Abstract
Traumatic injury or disease of the spinal cord and brain elicits multiple cellular and biochemical reactions that together cause or are associated with neuropathology. Specifically, injury or disease elicits acute infiltration and activation of immune cells, death of neurons and glia, mitochondrial dysfunction, and the secretion of substrates that inhibit axon regeneration. In some diseases, inflammation is chronic or non-resolving. Ligands that target PPARs (peroxisome proliferator-activated receptors), a group of ligand-activated transcription factors, are promising therapeutics for neurologic disease and CNS injury because their activation affects many, if not all, of these interrelated pathologic mechanisms. PPAR activation can simultaneously weaken or reprogram the immune response, stimulate metabolic and mitochondrial function, promote axon growth and induce progenitor cells to differentiate into myelinating oligodendrocytes. PPAR activation has beneficial effects in many pre-clinical models of neurodegenerative diseases and CNS injury; however, the mechanisms through which PPARs exert these effects have yet to be fully elucidated. In this review we discuss current literature supporting the role of PPAR activation as a therapeutic target for treating traumatic injury and degenerative diseases of the CNS.
Keywords: Alzheimer’s disease, astrocyte, experimental autoimmune encephalomyelitis (EAE), macrophage, multiple sclerosis, spinal cord injury
Abbreviations: ALS, amyotrophic lateral sclerosis; Arg1, Arginase 1; BMP, bone morphogenetic protein; 15d-PGJ2, 15-deoxy-Δ-12,14-prostaglandin J-2; EAE, experimental autoimmune encephalomyelitis; GR, glucocorticoid receptor; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MS, multiple sclerosis; NF-κB, nuclear factor κB; NGF, nerve growth factor; OPC, oligodendrocyte precursor cell; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; SCI, spinal cord injury; SHP-2, Src homology region 2-containing protein tyrosine phosphatase-2; TBI, traumatic brain injury; Th1, T helper type 1; TNFα, tumour necrosis factor α; UCP, uncoupling protein
INTRODUCTION
Neurodegenerative diseases [e.g. MS (multiple sclerosis), ALS (amyotrophic lateral sclerosis), and Alzheimer's disease] and traumatic or ischemic CNS injuries [e.g. SCI (spinal cord injury), stroke, TBI (traumatic brain injury)] all elicit neuroinflammatory cascades. Specifically, the collective effects of activated glia, inflammatory cytokines and chemokines, a compromised blood–brain/spinal cord barrier, and infiltrating leukocytes exacerbate axon damage and demyelination, mitochondrial dysfunction, and glial scar formation. The result is a tissue environment that favors cell death and inhibits mechanisms of endogenous repair (Norenberg et al., 2004; Fleming et al., 2006; Popovich and Longbrake, 2008). Since mature CNS neurons are post-mitotic and regenerate poorly, the destructive effects of trauma, disease and neuroinflammation render affected individuals permanently disabled.
PPARs (peroxisome proliferator-activated receptors) comprise a family of ligand-activated transcription factors that play a vital role in cellular processes such as cell differentiation and metabolism (Kersten et al., 2000; Bensinger and Tontonoz, 2008). They also are potent regulators of macrophage differentiation that, when activated, can attenuate pathology associated with various chronic neuroinflammatory diseases (Odegaard et al., 2008; Bouhlel et al., 2009; Chawla, 2010;). PPARs exist as three different isoforms, α, δ (also called β), and γ, and all are expressed by microglia, astrocytes, neurons and oligodendrocytes, albeit at different levels (Kliewer et al., 1994).
PPARs form obligate heterodimers with RXRs (retinoid X receptors), and ligand binding to either PPAR or RXR initiates gene transcription. PPAR–RXR heterodimers are termed ‘permissive’ because ligation of either component of the heterodimer can induce transcriptional activation of the receptor complex. This means that PPAR activation can be induced to varying degrees by ligands activating RXRs. Since the precise mechanisms by which RXR ligands affect PPAR signaling are not yet defined, it is important to note that RXR activation may not be identical with direct PPAR activation. Currently, FDA-approved agonists of PPARα and PPARγ are used to treat hyperlipidemia and Type II diabetes, respectively (Table 1). These same drugs are also ideal candidates for translational research in models of CNS trauma and disease (Lehmann et al., 1995; Staels et al., 1998).
Table 1. Commonly used PPAR agonists for CNS studies.
This table is a list of commonly utilized PPAR agonists that have been tested experimentally to attenuate neurological disease/injury. Instances of clinical use in humans are restricted to the following neurological conditions: Alzheimer's disease, multiple sclerosis, stroke, amyotrophic lateral sclerosis and Parkinson's disease.
