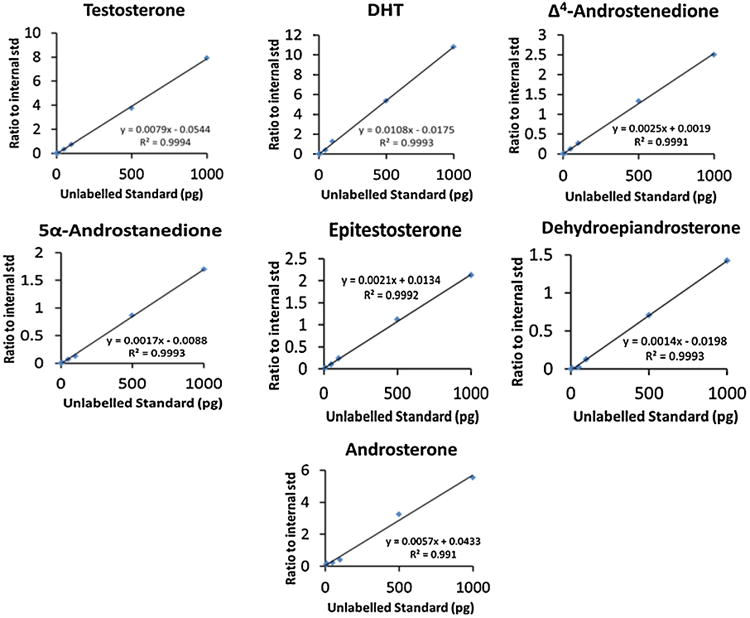
Calibration curves for quantifying each androgen metabolite using calibrators in the range from 2 to 1000 pg using CDS-FBS as a matrix. Formulas for each slope are displayed along with r2 values for each analyte.
3.3. Deconjugation and quantification of conjugated keto-androgens
The quantification of conjugated steroids was accomplished by way of enzymatic hydrolysis of the conjugates, followed by extraction, derivatization and analysis by LC/ESI/SRM/MS. Quantitative hydrolysis was ensured using commercially available DHEA-S and DHEA-G. Digestion with recombinant E. coli β-glucuronidase was straightforward and yielded quantitative recovery for 1000pg of DHEA-G (see Table S1 in Supplemental material). However, initial results with H. pomatia extract, widely used for its sulfatase activity, resulted in total conversion of DHEA-S to unconjugated Δ4-AD. This suggested that H. pomatia extract is contaminated with 3β-HSD or cholesterol oxidase activity and could confound studies, such as ours, in which the analytes of interest are 3β-HSD substrates. This problem has been previously addressed by addition of sodium ascorbate to the enzymatic hydrolysis reaction [25]. However, we found that this problem could be avoided by switching to Abalone entrails sulfatase. We conducted a DHEA-S digest with the Abalone entrails sulfatase, which yielded quantitative recovery of 1000 pg DHEA-S and no conversion to Δ4-AD (Table S1). However, Abalone entrails sulfatase contains some β-glucuronidase activity, as indicated by the manufacturer. We therefore employed a dual digest strategy to quantify the high levels of DHEA-S (μg/dL) and the lower levels of circulating glucuronide conjugates (ng/dL) in human serum. In one digest, only 1 μL of the 200 μL aqueous fraction from serum was taken for dual digest with Abalone entrails sulfatase/β-glucuronidase and E. coli β-glucuronidase. In a second digest, 40 μL of the aqueous fraction of the serum sample was taken for digest with E. coli β-glucuronidase alone. The second digest quantifies the glucuronide conjugates in the sample and was subtracted from the results of the first digest to yield the quantity of the sulfated conjugates in the sample. During post-MS data processing, the concentrations were adjusted for molecular weight and sampling volume.
