SUMMARY
Objective
This study evaluated the ability of benzalkonium chloride (BAC) to bind to dentine and to inhibit soluble recombinant MMPs and bound dentine matrix metalloproteinases (MMPs).
Methods
Dentine powder was prepared from extracted human molars. Half was left mineralized; the other half was completely demineralized. The binding of BAC to dentine powder was followed by measuring changes in the supernatant concentration using UV spectrometry. The inhibitory effects of BAC on rhMMP-2, -8 and -9 were followed using a commercially available in vitro proteolytic assay. Matrix-bound endogenous MMP-activity was evaluated in completely demineralized beams. Each beam was either dipped into BAC and then dropped into 1 mL of a complete medium (CM) or they were placed in 1 mL of CM containing BAC for 30 d. After 30 d, changes in the dry mass of the beams or in the hydroxyproline (HYP) content of hydrolyzates of the media were quantitated as indirect measures of matrix collagen hydrolysis by MMPs.
Results
Demineralized dentine powder took up 10-times more BAC than did mineralized powder. Water rinsing removed about 50% of the bound BAC, while rinsing with 0.5 M NaCl removed more than 90% of the bound BAC. BAC concentrations 0.5 wt% produced 100% inhibition of soluble recombinant MMP-2, -8 or -9, and inhibited matrix-bound MMPs between 55-66% when measured as mass loss or 76-81% when measured as solubilization of collagen peptide fragments.
Conclusions
BAC is effective at inhibiting both soluble recombinant MMPs and matrix-bound dentine MMPs in the absence of resins.
Keywords: MMPs, dentine, benzalkonium chloride, binding
1. Introduction
In adhesive dentistry, where there are usually no gaps between the adhesive restorative materials and tooth structure, antibacterial cavity disinfectants are usually applied after the acid-etching step in recognition of the fact that acid-etching often kills the majority of residual bacteria1. However, some manufacturers began adding acid-stable antimicrobial compounds to their acid-etchants. For instance, Bisco (Schaumburg, IL, USA) has added 1 wt% benzalkonium chloride (BAC) to their 37% phosphoric acid (i.e. ETCH-37 w/BAC, Bisco) for over a decade. They also manufacture 37% phosphoric acid gels without BAC (i.e. ETCH-37, Bisco).
Benzalkonium chloride (BAC) is a mixture of alkylbenzyl-dimethylammonium chlorides of various even-numbered alkyl chains (n = 8, 10, 12, 14, 16, 18). It is a nitrogenous cationic surface-acting agent containing a quaternary ammonium group. It is known to have three main uses; as a biocide (i.e. antimicrobial agent), as a cationic surfactant and as a phase transfer agent in the chemical industry. It is readily soluble in ethanol or acetone. Although the rate of dissolution in water is slow, aqueous solutions are easier to handle and are preferred. The margin of safety of BAC is very broad2.
Chan3 reported that dentine disks acid-etched with 37% phosphoric acid containing 1% BAC exhibited a zone of bacterial inhibition around the disk. Kanca4 investigated the bonding performance of One Step adhesive after acid-etching dentine or enamel with 37% phosphoric acid containing 1 wt% BAC (i.e. ETCH-37 w/BAC, Bisco) or plain 37% phosphoric acid (i.e. ETCH-37, Bisco). The etchant containing 1% BAC gave similar bond strengths to enamel and dentin compared to control etchant that showed phosphoric acid containing 1% BAC gave resin-dentin and resin-enamel bonds after 24 h bonds that were not significantly different from bonds made to dentine or enamel etched with 37% phosphoric acid free of BAC. He also showed that BAC did not affect immediate bond strengths with different etching or rinsing times.
Others have shown that 37% phosphoric acid containing 1% BAC had no adverse effect on bond strength of orthodontic brackets bonded to human enamel compared to BAC-free 37% phosphoric acid5.
The mean longevity of resin composite restorations has been reported to only be 5.7 yr, making it far less durable than amalgam restorations6. There are several reasons for the poor durability of resin-dentin bonds. Bonding formulations have become too hydrophilic7 in an attempt to facilitate the infiltration of adhesive comonomers into water-saturated dentine in etch-and-rinse adhesives. Although hydrophilic comonomer blends give high 24 h bond strength8, those bond strengths fall over time. Hydrophilic monomers create hydrophilic polymers that promote excessive water sorption into their matrices that plasticize the polymers and lower their mechanical properties9,10. A number of reports have documented large reductions in bond strength of resin-dentin bonds over 0.5-5 yr11-14. Ultrastructural studies have shown a loss of staining and cross-banding of collagen fibrils in the hybrid layers of etch-and-rinse adhesives over time15,16 indicating that the collagen fibrils are degrading. The degradation of acid-etched dentine was shown to be caused by the presence of endogenous MMPs in dentine that could be blocked by protease inhibitors or 0.2% chlorhexidine17. These MMPs include MMP-2 and -918-20.
Chlorhexidine (CHX), a potent cationic antimicrobial agent21, is also a potent inhibitor of MMP-2, -8 and -922. When used as a therapeutic primer, CHX can slow23-25 or stop16,26 reductions in resin-dentin bond strengths over time. It can also inhibit the degradation of collagen fibrils in the hybrid layer when examined by transmission electron microscopy16,27-29. As CHX is a cationic molecule like BAC, we speculated that BAC, another potent antimicrobial agent may also have anti-MMP activity.
