Abstract
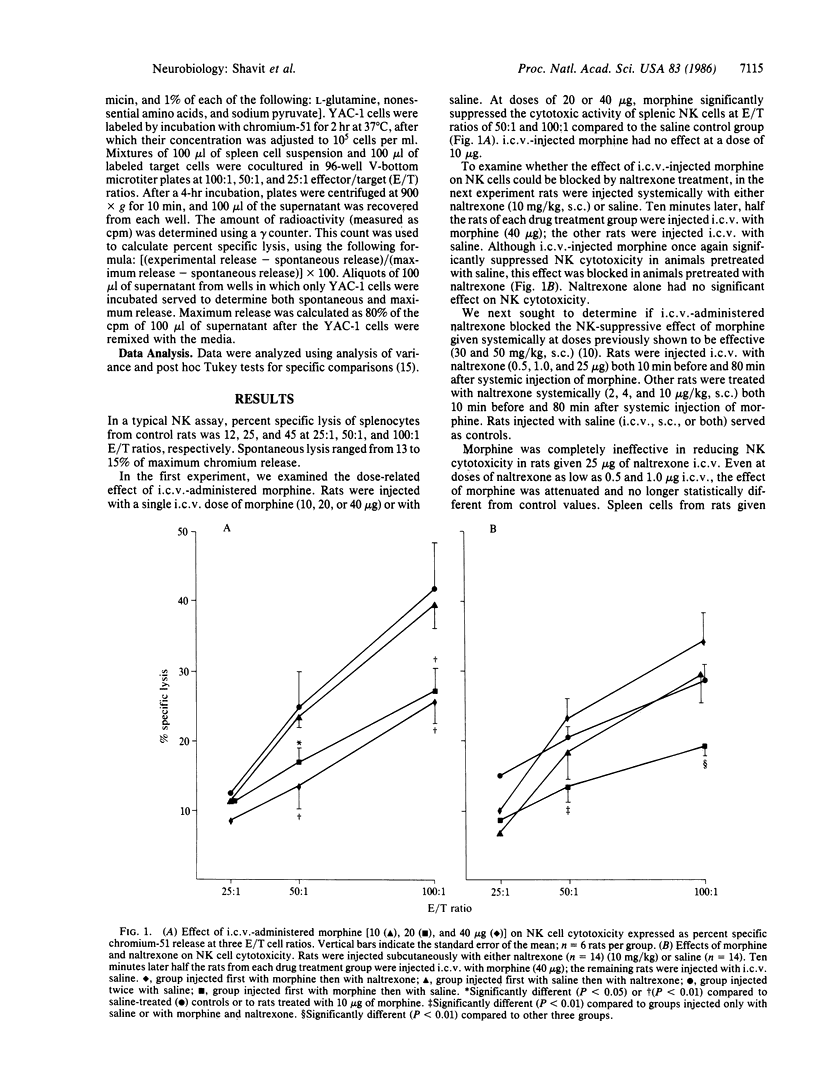
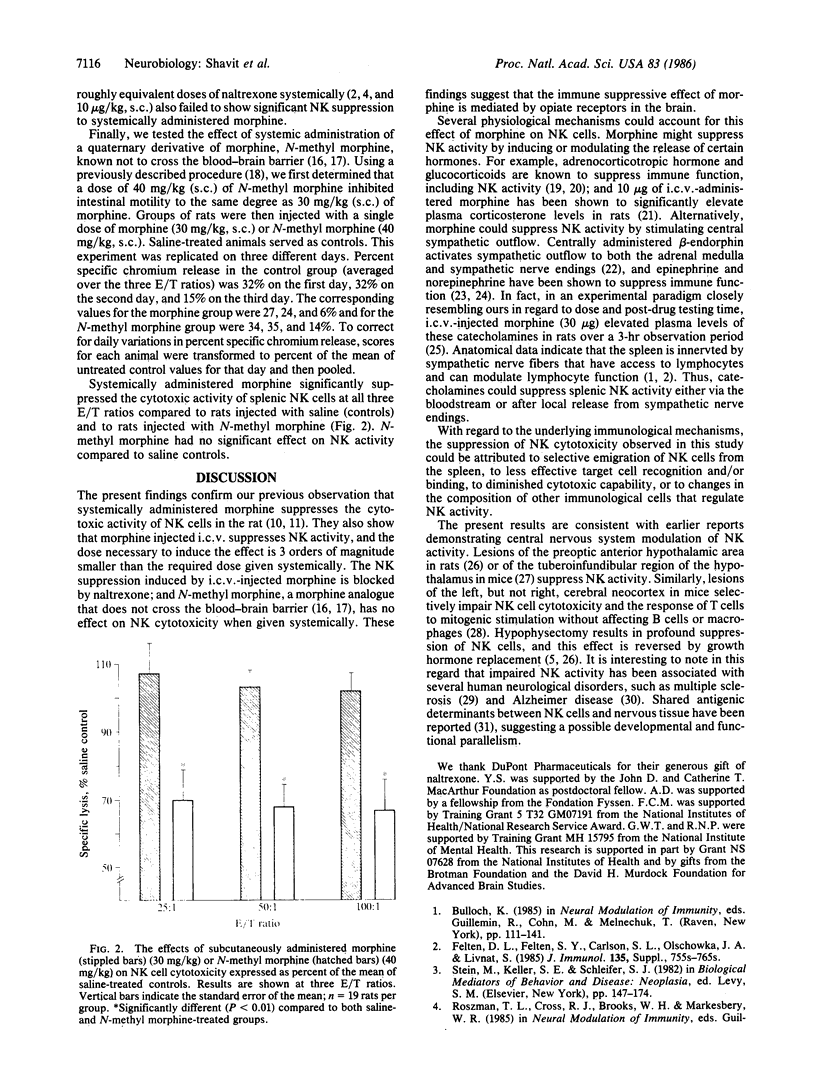
We previously reported that a single systemic injection of a high dose of morphine (greater than or equal to 20 mg/kg) transiently suppresses splenic natural killer cell cytotoxicity in rats. The present study examined the possibility that the immune-suppressive effect of morphine is mediated by opiate receptors in the brain. Supporting this hypothesis, we found that morphine (20 or 40 micrograms) injected into the lateral ventricle suppressed natural killer cell activity to the same degree as a systemic dose higher by three orders of magnitude. This effect was blocked by an opiate antagonist, naltrexone. Natural killer cell activity was unaffected by systemic administration of N-methyl morphine, a morphine analogue that does not cross the blood-brain barrier. These data implicate opiate receptors in the brain in morphine-induced suppression of natural killer cell cytotoxicity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benczur M., Petrányl G. G., Pálffy G., Varga M., Tálas M., Kotsy B., Földes I., Hollán S. R. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol. 1980 Mar;39(3):657–662. [PMC free article] [PubMed] [Google Scholar]
- Conway E. L., Brown M. J., Dollery C. T. Plasma catecholamine and cardiovascular responses to morphine and D-ala2-d-leu5-enkephalin in conscious rats. Arch Int Pharmacodyn Ther. 1983 Oct;265(2):244–258. [PubMed] [Google Scholar]
- Cox W. I., Holbrook N. J., Grasso R. J., Specter S., Friedman H. Suppression of the natural killer cell activity of murine spleen cell cultures by dexamethasone (41489). Proc Soc Exp Biol Med. 1982 Nov;171(2):146–150. doi: 10.3181/00379727-171-41489. [DOI] [PubMed] [Google Scholar]