3.4. Application of method to clinical specimens
The SID-LC/ESI/SRM/MS method was applied to the quantification of conjugated and unconjugated keto-androgens in human serum samples. The serum was collected from patients with localized high risk prostate cancer before and after their participation in a neoadjuvant TAPS clinical trial designed to maximally suppress androgen signaling. A complete data set is presented here to demonstrate the clinical utility of the assay and whether the profile of androgens differed based on the therapy administered. In patients from Arm 1 of the TAPS clinical trial, goserelin acetate and bicalutamide significantly reduced the mean concentration of serum T, but there were no significant reductions in the other analytes, but there is a modest increase in the mean serum concentration of unconjugated DHEA and Δ4-AD (Table 2 and Fig. S1A, B in Supplemental material). In patients from Arm 2 of the trial, goserelin acetate and dutasteride significantly reduced the mean amount of serum T, DHT and A, but again, a modest increase in was observed in DHEA and Δ4-AD (Table 2 and Fig. S1C, D). In patients from Arm 3 of the trial, administration of goserelin acetate, bicalutamide and dutasteride significantly reduced the mean amount of serum T and DHT (Table 2 and Fig. S1E, F). In patients undergoing total androgen pathway suppression, Arm 4 of the trial, goserelin acetate, bicalutamide, dutasteride and ketoconazole significantly reduced the mean amount of serum T, DHT, A, DHEA-G and DHEA-S (Table 2 and Fig. S1G, H). Percentage declines in these analytes in paired samples are shown in Fig. S3. A complete analysis of the TAPS serum and prostatic tissue androgens, and their correlation with clinical and pathological data has been submitted for publication elsewhere.
A robust bioanalytical method is a reproducible one. To that end, we have compared the keto-androgen measurements from the TAPS serum samples with measurements made by an independent analyst using a different, hydroxylamine derivatization strategy and different mass spectrometer. Despite differences in methodology (Girard T vs. hydroxylamine derivatization) and instrumentation, the percent changes in keto-androgen levels following drug treatment showed good concordance (Fig. S4) [32].
4. Discussion
We have described the development and validation of an SID-LC/ESI/SRM/MS method for the quantification of conjugated and unconjugated serum keto-androgens. Existing RIAs and ELISAs suffer from inaccuracy in quantification of androgens at low levels when compared to LC/ESI/SRM/MS methods [11,12]. Our method is robust down to a LLOQ of 0.2 pg on column for T (0.5 ng/dL in sample) and 1 pg on column for DHT (2.5 ng/dL in sample) Table 1. We have also validated an enzymatic hydrolysis protocol for the reliable quantification of androgen conjugates. We recapitulated earlier findings that H. pomatia extract leads to the conversion of DHEA-S to Δ4-AD [20,21]. By using a dual digest procedure of E. coli β-glucuronidase and Abalone entrails sulfatase/β-glucuronidase, we can accurately quantify sulfated and glucuronidated androgens. Measurements of DHEA-S could be performed on as little as 1 μL of serum.
The efficacy of abiraterone acetate in CRPC patients confirms the Geller hypothesis that these prostate tumors are still androgen dependent [26]. In CRPC, the tumor either acquires the ability to conduct de novo androgen biosynthesis from cholesterol or utilizes the circulating depot of adrenal androgens as precursors for T and DHT to drive tumor proliferation and metastasis [9,10,27–29]. The efficacy of ADT and the molecular mechanism of resistance to those therapies can be better assessed if sensitive methods such as those described here were applied to the patient. It is noteworthy that in the published clinical trials for treatment of CRPC patients with P450 17 inhibitors, rigorous SID-LC/ESI/SRM/MS methods were not used in their entirety and in some instances mixed methods were used including immunoassays for DHEA-S. Thus, the bioassays for assessing the clinical efficacy of new agents could be improved, and the concept that more effective suppression of the pool of DHEAS might provide additional benefit could then be tested with our method [30,31].