| Drug (other names) | Receptor target | FDA approved | Prescribed treatment | Toxicity/side effects | Clinical trial for CNS disease/trauma | References |
|---|---|---|---|---|---|---|
| Pioglitazone (Actos) | PPARγ | Yes | Type 2 diabetes | Associated with bladder tumors. Weight gain | Multiple sclerosis, Alzheimer's disease, stroke amyotrophic lateral sclerosis Parkinson's disease | Wilcox et al., 2007; Hanyu et al., 2009; Kaiser et al., 2009; Shukla et al., 2010; Geldmacher et al., 2011; Sato et al., 2011; Dupuis et al., 2012 |
| Rosiglitazone (Avandia) | PPARγ | Yes | Type 2 diabetes | Increased cardiovascular risk | Alzhiemer's disease | Watson et al., 2005; Kume et al., 2012 |
| Troglitazone (Rezulin, Resulin, Romozin, Noscal) | PPARγ | Formerly, taken off the market by FDA | Type 2 diabetes | Liver toxicity | N/A | |
| 15-deoxy-Delta(12,14)-prostaglandin J(2) | PPARγ | No | N/A | |||
| Telmisartan (Micardis) | PPARγ/PPARδ | Yes | Hypertension | Tacy/bradycardia, edema, hypotension | Alzheimer's disease, stroke | Diener et al., 2008; Yusuf et al., 2008; Bath et al., 2009; Kume et al., 2012 |
| Gemfibrozil (Lopid, Jezil, Gen-Fibro) | PPARα | Yes | Hyperlipidemia | Gastrointestinal distress, musculoskeletal pain, gallstones, increased risk of cancer, reduced blood K+ levels | N/A | |
| Fenofibrate (Tricor, Trilipix) | PPARα | Yes | Hyperlipidemia | Gastrointestinal distress, skin reactions, severly reduced high-density lipoprotein levels | N/A |
In vivo studies document that agonists for different PPAR isoforms typically improve outcomes in pre-clinical models of CNS injury or disease. For instance, in EAE (experimental autoimmune encephalomyelitis, an animal model of MS), several PPAR agonists have proven effective in delaying the onset and progression of disease (Niino et al., 2001; Diab et al., 2002, 2004; Feinstein et al., 2002; Gocke et al., 2009). PPARδ and PPARγ agonists also have shown benefits in experimental models of SCI, TBI and stroke (McTigue et al., 2007; Yi et al., 2008; Allahtavakoli et al., 2009; Sauerbeck et al., 2011; Thal et al., 2011; Villapol et al., 2012). Activation of these receptors attenuated inflammation and apoptosis, reduced lesion size and improved functional recovery; they also promoted oligodendrogenesis and differentiation (McTigue et al., 2007; Park et al., 2007; Yi et al., 2008; Allahtavakoli et al., 2009; Paterniti et al., 2010; Meng et al., 2011; Sauerbeck et al., 2011; Thal et al., 2011; Villapol et al., 2012). Neuropathology was exacerbated after CNS injury in mice deficient in PPARα, suggesting that endogenous PPAR ligands may limit neuropathology (Genovese et al., 2005). PPARα activation facilitated recovery after TBI, but surprisingly had no effect or worsened recovery after SCI (Besson et al., 2005; Chen et al., 2007, 2008; Almad et al., 2011) suggesting that PPAR activation may not be uniformly beneficial.
In animal models of ALS, a disease that causes paralysis and eventual death due to loss of upper and lower motor neurons, PPARγ agonists extend survival and attenuate motor neuron loss (Kiaei et al., 2005; Shibata et al., 2008). However, in a phase II double-blind controlled clinical trial, the PPARγ agonist pioglitazone did not increase survival in ALS patients (Dupuis et al., 2012).
Activation of PPARs also yielded conflicting data in rodent models of Alzheimer's disease and in human subjects. For example, in some, but not all studies, PPAR activation reduced amyloid deposition and reversed cognitive and memory decline (Yan et al., 2003; Pedersen and Flynn, 2004; Heneka et al., 2005; Nicolakakis et al., 2008; Escribano et al., 2010; Toledo and Inestrosa, 2010; Mandrekar-Colucci et al., 2012). The inconsistencies in the reported data may be due to use of different animal models of Alzheimer's disease, poor blood–brain barrier penetrance of PPAR agonists (i.e. inconsistent drug distribution) and widely variable dosing strategies (Maeshiba et al., 1997; Hemauer et al., 2010). Phase III clinical trials testing another PPARγ agonist, rosiglitazone, failed to show efficacy in patients with mild to moderate stages of Alzheimer's disease; however, the doses used in clinical trials were significantly lower than those shown to be beneficial in the rodent models (Gold et al., 2010).
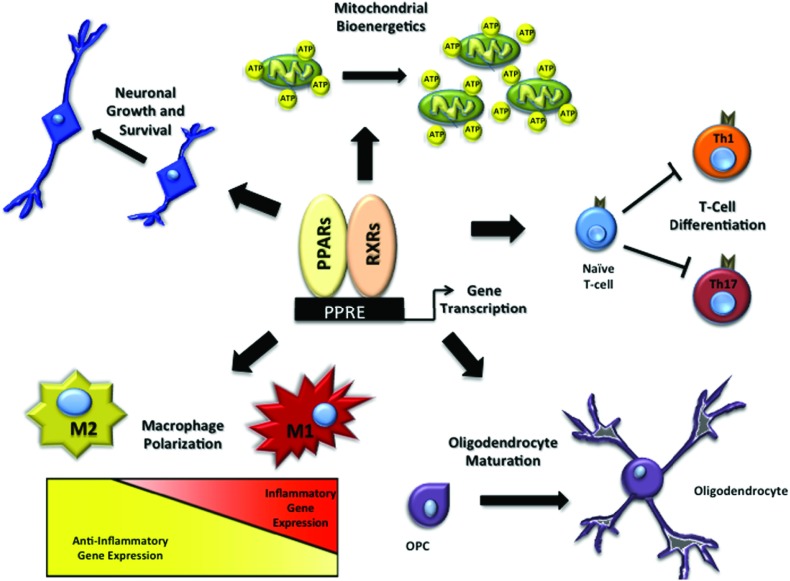
Clearly, PPAR activation has the potential to be beneficial in many neuropathological conditions. The mechanisms of action of PPAR agonists are so diverse that they may be advantageous at many stages of injury. Thus, the best timing and dose of agonists may vary depending on injury severity, progression of disease or the cellular target (i.e. neurons, microglia, oligodendrocytes), and may explain the conflicting results in studies listed above. A clearer understanding of how and where PPARs act will facilitate designing the most effective pre-clinical and clinical studies. This review will address the many mechanisms through which PPAR activation is known to alleviate pathology and improve neurological function in the damaged CNS (Figure 1).