The objectives of this work were to determine if BAC binds to dentine and if it can inhibit both soluble and matrix-bound MMPs. The test null hypotheses were that BAC does not bind to dentine and that it does not inhibit soluble or matrix-bound MMPs.
2. Materials and methods
2.1 Dentine powder
Mid-coronal dentine from unerupted extracted human third molars from 18-24 yr olds were obtained with informed consent using a protocol approved by the Human Assurance Committee of the Medical College of Georgia. The teeth were stored in 4°C water containing 0.02% sodium azide to prevent microbial growth, for no more than one month.
The occlusal enamel and deep dentine were removed using two parallel sections at right angles to the long axis of the tooth. The resulting 1-1.5 mm thick disks of mid-coronal dentine had their enamel periphery and mantle dentine removed with a highspeed handpiece and copious air-water spray. Using the same handpiece, the dentine disks were cut into four quarters. About 5 g of such dentine fragments were placed in a 25 mL capacity stainless steel screw-top jars with 4 stainless steel balls and immersed in liquid N2 for 10 min. After removal from the liquid N2, the cap was screwed on and the contents of the container was triturated at 30 Hz for 9 min in a Retsch ball-mill (Model MM201, Retsch, Newtown, PA, USA). The resulting dentine powder was passed through stacked sieves (Advantech Sonic Sifter, Advantech Mfg., New Berlin, MN, USA). The powder that was retained on a #400 mesh sieve gave an approximate particle size of <38 μm. This fraction was used for BAC-binding studies. Half of the powder remained mineralized while the other half was completely demineralized by adding 5 g aliquots of dentine powder to 25 mL volumes of 10 wt% phosphoric acid (pH 1.0) at 25°C with magnetic stirring for 8 hrs. At the end of this time, complete demineralization was confirmed radiographically.
After rinsing both mineralized and demineralized dentine powder with 0.05 M phosphate buffered saline (PBS), the powder was centrifuged at 3000 rpm for 30 min. The powder was then resuspended in 30 mL of distilled water to rinse away all traces of reaction products. Both mineralized and especially demineralized dentine powder releases products that give UV absorption at 225 nm, the wavelength used to quantitate BAC uptake30. The “background” absorbance of both types of the dentine powder was reduced to 0.09 absorbance units at 225 nm by rinsing the powder with distilled water every 8 h for 15 d. Then the powder was immediately tested for BAC binding experiments.
2.2 BAC binding experiments
Following the method of Blackburn et al.31, 0.05 g aliquots of rinsed dentine powder were transferred to microcentrifuge tubes containing 1 mL of standard solutions of BAC containing 0, 0.156, 0.313, 0.625, 1.25, 2.5, 5, 10, 20 or 40 mg/mL. The tubes were capped and tumbled for 30 min at 37°C to permit maximum binding of BAC by dentine powder. Preliminary studies indicated that there was no difference in binding between specimens tumbled for 1 or 30 min, so we used 30 min for convenience. Then the tubules were centrifuged at 3000 rpm for 10 min to separate the dentine powder from the supernatant. Three hundred μL of supernatant was removed from the tubules and placed in a UV transparent 96-well plate for measurement of its absorbance at 225 nm against water, in duplicate, in a Synergy HC plate reader. From the standard curve, the absorbance of the supernatant was converted to BAC concentration. There was no change in the concentration of standard solutions of BAC over time unless dentin powder was added to the solution. In the presence of dentine powder, the BAC concentration was always less than that of the standard BAC solution indicating that the dentine powder bound BAC. The BAC binding was expressed in mg BAC/g dry weight of dentine powder. This value was designated as the bound BAC at equilibrium. No further binding occurred at longer tumbling times. The binding of BAC to dentine powder was calculated as:
That is, the BAC concentration in standard solutions before exposure to powder (mg/mL) and minus the BAC concentration at equilibrium BAC concentration in solution (mg/mL) after exposure to powder, divided by the weight of the dry dentine powder (g).
2.3 BAC debinding experiments
After removing the unbound BAC in the supernatant solution from the dentine powder-bound BAC at each BAC concentration, the powder pellet was resuspended in either 1 mL of distilled water or 0.5 M NaCl, to determine how firmly BAC was bound. Sodium chloride solutions (0.5 M) are often used31 to test whether positively charged ligands like BAC can be displaced from the bound substrate by high concentrations of positively charged sodium ions. The tubes were capped and shaken vigorously at 4 Hz parallel to the long axis of the tubes for 1 min. Then the tubes were centrifuged at 3000 rpm to separate the powder from the supernatant. If the supernatant contained BAC, it indicated that water or 0.5 M NaCl could extract bound BAC from the powder.
2.4 Inhibition of soluble rhMMPs by BAC
To determine if BAC could inhibit human recombinant MMP-2, -8 and -9, we purchased these pure enzymes from AnaSpec Inc. (Fremont, CA, USA). The Sensolyte Generic MMP kit from AnaSpec Inc. (San Jose, CA, USA) contains a thiopeptide that is cleaved by MMPs to release a sulfhydryl group that reacts with 5,5’-dithiobis (2-nitrobenzoic acid) to produce a colored product (2-nitro-5-thiobenzoic acid) that is quantitated in a 96-well plate at 412 nm. By adding potential inhibitors at various concentrations, one can determine if they inhibit the MMPs over time. Because we used high MMP concentrations, we preincubated the inhibitor with the enzyme for 30 min before adding the substrate to prevent the enzymes from giving a burst of activity before being inhibited. All analyses were repeated 4 times.