- Crary B., Borysenko M., Sutherland D. C., Kutz I., Borysenko J. Z., Benson H. Decrease in mitogen responsiveness of mononuclear cells from peripheral blood after epinephrine administration in humans. J Immunol. 1983 Feb;130(2):694–697. [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions: neuromodulation of natural killer activity by lesioning of the anterior hypothalamus. Immunology. 1984 Feb;51(2):399–405. [PMC free article] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Carlson S. L., Olschowka J. A., Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985 Aug;135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- Forni G., Bindoni M., Santoni A., Belluardo N., Marchese A. E., Giovarelli M. Radiofrequency destruction of the tuberoinfundibular region of hypothalamus permanently abrogates NK cell activity in mice. Nature. 1983 Nov 10;306(5939):181–184. doi: 10.1038/306181a0. [DOI] [PubMed] [Google Scholar]
- Foster R. S., Jenden D. J., Lomax P. A comparison of the pharmacologic effects of morphine and N-methyl morphine. J Pharmacol Exp Ther. 1967 Jul;157(1):185–195. [PubMed] [Google Scholar]
- Haracz J. L., Bloom A. S., Wang R. I., Tseng L. F. Effect of intraventricular beta-endorphin and morphine on hypothalamic-pituitary-adrenal activity and the release of pituitary beta-endorphin. Neuroendocrinology. 1981 Sep;33(3):170–175. doi: 10.1159/000123224. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hochman P. S., Cudkowicz G. Suppression of natural cytotoxicity by spleen cells of hydrocortisone-treated mice. J Immunol. 1979 Sep;123(3):968–976. [PubMed] [Google Scholar]
- Parolaro D., Sala M., Gori E. Effect of intracerebroventricular administration of morphine upon intestinal motility in rat and its antagonism with naloxone. Eur J Pharmacol. 1977 Dec 15;46(4):329–338. doi: 10.1016/0014-2999(77)90227-8. [DOI] [PubMed] [Google Scholar]
- Saxena Q. B., Saxena R. K., Adler W. H. Regulation of natural killer activity in vivo. III. Effect of hypophysectomy and growth hormone treatment on the natural killer activity of the mouse spleen cell population. Int Arch Allergy Appl Immunol. 1982;67(2):169–174. [PubMed] [Google Scholar]