Figure 1. PPARs modulate multiple pathways in the CNS.
PPAR activation after CNS injury/trauma promotes recovery through multiple mechanisms by promoting (1) axon outgrowth, (2) mitochondrial bioenergetics, (3) inhibition of Th1 and Th17 T-cell differentiation, (4) OPC maturation, and (5) polarization of macrophages from an inflammatory ‘M1’ to an anti-inflammatory ‘M2’ activation state.
PPARs AND MACROPHAGE POLARIZATION
Microglia are the primary immune effector cells of the CNS. Pathological changes in the brain or spinal cord cause rapid microglial migration to the affected area where they undergo phenotypic and morphologic transformation. If sufficiently activated, these cells also release chemotactic and inflammatory cytokines that signal the recruitment of monocytes from the circulation into the pathological CNS (Davalos et al., 2005; Nimmerjahn et al., 2005).
The phenotype and corresponding function of macro-phages and microglia are shaped by a cadre of signals present in pathological tissue (Gordon and Martinez, 2010). These signals collaborate to instruct a population of cells that, at any given time, can be quite heterogeneous. To simplify this intrinsic complexity, working models of microglia and macrophage function are often used in which the cells are broadly defined using nomenclature and phenotypic signatures developed from in vitro models. For example, ‘classically’ activated M1 macrophages and ‘alternatively’ activated M2 macrophages are distinct macrophage subsets that can be generated in vitro using defined stimuli (Mosser and Edwards, 2008). Classical activation of macrophages is associated with antigen presentation and the production of inflammatory cytokines, chemokines and reactive oxygen species. Chronic persistence of M1 macrophages is thought to exacerbate disease and tissue pathology (Horn et al., 2008; Busch et al., 2009; Kigerl et al., 2009; Martinez et al., 2009; Hu et al., 2012). In contrast, M2 macrophages produce immune regulatory cytokines including TGF-β (transforming growth factor β), IL (interleukin)-10, IL-13 and IL-4 as well as wound healing molecules such as Arg1 (Arginase 1), YM1, MR (mannose receptor, CD206) and FIZZ1 (RELMα).
Using canonical molecular indicators of macrophage phenotype, recent studies have identified M1 and M2 macrophages in the pathological brain and spinal cord (Kigerl et al., 2009; Mandrekar-Colucci and Landreth, 2010; Kumar et al., 2013). In many models of neurologic disease, the magnitude of pathology or functional loss correlates with a robust M1 macrophage response, and blocking inflammatory signaling often confers neuroprotection (Colton and Wilcock, 2010; David and Kroner, 2011; Shechter and Schwartz, 2013). Similarly, functional inhibition or acute depletion of macrophages in rats and mice after traumatic SCI is neuroprotective and promotes functional recovery (Giulian and Robertson, 1990; Blight, 1994; Popovich et al., 1999; Gris et al., 2004)
Recent data indicate that PPARγ and PPARδ are critical transcriptional ‘gatekeepers’, i.e. they control transcriptional modules that influence macrophage phenotype. PPAR activation inhibits the expression of M1 genes in cells exposed to M1-type stimuli and enhances the expression of M2 markers in the presence of M2 stimuli. In particular, activation of these PPARs in macrophages induces the M2 markers Arg1, CD206, YM1 and FIZZ1; these effects are lost in mice deficient for either receptor (Bouhlel et al., 2007; Odegaard et al., 2007, 2008; Gallardo-Soler et al., 2008; Kang et al., 2008). PPAR activation produces similar effects in microglia (Storer et al., 2005a; Xu et al., 2005b; Ramanan et al., 2009; Antonietta Ajmone-Cat et al., 2012). For instance, PPAR activation of microglia promotes phagocytosis of pathological protein aggregates and is neuroprotective in models of MS and Alzheimer's disease (Mandrekar-Colucci et al., 2012; Yamanaka et al., 2012). Activation of all three PPAR isoforms inhibits NF-κB (nuclear factor κB)-mediated induction of inflammatory cytokine genes (Chawla, 2010). PPARγ achieves this through ligand-activated sumolyation of the receptor, which then binds to and stabilizes the interaction between NF-κB and its co-repressor complex, thereby preventing the transcription of inflammatory cytokines (Pascual et al., 2005).