The preliminary BAC inhibitory dose-response was performed using MMP-9. Once the effective BAC concentrations were determined, those higher concentrations were used to inhibit the activity of MMP-2 and -8.
2.5 Assay of endogenous matrix-bound MMPs in beams of demineralized dentine
A recently developed assay32 uses beams of human coronal dentine (1 × 2 × 6 mm) that are completely demineralized in 10 wt% phosphoric acid for 8 hrs at 25°C. Using a miniature 3-point flexure fixture, the stiffness of the beams was measured after demineralization. Mineralized dentine has a stiffness of about 17-18,000 MPa, while completely demineralized dentine has a stiffness of 2-3 MPa. By measuring the flexural stiffness of each beam after demineralization but before incubation, the beams were grouped into sets of 10 beams that have mean ± SD stiffnesses that are not significantly different from each other when compared by one-way ANOVA. The control beams were separately incubated in a complete medium (CM) containing 2.5 mM CaCl2, 0.05 mM ZnCl2, 5 mM HEPES buffer pH 7.4, and 0.3 mM NaN3 to prevent microbial growth over 30 d at 37°C.
Experimental beams were dipped in 0.5 or 1.0 wt% BAC for 10 or 60 s, blotted briefly to remove excess and incubated in the same medium. One experimental control group was incubated in 1 mL of CM alone or containing 1.0 wt% BAC. All beams were separately incubated at 37°C in a shaking (1 Hz) water bath (Precision Model 2873, Thermo Scientific, Marietta, OH, USA) in 1 mL of media in a polypropylene tube with a rubber “O” ring in the screw cap to prevent evaporation of water or inhibitors. The following variables were measured: decreases in dry mass at 4 wks and release of hydroxyproline from hydrolyzates of solubilized collagen peptides in the CM after 30 days of incubation.
2.6 Measurement of changes in dry mass of matrix over time
After incubating the beams for 30 d, the beams were rinsed with water for 10 min to remove all media salts. They were then placed in sealed containers of anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Company, Ltd., Xenia, OH, USA) overnight. The next day, the dry mass of each beam was measured using a microanalytical balance to the nearest 0.01 mg. Pilot experiments indicated that these procedures yielded constant dry masses. These dry masses were measured on each beam after 0 and 30 d. Each beam was rehydrated in water for 1 hr before being returned to its original medium for further incubation. Our previous work showed that this was sufficient time for complete re-expansion of the dried beams33. Loss of dry mass over time provides an indirect measurement of solubilization of matrix by endogenous MMP activity. The percent inhibition of endogenous proteases by BAC was calculated by subtracting the BAC-inhibited activity from the uninhibited controls, divided by the uninhibited activity × 100.
The second index of matrix degradation over time was obtained by measuring the amount of collagen peptide fragments that were solubilized over 30 d of incubation. That is, each beam was incubated in 1 mL of medium. Any collagen peptide fragments that accumulated in the medium over 30 d could be quantitated by removing 400 μL of the 1 mL and mixing it with an equal volume of concentrated HCl to yield a final acid concentration of 6 N HCl in glass ampules (Wheaton, Millville, NJ, USA). Ampules were automatically sealed using a Ampulmatic Ampule Sealer (Bioscience, Inc., Allentown, PA, USA). The contents were hydrolyzed to amino acids in an oil bath at 118°C for 18 hr. After cooling, the glass vials were opened via the prescored lines and placed in large glass dessicators containing NaOH pellets to trap HCl vapor and anhydrous calcium sulfate to trap water vapor. After 1 week in the vacuum dessicators, the dry contents of the vials were analyzed for hydroxyproline using the colorimetric assay of Jamall et al.34. After color development, the absorbance of all specimens and standards was measured at 558 nm in a Shimadzu UV/VIS spectrometer.
2.6 Statistics
Because the distribution of the data was not normal and the equality of variances was violated, the data were analyzed with the Kruskal-Wallis test using Dunn’s multiple comparison test at α = 0.05.
3. Results
3.1 Binding of BAC by demineralized human dentine powder
As the BAC in the medium increased, the amount of BAC bound to demineralized dentine powder rose rapidly and then reached a plateau at about 78 mg BAC/g dry dentine powder, indicating that saturation of dentine binding of BAC occurred (Fig. 1). When similar analyses were done with mineralized dentine powder, the BAC uptake was only one-tenth that of demineralized dentine powder (data not shown).
Fig. 1.