- Schuller-Petrovic S., Gebhart W., Lassmann H., Rumpold H., Kraft D. A shared antigenic determinant between natural killer cells and nervous tissue. Nature. 1983 Nov 10;306(5939):179–181. doi: 10.1038/306179a0. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Lewis J. W., Terman G. W., Gale R. P., Liebeskind J. C. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984 Jan 13;223(4632):188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Terman G. W., Lewis J. W., Zane C. J., Gale R. P., Liebeskind J. C. Effects of footshock stress and morphine on natural killer lymphocytes in rats: studies of tolerance and cross-tolerance. Brain Res. 1986 May 7;372(2):382–385. doi: 10.1016/0006-8993(86)91149-2. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Terman G. W., Martin F. C., Lewis J. W., Liebeskind J. C., Gale R. P. Stress, opioid peptides, the immune system, and cancer. J Immunol. 1985 Aug;135(2 Suppl):834s–837s. [PubMed] [Google Scholar]
- Sklar L. S., Anisman H. Stress and cancer. Psychol Bull. 1981 May;89(3):369–406. [PubMed] [Google Scholar]
- Smith T. W., Buchan P., Parsons D. N., Wilkinson S. Peripheral antinociceptive effects of N-methyl morphine. Life Sci. 1982 Sep 20;31(12-13):1205–1208. doi: 10.1016/0024-3205(82)90343-5. [DOI] [PubMed] [Google Scholar]
- Solomon G. F., Amkraut A. A. Psychoneuroendocrinological effects on the immune response. Annu Rev Microbiol. 1981;35:155–184. doi: 10.1146/annurev.mi.35.100181.001103. [DOI] [PubMed] [Google Scholar]
- Van Loon G. R., Appel N. M., Ho D. Beta-endorphin-induced increases in plasma epinephrine, norepinephrine and dopamine in rats: inhibition of adrenomedullary response by intracerebral somatostatin. Brain Res. 1981 May 11;212(1):207–214. doi: 10.1016/0006-8993(81)90053-6. [DOI] [PubMed] [Google Scholar]