In Alzheimer's disease, which is characterized by chronic neuroinflammation, PPARγ activation attenuates neuroinflammation and augments expression of M2 macrophage markers, indicating that peripheral administration of PPAR agonists can influence an active and chronic inflammatory milieu in the CNS (Mandrekar-Colucci et al., 2012). PPAR activation is beneficial in other pathological conditions including TBI, SCI, EAE, stroke and ALS (Kiaei et al., 2005; Schutz et al., 2005; Drew et al., 2006; Sundararajan et al., 2006; Yi et al., 2008; Villapol et al., 2012). In EAE, infiltration of monocytes correlates with progression to the severe paralytic stages of disease (Ajami et al., 2011). Treating EAE animals with PPAR agonists is anti-inflammatory and slows disease progression; however, whether PPARs act solely by altering macrophage polarization in this model has not been confirmed (Niino et al., 2001; Diab et al., 2002, 2004; Feinstein et al., 2002; Gocke et al., 2009).
The molecular phenotype of microglia or macrophages affects the ability of these cells to phagocytose debris. For instance, activation of PPARδ in macrophages promotes clearance of apoptotic cells (Mukundan et al., 2009). This occurs through increased expression of opsonins (e.g. complement C1q) by macrophages, which increases phagocytosis of apoptotic cells (Mukundan et al., 2009). Similarly, PPARγ activation in microglia promotes phagocytosis by up-regulating the scavenger receptor CD36 (Yamanaka et al., 2012). Considering traumatic CNS injuries produce large amounts of myelin and cell debris, PPAR-induced M2 polarization of macrophages and microglia may be beneficial by promoting removal of debris in addition to the other mechanisms mentioned above.
Finally, it should be noted that while the in vitro-derived M1 and M2 nomenclature is widely used to describe macrophage activation states, these classifications are imperfect and reflect only a subset of states existing on a continuum of macrophage activation (Mosser and Edwards, 2008). Thus, characterizing M1 as ‘bad’ and M2 as ‘good’ macrophages is overly simplistic since both types have important functions and it is likely that an imbalance in their ratios causes pathology, especially if the imbalance is prolonged. Chronic inflammation involving both M1 and M2 macrophages is documented in many CNS diseases and injuries such as Alzheimer's disease, MS, SCI, TBI, and stroke (Colton et al., 2006; Kigerl et al., 2009; Mikita et al., 2011; Hu et al., 2012; Kumar et al., 2013). Therapeutically targeting PPARs may help to ‘re-balance’ these two phenotypes in the injured CNS and promote neuroprotection.
PPARs AND ASTROCYTES
Astrocytes are highly reactive cells, and in the pathological state, they can release damaging molecules that cause neuron loss (Bal-Price and Brown, 2001). The ability of astrocytes to promote inflammation and their responsiveness to PPAR agonists positions these cells to play a critical role in the progression and treatment of neurological disease. PPAR agonists attenuate pathological astrocyte activation and improve disease progression (Diab et al., 2002, 2004; Storer et al., 2005a, 2005b; Mandrekar-Colucci and Landreth, 2010; Hong et al., 2012). Given that astrocytes play an important role in most CNS disorders, targeting them with PPAR agonists may prove effective in multiple settings.
Astrocytes can regulate how they respond to PPAR ligands through changes in receptor expression. In LPS (lipopolysaccharide)-stimulated astrocytes, PPARγ activation leads to a positive feed-forward signal that increases expression of PPARδ, and PPARδ activation increases expression of PPARα (Aleshin et al., 2009). In turn, PPARα provides a negative-feedback signal inhibiting PPARδ expression (Aleshin et al., 2009). This coordinated signaling helps regulate how PPAR activation influences inflammation (Aleshin et al., 2009). Since the environment surrounding astrocytes can change dramatically with injury or disease, the ability for astrocytes to alter their responses to PPAR ligands allows more precise control of inflammation.
Modulating inflammation is one of the best-studied roles of PPAR activation in astrocytes and has been examined in multiple experimental models (Diab et al., 2002; Giri et al., 2004; Storer et al., 2005a, 2005b; Xu and Drew, 2007; Xu et al., 2007; Lee et al., 2008; Tjalkens et al., 2008; Pineau et al., 2010; Cowley et al., 2012; Hong et al., 2012). In the spinal cord, the PPARγ agonist pioglitazone reduces astrocyte activation in a receptor-dependent manner (Jia et al., 2013). Similarly, the PPARγ agonists 15d-PGJ2 (15-deoxy-Δ-12,14-prostaglandin J-2) and rosiglitazone, and PPARα agonists gemfibrozil and fenofibrate reduced levels of the IL-12 family of cytokines, nitric oxide, IL-6, IL-1β and MCP-1 in primary astrocyte cultures exposed to LPS (Xu and Drew, 2007; Xu et al., 2006, 2007). It is important to note that each drug has different effects on cytokine expression, which can be advantageous since similarly acting drugs can be combined for more potent effects (Diab et al., 2004). Furthermore, attenuating inflammatory signals reduces disease severity in models of MS, even after the onset of clinical symptoms (Diab et al., 2002, 2004). Notably, these beneficial effects can occur through PPAR-dependent and PPAR-independent mechanisms. For example, pioglitazone reduced intraspinal astrocyte activation in a receptor dependent manner in the sciatic nerve transection model (Jia et al., 2013), while 15d-PGJ2, another PPARγ agonist, promoted astrocyte-mediated neuroprotection independent of PPARγ (Giri et al., 2004; Haskew-Layton et al., 2013).