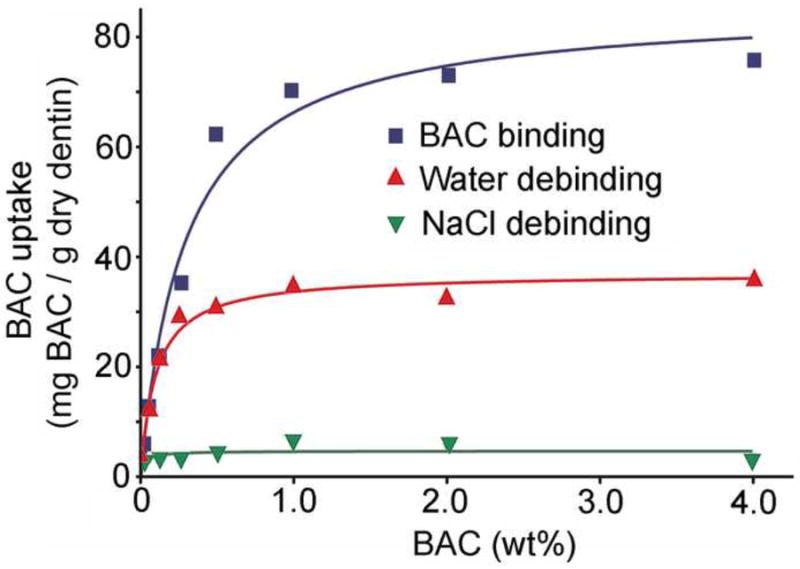
Uptake and debinding of BAC by demineralized dentine powder. Fifty mg of powder was incubated with 1 mL of increasing concentrations of aqueous solution of BAC. Thirty min after binding, the supernatant solution was removed and replaced with water (middle curve) or 0.5 M NaCl (lower curve) to determine how much debinding would occur. N = 4.
When the dentine powder-bound BAC was extracted with water, about 56% of the dentine-bound BAC was extracted from the powder. The water-extracted lower plateau was about 35 mg BAC/g dry weight of dentine powder. When dentine-bound BAC was extracted with 1 mL of 0.5 M NaCl, about 94% of the BAC was extracted from the dentine powder (Fig. 1). Qualitatively similar results were obtained with mineralized dentine but the absolute values were only one-tenth much (data not shown).
When soluble rhMMP-9 was incubated with increasing concentrations of aqueous BAC, the enzyme was increasingly inhibited (Fig. 2), reaching 100% inhibition at between 0.5-1 wt% BAC (Fig. 2). There was little inhibition of soluble MMPs at BAC concentrations of 0.05 wt% or lower (Fig. 2). All three MMPs were then incubated with higher BAC concentrations. BAC concentrations of 0.5-1.0 wt% inhibited all rhMMPs 100%. All three MMPs were inhibited to the same extent by similar BAC concentrations (Fig. 3). These simple assays prove the efficacy of BAC in inhibiting soluble MMPs. However, most MMPs in dentine are not soluble but are bound to the collagen matrix18-20.
Fig. 2.
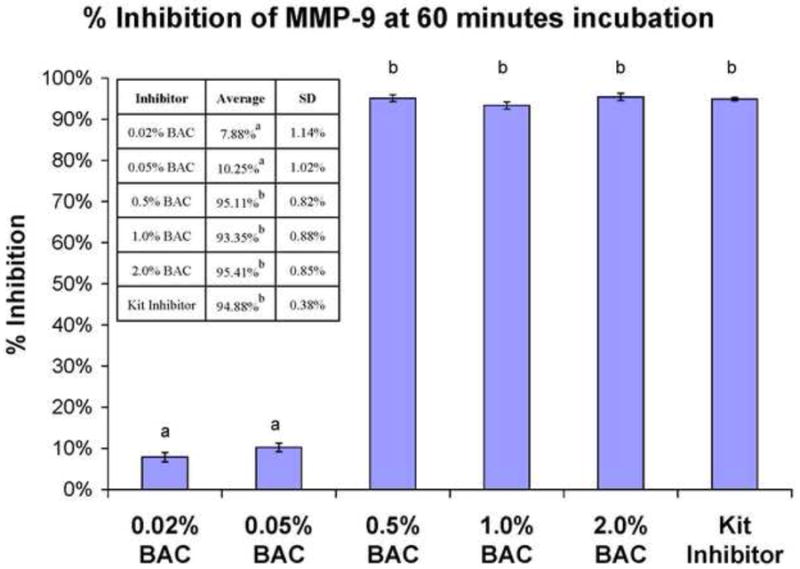
Percent inhibition of rhMMP-9 by increasing concentrations of BAC using the SenSolyte Generic MMP assay kit. Height of bars is the mean; brackets indicate ± SD. BAC concentration is given in weight %. Table insert gives digital data. N = 8. Groups identified by different letters are significantly different (p<0.05).
Fig. 3.
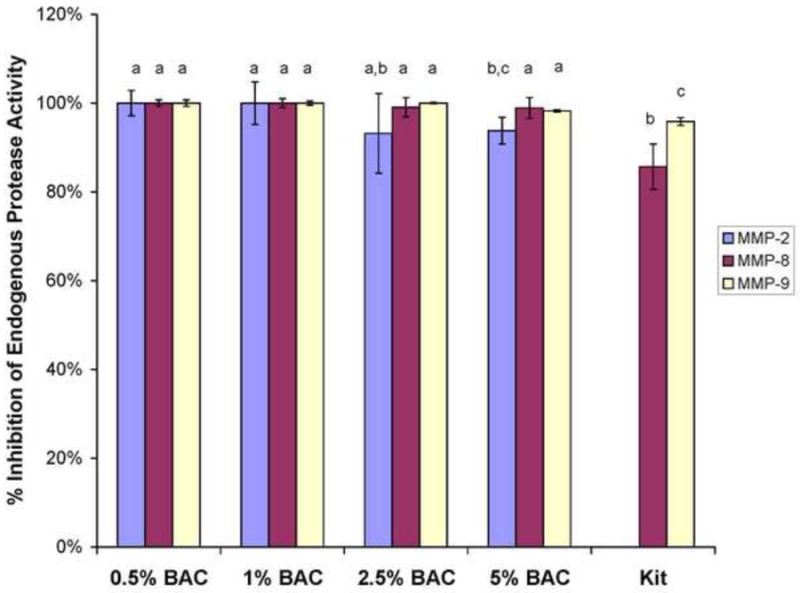
Percent inhibition of MMP-2, -8 and -9 by increasing concentrations of BAC using the SenSolyte generic MMP assay kit. Height of bars are the mean, brackets ± 1 SD, N = 5. Groups identified by different letters are significantly different (p<0.05). Kit = kit inhibitors for MMP-8 and -9.