The clinical utility of the SID-LC/ESI/SRM/MS method for the quantification of keto-androgens was validated by its application to human serum samples from patients enrolled in the TAPS clinical trial. The data showed significant differences in the reduction of androgen metabolites when the four arms of the TAPS trial were compared. Especially notable is the ketoconazole arm of the clinical trial, in which we observed a significant reduction in the DHEA-S levels. Ketoconazole is a non-specific inhibitor of cytochrome P450s, including P450 17. However, due to its effects on downstream glucocorticoid and mineralocorticoid metabolism, these patients are also administered prednisone. In patients receiving ketoconazole and prednisone, unconjugated DHEA levels actually increased, suggesting a possible inhibition of adrenal steroid sulfotransferase activity due to off-target effects of one of the agents. These studies show that a significant depot of DHEA and the sulfate conjugate remain unless ketoconazole is included. It is noteworthy that in the ketoconazole arm, patients received ketoconazole and prednisone. Whereas ketoconazole can lower DHEA levels by inhibition of P450 17, prednisone could have a similar effect by feedback inhibition of the adrenal–hypothalamic–pituitary axis. Our studies also indicate that measurements of unconjugated DHEA to assess the clinical efficacy of new P45017 inhibitors may be unreliable since unconjugated DHEA represents 0.1–0.2% of the total DHEA. The TAPS trial was initiated before abiraterone acetate was available, it will therefore be interesting to compare the efficacy of ketoconazole with abiraterone, which is a much more potent and specific inhibitor of P450 17.
Other arms of the TAPS trial also deserve comment. All arms contained goserelin and T levels were reduced to castrate levels as expected for a GnRH agonist. The arms that contained dutasteride resulted in a significant decrease in serum 5α-reduced steroids, e.g. DHT and a downward trend in androsterone levels. When the arms containing goserelin and dutasteride are compared with the arms that contained goserelin, dutasteride and bicalutamide, the effect of these drug combinations were similar. These data would suggest that the addition of bicalutamide has no beneficial effect on serum ketoandrogens as might be expected with an AR antagonist. Only the arm that contained ketoconazole reduced DHEA-S. Our study shows that measurement of serum androgens provides insight into drug response.
With regard to the quantification of conjugated androgens, a major concern during the development of this method was that the levels would saturate the MS detector, as baseline levels of DHEA-S are at least three orders of magnitude higher than unconjugated androgens. Therefore, we took the precaution of only sampling a fraction of the aqueous fraction for analysis to maintain levels within the linear range: 1 μL for sulfatase and glucuronidase dual digestion and 40 μL for the glucuronidase digestion. A reduced sample volume ensured that the DHEA released from the serum samples, upon enzymatic de-conjugation, were within the linear range of the calibration curve and also did not saturate the MS detector. This allowed for quantification of the conjugated and unconjugated keto-androgens using a single MS protocol. Using this methodology, serum DHEA-S concentrations from the TAPS patients were found to be within the reference range of age-matched individuals [33]. Furthermore, the blinded study found the ketoconazole arm to be the only treatment group to confer a statistically significant (p < 0.05) reduction in serum DHEA-S. These findings and the exquisite sensitivity of the assay for DHEA conjugates suggest that our assay could have widespread utility.
Quantification of the androgen metabolome, which is our aim, may facilitate personalized medicine in CRPC patients and identify mechanisms of drug resistance. Androgen metabolite profiles could be used in conjunction with transcriptomic analysis of tumor biopsy material to identify the upregulation of steroidogenic pathways that may have adapted to ADT in a specific tumor and may ultimately inform the clinician as to which pharmacological inhibitors to use for a given patient.
Supplementary Material
Acknowledgments
We thank Dr. Clementina Mesaros for her assistance with LC-MS.