In models of Alzheimer's disease, activation of PPARs in astrocytes is protective against amyloid-β accumulation (Kalinin et al., 2009; Valles et al., 2010; Wang et al., 2010; Benito et al., 2012; Mandrekar-Colucci et al., 2012). Given the well-established effects of PPAR ligands in other cell types, it is not surprising that these effects occur both through astrocytes and microglia (Wang et al., 2010; Mandrekar-Colucci et al., 2012). The ability of astrocytes to attenuate amyloid-β-induced toxicity depends on the activation and presence of PPARs (Valles et al., 2010; Benito et al., 2012). Exposing astrocytes with reduced PPAR expression to amyloid-β exacerbated production of the inflammatory molecules TNFα (tumour necrosis factor α), IL-6, iNOS (inducible nitric oxide synthase), and COX-2 (cyclo-oxygenase 2) compared with wild-type astrocytes (Benito et al., 2012). Furthermore, treating with a PPARα or PPARγ agonist attenuated the increased inflammatory response in amyloid-β-treated astrocytes (Benito et al., 2012). Encouragingly, the reduced amyloid-dependent toxicity led to improved cognition (Mandrekar-Colucci et al., 2012).
PPARs AND T-CELL ACTIVATION
T-cells cross the blood–brain/spinal barrier and secrete various cytokines, including IFNγ (interferon γ), IL-17, and TNFα, all of which can damage myelin and neurons. These processes play an integral role during neurological insult. For example, MS is mediated primarily by autoreactive T-cells of the Th1 (T helper type 1) or Th17 phenotype (Trinchieri et al., 2003; Fletcher et al., 2010). Given that T-cells express PPARα and PPARγ, these PPARs can influence the adaptive immune system by modifying the activity of these cells (Marx et al., 2002). Several studies show that agonists for all three PPAR isoforms inhibit Th1-cell expansion and cytokine production, and in some cases, can concomitantly increase expression of Th2 cytokines (Niino et al., 2001; Diab et al., 2002, 2004; Feinstein et al., 2002; Gocke et al., 2009; Kanakasabai et al., 2010). This may explain why Th1 responses are enhanced in PPARγ-deficient mice, and EAE pathology is exacerbated in mice treated with PPARγ antagonists (Natarajan et al., 2003; Raikwar et al., 2005).
PPARs AND OLIGODENDROCYTES
Oligodendrocytes are the myelinating cells of the CNS and are highly susceptible to various components of the pathologic cascades that occur in most or all neurological diseases (McTigue and Tripathi, 2008). Oligodendrocytes are vulnerable to inflammatory mediators (e.g. cytokines and chemokines) and because of their high intracellular iron and low levels of antioxidant molecules, they are exquisitely sensitive to oxidative damage from reactive oxygen and nitrogen species (Thorburne and Juurlink, 1996; Juurlink et al., 1998). Loss of myelinating oligodendrocytes exposes axons to the injury milieu, which can lead to axon degeneration and, in some cases, neuronal death.
Within the CNS, NG2+ OPCs (oligodendrocyte precursor cells) can differentiate into mature myelinating oligodendrocytes following injury or demyelination (McTigue and Tripathi, 2008). However, the mechanisms that regulate these processes after injury or insult are not well understood (McTigue et al., 1998; McTigue and Tripathi, 2008; Whittaker et al., 2012). In models of MS, clinical symptoms and demyelination are exacerbated in PPARγ heterozygous mice (Natarajan et al., 2003). Work by De Nuccio et al. (2011) points to a potential mechanism; they show that PPARγ agonists promote OPC differentiation by inducing mitochondrial respiratory chain activity and oscillatory Ca2+ waves, which are crucial for oligodendrocyte differentiation. Furthermore, PPARγ activation directly promotes differentiation of rat OPCs into mature oligodendrocytes (Bernardo et al., 2009). Since myelin is composed mostly of lipid and since PPARs play a major role in lipid metabolism, it is not surprising that PPARs regulate the differentiation and function of oligodendrocytes (Saluja et al., 2001; Leisewitz et al., 2008; Kanakasabai et al., 2012). Statins (cholesterol-reducing drugs) also promote oligodendrocyte maturation by inducing PPARγ, an effect that is blocked by PPARγ antagonism (Sim et al., 2008). Thus, targeting oligodendrocytes through PPARs will likely enhance the production and maturation of OPCs, repopulate lost oligodendrocytes and maintain myelination and the integrity of axons during CNS pathology.