When the inhibitory effects of BAC were evaluated using demineralized dentine beams containing bound MMPs, the control beams incubated in BAC-free medium lost 28% of the dry mass over 30 days (Fig. 4). When 1 wt% BAC was added to the incubation medium for 30 days, the loss of dry mass was only 4%, a significant reduction in mass loss (p<0.05). The percent inhibition of the endogenous proteases by the continuous presence of BAC was (28% - 4%) ÷ 28 × 100 = 85.7% (Fig. 4). Clinically, one can not continuously bathe collagen in BAC. Instead, with etch-and-rinse adhesives, one would etch dentine with 37% phosphoric acid etchant containing 1% BAC (ETCH-37 w/BAC, Bisco or use an acid-free of BAC, but treat the acid-etched dentine with a therapeutic primer/adhesive containing 1% BAC. In our demineralized beam model, we dipped water-saturated beam in BAC to allow the BAC to diffuse into the water-filled spaces between the collagen fibrils and within the dentinal tubules for 10 or 60 s. The BAC-dipped beams were then dropped into 1 mL of the BAC-free complete medium. When the demineralized dentine beams were dipped into 0.5 wt% BAC for 10 or 60 s and then dropped into the incubation medium, the percent inhibition fell significantly (p<0.05) from 85.7 to 55.4% and 57.1%, respectively (Fig. 4). When beams were dipped into 1.0 wt% BAC for 10 or 60 s, the percent inhibition was 63.6 and 66.1%, respectively (p<0.05 compared to the BAC-free complete medium (CM) (negative control).
Fig. 4.
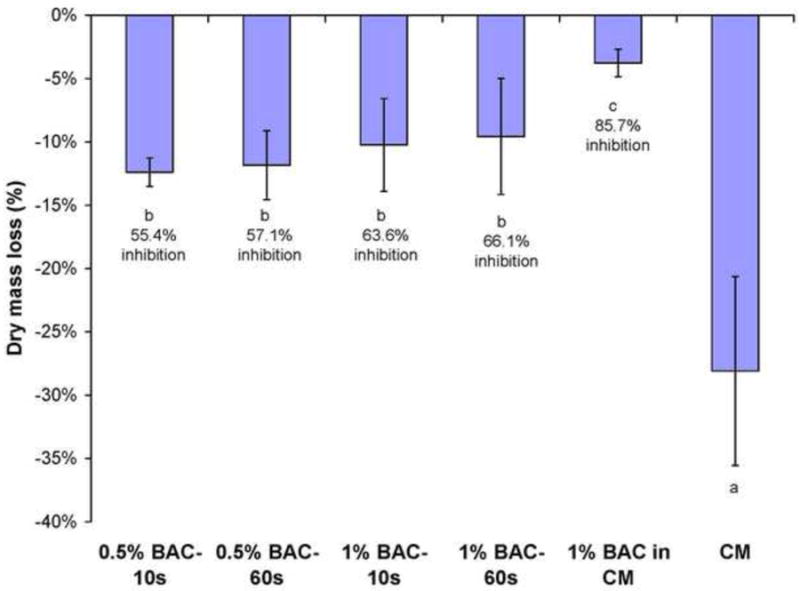
Percent loss of dry mass of demineralized beams incubated in complete medium (CM) for 30 d. Height of bar is mean; brackets indicate ± 1 SD. N = 10. The value for complete medium represents zero inhibition. The value for various experimental BAC treatments subtracted from the loss of dry mass of the control/loss of dry mass of control × 100 = percent inhibition of endogenous proteinases. Groups identified by different letters are statistically significantly differently (p<0.05).
Using a second independent evaluation of the inhibition of the endogenous protease activities of demineralized dentine beams, 400 μL aliquots of the incubation media were hydrolyzed in 6 N HCl to amino acids and the amount of hydroxyproline determined. Hydroxyproline (HYP) is a unique amino acid to collagens. The presence of HYP in the hydrolyzate indicates that collagen peptide fragments solubilized from the beams into the medium over time.
Control beams incubated in complete medium for 30 days solubilized sufficient collagen peptides to produce 34 μg of hydroxyproline per mg dry weight of dentine. Beams that were incubated in 1 wt% BAC in the media for 30 days released 6.25 μg of hydroxyproline/mg dry matrix, a significant reduction (p<0.05). This represents a percent inhibition of endogenous protease activity of 81.6% (Fig. 5).