Abbreviations
- A
androsterone
- Adione
5α-androstane-3,17-dione
- Δ4-AD
Δ4-androstene-3,17-dione
- ADT
androgen deprivation therapy
- CD-FBS
charcoal dextran stripped fetal bovine serum
- CRPC
castration resistant prostate cancer
- CV
coefficient of variation
- DHEA
dehydroepiandrosterone
- DHEA-G
dehydroepiandrosterone glucuronide
- DHEA-S
dehydroepiandrosterone sulfate
- DHT
5α-dihydrotestosterone
- ELISA
enzyme-linked immunosorbent assay
- Epi-T
epitestosterone
- GnRH
gonadotropin releasing hormone
- HSD
hydroxysteroid dehydrogenase
- LC
liquid chromatography
- LLOQ
lower limit of quantification
- m/z
mass to charge ratio
- ND
not detected
- NS
not significant
- PSA
prostate specific antigen
- QC
quality control
- RIA
radioimmunoassay
- SID-LC/ESI/SRM/MS
stable isotope dilution liquid chromatography electrospray ionization selected reaction monitoring mass spectrometry
- T
testosterone
- TAPS
Targeted Androgen Pathway Suppression clinical trial
- TFA
trifluoroacetic acid
- UGT
UDP glucuronosyltransferase
Footnotes
Grants Supporting the Work: This work was supported by National Institutes of Health grants, P30-ES013508 and R01-CA90744 (and ARRA supplement) awarded to TMP; P01-CA163227-01A1 and a Prostate Cancer Foundation Challenge Award grant awarded to SB, PSB, BM and TMP; and an NCI Cancer Pharmacology postdoctoral fellowship to DT funded by 5R25-CA101871.
Clinical Trial Registration No: Maximal Suppression of the Androgen Axis in Clinically Localized Prostate Cancer (TAPS): NCT00298155.
Financial disclosure statement: Brett Marck, DL, DT, EAM, IAB, LT, MB, and RV have nothing to declare. BM received funding from Glaxo–Smith Kline, PN served as a consultant for Jannsen Pharmaceuticals, MET received consulting and research support from Medivation, JNJ and Tokai, SB consulted for Johnson & Johnson, Tokai and Medivation, and TMP served as a consultant for Tokai.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsbmb.2013.06.014.
References
- 1.Huggins C, Hodges CV. Prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–297. [Google Scholar]
- 2.Mostaghel EA, Plymate S. New hormonal therapies for castration-resistant prostate cancer. Endocrinology and Metabolism Clinics of North America. 2011;40(3):625–642. doi: 10.1016/j.ecl.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim MM, Hoffman KE, Levy LB, Frank SJ, Pugh TJ, Choi S, Nguyen QN, McGuire SE, Lee AK, Kuban DA. Improvement in prostate cancer survival over time: a 20-year analysis. Cancer Journal. 2012;18(1):1–8. doi: 10.1097/PPO.0b013e3182467419. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, Logo-thetis CJ, Kheoh T, Kilian C, Haqq CM, Molina A, Small EJ. Phase II study of abiraterone acetate in chemotherapy-naïve metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clinical Cancer Research. 2011;17(14):4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, Mainwaring P, Harland S, Goodman OB, Jr, Sternberg CN, Li JH, Kheoh T, Haqq CM, de Bone JS COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology. 2012;13(10):983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Main-waring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. New England Journal of Medicine. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends in Endocrinology & Metabolism. 2004;15(9):432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Research. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Research. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. Journal of Clinical Endocrinology and Metabolism. 2004;89(2):534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 12.Moal V, Mathieu E, Reynier P, Malthiery Y, Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clinica Chimica Acta. 2007;386(1–2):12–19. doi: 10.1016/j.cca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Wudy SA, Wachter UA, Homoki J, Teller WM, Shackleton CH. Androgen metabolism assessment by routine gas chromatography/mass spectrometry profiling of plasma steroids: Part 1, unconjugated steroids. Steroids. 1992;57(7):319–324. doi: 10.1016/0039-128x(92)90050-j. [DOI] [PubMed] [Google Scholar]
- 14.Titus M, Tomer KB. Androgen quantitation in prostate cancer tissue using liquid chromatography tandem mass spectrometry. Methods in Molecular Biology. 2011;776:47–57. doi: 10.1007/978-1-61779-243-4_3. [DOI] [PubMed] [Google Scholar]