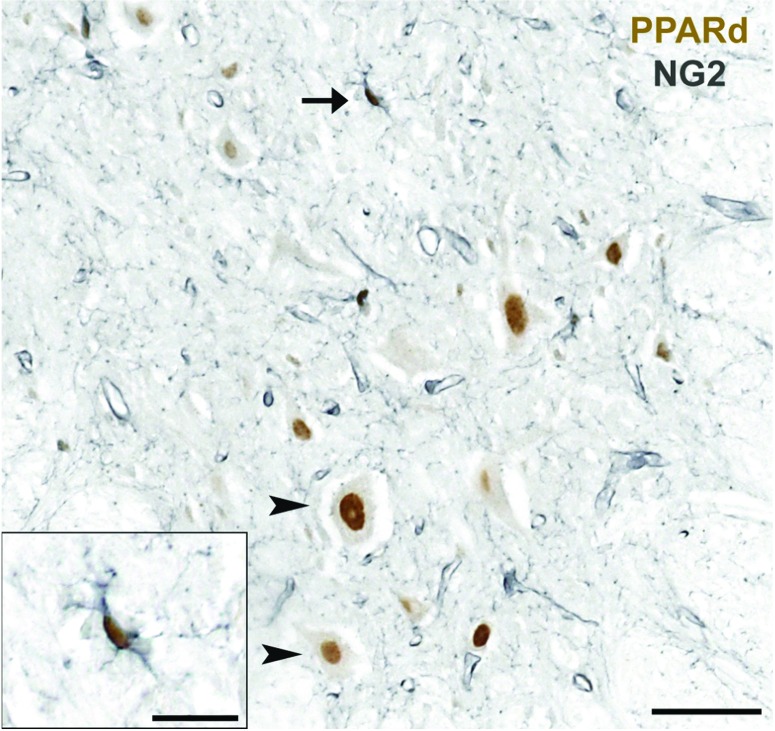
Like PPARγ, PPARδ also appears to be important in oligodendrocyte lineage cell regulation. It is expressed by OPCs in the adult CNS (Figure 2) and, after SCI, the number of PPARδ-expressing OPCs increases along the lesion border where robust oligodendrocyte genesis occurs (Tripathi and McTigue, 2007; Almad and McTigue, 2010). In EAE, PPARδ promotes oligodendrocyte differentiation by limiting the effects of BMPs (bone morphogenetic proteins). Oligodendrocytes express BMPs and their receptors, and during CNS development, BMPs restrict OPC maturation (Gross et al., 1996; Hardy and Friedrich, 1996). PPARδ activation counteracts BMP signaling by increasing the expression of noggin, a BMP antagonist produced by astrocytes. In the presence of noggin, BMPs are inhibited and the number of myelin-producing oligodendrocytes is increased (Simonini et al., 2010). Thus, these findings further indicate a direct role for PPARδ in the regulation of oligodendrocytes.
Figure 2. NG2+ oligodendrocyte progenitor cells express PPARδ.
Spinal cord section from a normal rat spinal cord gray matter (ventral horn) immunolabeled for PPARδ (brown) and NG2 (gray), a marker for oligodendrocyte progenitor cells. In the normal spinal cord, PPARδ is expressed by NG2 cells (arrow, inset) and is also visible in NG2-negative motor neurons (arrowheads). Scale bar=50 μm; scale bar in inset=20 μm.
Even with evidence showing a role for PPARs in oligodendrocyte regulation, conflicting data regarding the efficacy of these agonists on promoting OPC maturation or differentiation have been documented. In one study, undifferentiated C6 glioma cells were shown to up-regulate oligodendrocyte markers in response to agonists that target PPARγ, but not PPARδ or PPARα. In the same study, however, overexpression of PPARδ committed C6 cells to oligodendrocyte fate through the up-regulation of PPARγ (Leisewitz et al., 2008). These studies suggest that oligodendrocyte differentiation may depend on the coordinate activation of PPARs.
Activating PPARs may also enhance oligodendrocyte survival in the injured or diseased CNS. Oligodendrocytes express all three PPAR isoforms and PPAR activation promotes differentiation and myelin gene expression (Granneman et al., 1998; Saluja et al., 2001; Roth et al., 2003; Woods et al., 2003; Jana et al., 2012). Indeed, genes for many of the myelin proteins contain PPAR response elements indicating they can be directly targeted by PPARs (Jana et al., 2012). For example, the PPARα agonist gemfibrozil stimulates expression of the myelin genes MBP (myelin basic protein), MOG (myelin oligodendrocyte glycoprotein), PLP (proteolipid protein) and CNPase. Using chromatin immunoprecipitation assays, it is possible to show that gemfibrozil enhances binding of PPARδ rather than PPARα, its receptor target, to promoters of myelin genes in human oligodendrocytes (Jana et al., 2012). Moreover, activation of PPARδ promotes myelin protein expression (Saluja et al., 2001).
Additionally, CNS pathology can alter PPAR expression by oligodendrocyte lineage cells. For instance, PPARδ expression is enhanced in OPCs and oligodendrocytes after SCI, suggesting these cells would be responsive to PPAR signaling (Almad and McTigue, 2010). Indeed, activation of PPARγ and PPARδ after SCI decreases lesion area, increases myelination and promotes locomotor activity (McTigue et al., 2007; Park et al., 2007; Paterniti et al., 2010). PPARγ activation also protects myelin in an in vitro model of inflammatory demyelination (Paterniti et al., 2010). Similarly, PPAR activation in the EAE model delays onset and reduces the severity of clinical symptoms (Niino et al., 2001; Diab et al., 2002; Feinstein et al., 2002; Genovese et al., 2005; Gocke et al., 2009).