Fig. 5.
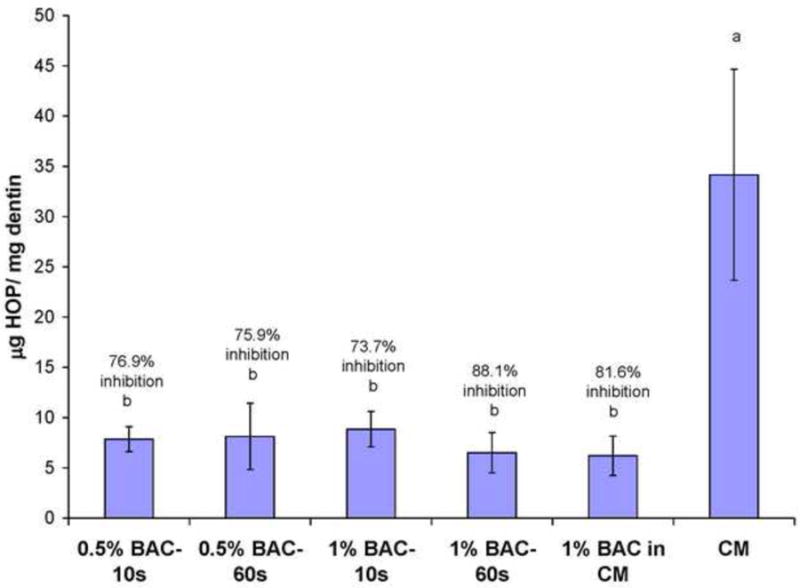
Hydroxyproline (HYP) release from solubilized collagen peptide fragments in incubation medium. Values are μg HYP/mg dry matrix. Beams incubated in CM without inhibitors released 34 ug HYP/mg dry matrix in 30 d. Heights of bars are the means; brackets ± 1 SD. N = 10. 1% BAC in CM represents the HYP released by beams incubated in CM containing 1 wt% BAC continuously for 30 days. 0.5 wt% BAC-10 s indicates the HYP release from beams dipped into 0.5 wt% BAC for 10 s, blotted and then dropped into 1 mL of AM. Percent inhibition was calculated as described in Fig. 3. Groups identified by different letters are significantly different (p<0.05).
When the experimental beams were dipped into 0.5 wt% BAC for 10 or 60 s, and then dropped into BAC-free media, the release of hydroxyproline was 7.86 and 8.21 μg/mg dry wt, respectively, giving significant (p<0.05) percent inhibitions of endogenous proteases in dentine of 76.9 and 75.9%, respectively (Fig. 5).
When the experimental beams were dipped into 1.0 wt% BAC for 10 or 60 s and then stored in BAC-free media for 30 days, their release of hydroxyproline was 8.93 and 6.43%, respectively for a percent inhibition of 73.7 and 81.1% (p<0.05), respectively (Fig. 5).
4. Discussion
The results of this work require rejection of the test null hypotheses that BAC does not bind to dentine and that it does not inhibit soluble or matrix bound MMPs. The results indicated that 0.5 wt% BAC or higher inhibited soluble MMPs 100%. When dentine beams were dipped in 0.5% BAC for 10 s, the BAC inhibited the endogenous proteases in dentine between 55-66%. This means that clinically, one needs only to treat dentine for 10 s to achieve significant inhibition of dentine proteases by BAC. This is because BAC binds to demineralized dentine (Fig. 1). The total binding curve in the top line represents BAC binding to collagen, plus BAC diffusing into the interstitial water in the dentinal tubules and interfibrillar spaces. That BAC is not truly bound but is dissolved or trapped in that water. It was easily removed by water rinsing as shown by the loss of about 50% of the “bound” BAC after a water rinse. The middle curve represents the amount of residual BAC that was electrostatically bound to collagen. When that electrostatically bound cationic BAC was mixed with 0.5 M NaCl, the sodium ions displaced the BAC from the negatively charged binding sites on collagen. These were probably the carboxylic acid residues of glutamic and aspartic acids.
When demineralized dentin beams were dipped into 1.0 wt% BAC for 10 or 60 s, we speculate that the BAC diffused into the unbound water trapped within the matrix. Careful measurement of the wet versus dry weight of such beams reveals that their water content is 68.5% ± 1.3% (Carrilho M, Pashley DH, unpublished observations). If BAC could reach diffusional equilibrium from the dipping solution into the water-filled interstitial spaces of the matrix in 60 s, then the beam could transfer that amount of BAC to the 1 mL incubation medium, where it would remain for 30 days. The question is what would be the medium BAC concentration. The volume of water in the 1 × 2 × 6 mm beam would be 12 mm3 × 0.685 = 8.22 mm3 or 8.22 μL. A BAC concentration of 1.0 wt% = 10 μg/μL. The 8.22 μL of interstitial water could hold 8.22 μL × 10 μg/μL = 82.2 μg of BAC. If all of this BAC diffused out of the beam into the 1 mL of incubation medium, it would give a final medium BAC concentration of 82.2 μg BAC/mL or 0.082 mg BAC/mL or 8.2 mg % or 0.0082 wt %. Such a concentration of BAC in the incubation medium would not inhibit MMPs (Fig. 2). Yet, dipping the demineralized dentin beams in 1.0 wt% BAC inhibited endogenous proteases 55-66%. This result suggests that when the beam was dipped into 1% BAC, it first diffused into the unbound water in the beam and then diffused from that water to bind to the collagen matrix (Fig. 1). Perhaps as much as half of that 82.2 μg of BAC was eluted into the incubation medium but achieved concentrations too low to inhibit MMPs. We speculate that at least half of the BAC that was taken up remained bound to the matrix and to the MMPs bound to the matrix, for at least 30 days. However, the concentration of BAC on the MMPs may have been below the concentration necessary to maximally inhibit their activity.