- 15.Shackleton CHL, Chuang H, Kim J, de la Torre X, Segura J. Electrospray mass spectrometry of testosterone esters: potential for use in doping control. Steroids. 1997;62(7):523–529. doi: 10.1016/s0039-128x(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW. Ketosteroid profiling using Girard T derivatives and electrospray ionization tandem mass spectrometry: direct plasma analysis of androstene-dione, 17-hydroxyprogesterone and cortisol. Rapid Communications in Mass Spectrometry. 2005;19(2):193–200. doi: 10.1002/rcm.1771. [DOI] [PubMed] [Google Scholar]
- 17.Arai S, Miyashiro Y, Shibata Y, Tomaru Y, Kobayashi M, Honma S, Suzuki K. Effect of castration monotherapy on the levels of adrenal androgens in cancerous prostatic tissues. Steroids. 2010;79(3):301–308. doi: 10.1016/j.steroids.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita K, Miyashiro Y, Maekubo H, Okuyama M, Honma S, Takahashi M, Numazawa M. Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2009;74(12):920–926. doi: 10.1016/j.steroids.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Gomes RL, Meredith W, Snape CE, Sephton MA. Analysis of conjugated steroid androgens: deconjugation, derivatization and associated issues. Journal of Pharmaceutical and Biomedical Analysis. 2009;49(5):1133–1140. doi: 10.1016/j.jpba.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messeri G, Cugnetto G, Moneti G, Serio M. Helixpomatia induced conversion of some 3 β-hydroxysteroids. Journal of Steroid Biochemistry. 1984;20(3):793–796. doi: 10.1016/0022-4731(84)90088-8. [DOI] [PubMed] [Google Scholar]
- 21.Hauser B, Schulz D, Boesch C, Deschner T. Measuring urinary testosterone levels of the great apes – problems with enzymatic hydrolysis using Helix pomatia juice. General and Comparative Endocrinology. 2008;158(1):77–86. doi: 10.1016/j.ygcen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Ciccimaro E, Blair IA. Stable-isotope dilution LC–MS for quantitative biomarker analysis. Bioanalysis. 2010;2(2):311–341. doi: 10.4155/bio.09.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids. 2010;75(4–5):297–306. doi: 10.1016/j.steroids.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangiah K, Shah SJ, Vachani A, Ciccimaro E, Blair IA. Liquid chromatography/mass spectrometry of pre-ionized Girard P derivatives for quantifying estrone and its metabolites in serum from postmenopausal women. Rapid Communications in Mass Spectrometry. 2011;25(9):1297–1307. doi: 10.1002/rcm.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christakoudi S, Cowan DA, Taylor NF. Sodium ascorbate improves yield of urinary steroids during hydrolysis with Helix pomatia juice. Steroids. 73:309–319. doi: 10.1016/j.steroids.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Geller J. Prolonging survival in metastatic prostate cancer: the case for adrenal androgens – overview and summary of therapeutic controversies in prostatic cancer. Journal of Clinical Endocrinology and Metabolism. 1995;80(4):1074–1078. doi: 10.1210/jcem.80.4.7714070. [DOI] [PubMed] [Google Scholar]
- 27.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AB, Simon NI, Wang H, Chen S, Balk S. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Research. 2011;71(20):6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labrie F, Cusan L, Gomez J, Luu-The V, Candas B, Belanger A, Labrie C. Major impact of hormonal therapy in localized prostate cancer – death can already be an exception. Journal of Steroid Biochemistry and Molecular Biology. 2004;92(5):327–344. doi: 10.1016/j.jsbmb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Mohler JL, Titus MA, Bai S, Kennerley BJ, Lih FB, Tomer KB, Wilson EM. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Research. 2011;71(4):1486–1496. doi: 10.1158/0008-5472.CAN-10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. Journal of Clinical Oncology. 2010;28(9):1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attard G, Reid AHM, Yap TA, Raynaud R, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of Clinical Oncology. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 32.Roth MY, Page ST, Lin K, Anawalt BD, Matsumoto AM, Snyder CN, Marck BT, Bremner WJ, Amory JK. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. Journal of Clinical Endocrinology and Metabolism. 2010;95(8):3806–3813. doi: 10.1210/jc.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.nlm.nih.gov/medlineplus/ency/article/003717.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.