Enhanced myelination and oligodendrocyte survival following PPAR activation could occur independent of changes in myelin gene expression. PPAR activation suppresses synthesis of inflammatory cytokines/chemokines and reactive oxygen (ROS) and nitrogen (RNS) species, all of which are toxic to oligodendrocytes (Springer et al., 1997; Zhao et al., 2006; McTigue, 2008). These inflammatory mediators are inhibited by PPAR activation in macrophages and astrocytes (Ricote et al., 1998; Bernardo et al., 2000; Xu et al., 2006). PPARγ activation increases cellular antioxidants, including catalase and copper-zinc superoxide dismutase, both of which are expressed at low levels in oligodendrocyte lineage cells (Juurlink et al., 1998; Bernardo et al., 2009). Treating OPCs with the PPARγ antagonist GW9662 abolished the anti-inflammatory and antioxidant effects of PPAR agonists, demonstrating a direct role for PPAR signaling in these signaling cascades (Bernardo et al., 2009).
Oligodendrocytes are vulnerable to glutamate excitotoxicity (McAdoo et al., 1999; Pitt et al., 2000; Xu et al., 2005a). High levels of extracellular glutamate are believed to contribute to several neurological diseases and after CNS injury (McAdoo et al., 1999; Pitt et al., 2000; Bogaert et al., 2010; Hinzman et al., 2010, 2012; Thomas et al., 2012; Mehta et al., 2013). Indeed, glutamate antagonists are neuroprotective in many pre-clinical models of neurologic disease (Wrathall et al., 1997; Rosenberg et al., 1999; Faden et al., 2001). Like traditional glutamate antagonists, PPAR activation may also attenuate excitotoxicity. For instance, the PPARγ agonist rosiglitazone increases expression of the glutamate transporter GLT1/EEAT2 mRNA and protein in cultured astrocytes (Romera et al., 2007). An increase in functional GLT1 would promote glutamate uptake by astrocytes thereby reducing extracellular levels. However, when tested in vivo in a model of focal cerebral ischemia, rosiglitazone did not affect GLT1/EAAT2 expression (Verma et al., 2011). Thus, the effects may be context-dependent or require a more rigorous analysis of dosing schedule or pharmacokinetics.
Collectively, data from these studies suggest that PPARs likely act in concert to promote oligodendrocyte survival and OPC differentiation and may represent a novel molecular target, that, if activated appropriately, could promote oligodendrocyte replacement and remyelination in the injured or diseased CNS.
PPARs: NEURON SURVIVAL AND AXON REGENERATION
Neuron loss is a devastating and permanent effect of CNS trauma or disease. Several studies have shown neuroprotective effects of PPARs, most notably PPARγ. For instance, lipid peroxidation was shown to raise PPARγ levels in motor neurons in a model of ALS (Benedusi et al., 2012). This was believed to be a self-protective mechanism since PPARγ activation promotes the expression of lipid detoxifying genes such as lipoprotein lipase and glutathione transferase a-2 (Benedusi et al., 2012). PPARγ also may regulate neuronal responses to ischemia since conditional deletion of PPARγ in neurons increases their susceptibility to ischemia in vitro (Zhao et al., 2009). Further, the PPARγ agonist troglitazone enhances survival of rat motor neurons in culture and PPARγ activation by 15d-PGJ2 protects PC12 cells from nitrosative-induced cell death (Lim et al., 2004). In vivo, activating PPARγ in a middle cerebral artery occlusion model of stroke reduces infarct size and lowers cyclin D1, a protein involved in programmed cell death (Pei et al., 2010). Additionally, PPARγ activation can stabilize mitochondria and protect neurons against apoptotic cell death and oxidative stress by upregulating the anti-apoptotic protein bcl-2 (Fuenzalida et al., 2007). At least part of the neuroprotective effects of PPARγ involves synergistic signaling with neurotrophins. For instance, NGF (nerve growth factor)-induced neuronal differentiation is mediated through activation of PPARγ in a TrkA-dependent manner. Further, PPARγ activation increases NGF and BDNF levels after SCI (Fuenzalida et al., 2005; Meng et al., 2011). Together these studies suggest that activation of PPARs, and in particular PPARγ, may be neuroprotective and promote neuronal survival.
Injured axons have a limited capacity to spontaneously regenerate. Therefore, interventions that enhance or stimulate axon growth may further increase recovery or minimize the functional deficits caused by CNS injury. A few studies have reported that PPAR activation promotes axonal growth in neuronal cell lines and primary DRG (dorsal root ganglion) neuron cultures. Specifically, the PPARγ agonists pioglitazone and 15d-PGJ2 increase the number and lengths of neurites (Jung et al., 2003; Miglio et al., 2009). These effects may occur through modulating RhoA, which is increased in injured neurons and limits axon regeneration after CNS injury (Dubreuil et al., 2003; Madura et al., 2004). Ibuprofen (which activates PPARγ at micromolar levels) inhibits RhoA and stimulates corticospinal and serotonergic axon sprouting after spinal cord transection in rats (Lehmann et al., 1997; Fu et al., 2007). Work by others showed that the growth-promoting effects of ibuprofen involved PPARγ activation and its ability to inhibit RhoA activation (Dill et al., 2010). This effect may be mediated by SHP-2 (Src homology region 2-containing protein tyrosine phosphatase-2), which is involved in the PPARγ-dependent inhibition of RhoA (Wakino et al., 2004). However, the potential role of SHP-2 in PPAR-mediated neurite outgrowth has not yet been studied in the CNS. Like their ability to promote neuronal survival, these studies show that PPAR activation can positively affect axon regeneration.