Clinically, if after binding BAC to collagen, no water rinse is employed, the BAC is likely to be trapped on collagen by infiltrating solvated comonomer mixtures that flow over the BAC bound to collagen. After polymerization, it is probable that this BAC will remain trapped under that resin for a very long time. Future in vitro and in vivo studies will have to be done to determine how long BAC remains bound to resin-bonded dentine.
The mechanism of antimicrobial action of BAC is thought to be due to disruption of intermolecular interactions. Enzymes are particularly susceptible to inactivation by BAC when its surfactant properties dissociates the tertiary 3-D structures of enzymes35,36. We speculate that this is how BAC inactivates matrix-bound MMPs. BAC first binds to collagen and then to MMPs bound to the collagen. It is clear that 0.5-1.0 wt% BAC inhibits MMPs 95-100%. In the current study, we showed that BAC-dipping treatment of dentine matrices irreversibly inactivates between 50-75% of matric-bound proteases. These values are lower than the 100% inhibition obtained using soluble MMPs.
The lower inhibition of matrix-bound proteases in the continuous presence of 1 wt% BAC (85.7%, Fig. 4, 81.6%, Fig. 5) compared to 100% inhibition of rhMMPs by 1% BAC (Fig. 3) may be due to the presence of active caseine cathepsins in the matrix37. If they are not inhibited by BAC, their continued activity would cause loss of dry mass and liberation of collagen peptide fragments even in the continued presence of 1% BAC that may have completely inhibited the matrix-bound MMPs. This continual hydrolysis of collagen by cathepsins would lower the calculated inhibition from a theoretical value of 100% to 85.7 or 81.6% (Figs. 4 and 5, respectively). Thus, the use of demineralized dentin beams as an endogenous protease model may underestimate the true inhibitory effect of BAC. Future experiments will include beams incubated with the cathepsin inhibitor E-64 alone compared to BAC plus E-64.
If one assumes that 1 wt% BAC inhibits matrix-bound MMPs 100%, then the contribution of caseine cathepsins to total matrix hydrolysis would be 100%-81.6% = 18.4%. In addition to collagen hydrolysis, cathepsins may activate MMPs38.
When demineralized dentin beams were dipped in BAC for 10 or 60 s, the percent inhibition of endogenous MMPs fell from 85.7% of dry mass to 55.4 to 66.1% depending on the BAC concentration. We speculate that this was due to the dilution of BAC in the beams by BAC-free incubation medium that lowered the matric BAC concentration below the optimal MMP inhibitory concentration.
In vivo, if one acid-etches dentine with 37% phosphoric acid containing 1 wt% BAC, as the mineral phase of the dentine matrix as being solubilized by acid, we would expect that BAC is simultaneously binding to the collagen and its associated noncollagenous proteins like MMPs. During subsequent water rinsing, about 50% of the BAC that was trapped in the water phase of the matrix would debind. If the protein-bound BAC was infiltrated with solvated primers or adhesives, the BAC should become sealed in place by the adhesive polymers after polymerization. Once sealed in place, it seems unlikely that any salivary ions could displace BAC from the MMPs-bound to resin-infiltrated collagen. This idea can be tested by comparing the long-term resin-dentine bond strengths of BAC-containing products to BAC-free products, both in vivo and in vitro in future experiments. Those studies will determine whether BAC can increase the durability of resin-dentin bonds.
5. Conclusion
Benzalkonium chloride (BAC) binds strongly to demineralized dentin. BAC concentrations of 0.5-1.0 wt% or more inhibit rhMMP-2, 8 and 9 100%. Similar BAC concentrations inhibit matrix-bound MMPs between 55-76% for a 30 d period. Thus, BAC must be added to a growing list of quaternary ammonium compounds that can inhibit MMPs in addition to their antibacterial effects.
Acknowledgments
The authors are grateful to Michelle Barnes for secretarial support. This work was supported, in part, by grant R01 DE015306-06 to DHP (PI) from the National Institute of Dental and Craniofacial Research and Grant # 8126472 (PI. Arzu Tezvergil-Mutluay) from Academy of Finland
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Komori PCP, Pashley DH, Tjäderhane L, Breschi L, Mazzoni A, de Goes MF, Wang L, Carrilho MR. Effect of 2% chlorhexidine digluconate on the bond strength to normal versus caries-affected dentin. Oper Dent. 2009;34:157–65. doi: 10.2341/08-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marple B, Roland P, Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: An overview of conflicting data and opinions. Otolaryngal Head Neck Surg. 2004;130:131–41. doi: 10.1016/j.otohns.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Chan D. Residual antimicrobial action of benzalkonium chloride-containing etchant. Journal of Dental Research. 1994;73:226. Abst 995. [Google Scholar]
- 4.Kanca J. One Step bond strength to enamel and dentin. American Journal of Dentistry. 1997;9:5–8. [PubMed] [Google Scholar]
- 5.Vincente A, Bravo LA. Influence of an etchant and a desensitizer containing benzalkonium chloride on shear bond strength of brackets. Journal of Adhesive Dentistry. 2008;10:205–9. [PubMed] [Google Scholar]
- 6.NIDCR Strategic Plan 2009-2013. http://www.nidcr.nih.gov/research/research/priorities/strategicplan. [PubMed]
- 7.Tay FR, Pashley DH. Have dentine adhesives become too hydrophilic. Journal of the Canadian Dental Association. 2003;69:726–31. [PubMed] [Google Scholar]
- 8.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effect of resin hydrophilicity on dentin bond strength. Journal of Dental Research. 2006;85:1016–21. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg F, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–59. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka K, Tagami J, Nishitani Y, Yoshiyama M, Carrilho M, Tay FR, Agee KA, Pashley DH. Effect of wet versus dry testing on the mechanical properties of hydrophilic primer polymers. European Journal of Oral Sciences. 2007;115:1–7. doi: 10.1111/j.1600-0722.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 11.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, Pashley DH. Durability of resin-dentin bonds. Journal of Adhesive Dentistry. 1999;1:211–18. [PubMed] [Google Scholar]
- 12.DeMunck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G. Four year water degradation of total-etch adhesives bonded to dentin. Journal of Dental Research. 2003;82:136–40. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong SR, Vargas MA, Chung L, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Operative Dentistry. 2004;29:705–12. [PubMed] [Google Scholar]
- 14.Hosaka K, Nakajima M, Takahashi M, Itoh S, Ikeda M, Tagami J, Pashley DH. Relationship between mechanical properties of one-step, self-etch adhesives and water sorption. Dental Materials. 2010;26:360–67. doi: 10.1016/j.dental.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Godoy F, Tay FR, Pashley DH, Feitzer A, Tjäderhane L, Pashley EL. Degradation of resin-bonded dentin after 3 years of storage. American Journal of Dentistry. 2007;19:109–13. [PubMed] [Google Scholar]
- 16.Carrilho MRO, Geraldeli S, Tay F, deGoes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley DH. In vivo preservation of the hybrid layer by chlorhexidine. Journal of Dental Research. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 17.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. Journal of Dental Research. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoni A, Mannello F, Tay FR, Toni GAM, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. Journal of Dental Research. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 19.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52:121–27. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Santos J, Carrilho M, Tervahartiala T, Sorsa T, Breschi L, Mazzoni A, Pashley DH, Tay FR, Ferraz C, Tjäderhane L. Determination of matrix metalloproteinases in human radicular dentin. Journal of Endodontics. 2009;35:686–89. doi: 10.1016/j.joen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clinical and Diagnotic Laboratory Immunology. 1999;6:437–39. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrilho MRO, Carvalho RM, de Goes MF, di Hipolito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine partially preserves long-term dentin bond strength in vitro. J Dent Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breschi L, Cammelli F, Visintini E, Mazzoni A, Vita F, Carrilho M, Cadenaro M, Foulger S, Tay FR, Pashley DH, Di Lenarda R. Influence of chlorhexidine concentrations on the durability of etch-and-rinse dentin bonds: A 12-month in vitro study. Journal of Adhesive Dentistry. 2009;11:191–98. [PMC free article] [PubMed] [Google Scholar]
- 25.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Tay FR, Dorigo EDS, Pashley DH. Chlorhexidine stabilizes the adhesive interface: A 2-yr in vitro study. Dental Materials. 2010;26:320–25. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Tan J, Chen L, Li D, Tan Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. Journal of Dentistry. 2009;37:807–12. doi: 10.1016/j.jdent.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical breakdown of dentin hybrid layers in vivo. Journal of Dental Research. 2005;84:741–46. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 28.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Operative Dentistry. 2007;32:107–11. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 29.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, Pashley DH. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Operative Dentistry. 2009;34:381–85. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjäderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–78. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackburn RS, Harvey A, Kettle L, Manian AP, Payne JD, Russell SJ. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. Journal of Physical Chemistry B. 2007;111:8775–84. doi: 10.1021/jp070856r. [DOI] [PubMed] [Google Scholar]
- 32.Carrilho MRO, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin stiffness associated with solubilization of collagen. Journal of Biomedical Materials Research B: Applied Biomaterials. 2009;90B:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol infiltration and solvent evaporation. Journal of Biomedical Materials Research A. 2006;79A:349–58. doi: 10.1002/jbm.a.30752. [DOI] [PubMed] [Google Scholar]
- 34.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroproline in biological tissues. Analytical Biochemistry. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 35.Feldbau E, Swabe C. Selective inhibition of serine proteases by alkyldimethylbenzylammonium chloride. Biochemistry. 1971;10:2131–38. doi: 10.1021/bi00787a027. [DOI] [PubMed] [Google Scholar]
- 36.Schwabe C. Peptide hydrolases in mammalian connective tissue. II. Leucine aminopeptidase Purification and evidence for subunit structure. Biochemistry. 1969;8:783–94. doi: 10.1021/bi00831a005. [DOI] [PubMed] [Google Scholar]
- 37.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–60. [PubMed] [Google Scholar]