PPARs AND NEUROPATHIC PAIN
Neuropathic pain is a debilitating consequence of CNS injury, MS, and other neurological diseases. Currently, there is no cure for chronic neuropathic pain and most analgesics are ineffective. Interestingly, emerging pre-clinical data indicate that agonists for PPARα and PPARγ attenuate neuropathic pain following peripheral nerve injury (Churi et al., 2008; Maeda et al., 2008; Taylor, 2009; Takahashi et al., 2011; Ruiz-Medina et al., 2012). The anti-inflammatory effects of PPAR activation, occurring through genomic and non-genomic PPAR-dependent mechanisms, may mediate such effects. For example, intrathecal injection of the PPARγ agonists 15d-PGJ2 or rosiglitazone rapidly (<5 min) attenuated pain-like behaviors in rodents; the effects were PPARγ-dependent since co-administering PPARγ antagonists blocked the effects (Churi et al., 2008). It is thought that because actions occur within minutes of drug administration that these analgesic effects must be non-genomic (Fehrenbacher et al., 2009).
PPARs also may affect pain sensations by modulating glucocorticoid action. Glucocorticoids are steroid hormones released during periods of acute or sustained stress, both physiological and psychological, and signal via the GR (glucocorticoid receptor), another nuclear receptor. Glucocorticoids can pass the blood–brain/spinal barrier and excess levels are detrimental to neuronal survival in the brain, inducing synaptic loss, atrophy of the hippocampus and cognitive deficits. In the CNS, glucocorticoid signaling enhances pain-like behaviors and is up-regulated in parallel with inflammatory cytokines after injury (Blackburn-Munro and Blackburn-Munro, 2003). PPARγ activation inhibits the autonomic and neuroendocrine responses to stress in rats and may explain why activation of this receptor reduces circulating corticosterone levels (Ryan et al., 2012). A 5-day treatment with rosiglitazone attenuates corticosterone levels, heart rate, and expression of c-Fos (a marker of neuronal activation) in the hypothalamus of rats that were subjected to restraint stress (Ryan et al., 2012). Rosiglitazone also decreased circulating corticosterone levels in a mouse model of Alzheimer's disease (Escribano et al., 2009). While the mechanisms through which PPARs attenuate corticosterone remain to be fully elucidated, PPARα activation does interfere with GR-dependent gene expression by blocking the recruitment of RNA polymerase II to the glucocorticoid response elements on the promoter of GR target genes (Bougarne et al., 2009). While the effects of glucocorticoids after injury are complicated, it is apparent in these studies that PPARs play an integral role in this signaling pathway.
PPARs AND MITOCHONDRIAL BIOENERGETICS
Mitochondrial dysfunction is common in the CNS with stroke, SCI, TBI, ALS, MS, Huntington's disease and Alzheimer's disease (Mecocci et al., 1996; Mattiazzi et al., 2002; Wiedemann et al., 2002; Korde et al., 2005, 2007; Vijayvergiya et al., 2005; Dutta et al., 2006; Singh et al., 2006; Sullivan et al., 2007; Robertson et al., 2007; Regenold et al., 2008; Vyshkina et al., 2008; Martin et al., 2009; Pandya et al., 2009; Patel et al., 2009; Readnower et al., 2011; Sauerbeck et al., 2011; Zhao et al., 2011; Lunnon et al., 2012). In these conditions, mitochondrial dysfunction correlates with cell death, functional impairment, and cognitive deficit. This is intuitively obvious since energy production by mitochondria is essential for survival of all cells, including neurons (Nicholls and Budd, 2000; Stephans et al., 2002; Borland et al., 2008). Oligodendrocytes also have high energy demands since they must maintain large amounts of plasma membrane as myelin. Damage to oligodendrocyte mitochondria impairs energy metabolism resulting in reduced myelin production and compaction, and ultimately hypo-myelination or complete axon demyelination (Kalman et al., 1997). Accordingly, finding new therapies that protect mitochondria should help protect neurons and oligodendrocytes in most, if not all, forms of CNS disease.
PPAR agonists have been extensively studied for their role in modulating metabolism and energy production (Alaynick, 2008; Sugden et al., 2009). Activation of PPAR receptors by fatty acids promotes mitochondrial β-oxidation allowing for greater cellular energy production (Gulick et al., 1994). Many of the effects of PPARs on bioenergetics occur through regulation of gene expression. Specifically, activation of PPARδ increases production of mTFA (mitochondrial transcription factor), UCPs (uncoupling proteins) 2 and 3 (UCP2/3), and lipoprotein lipase (Muoio et al., 2002; Dressel et al., 2003; Jiang et al., 2010). Similarly, PPARα activation increases the transport and utilization of fatty acids needed during β-oxidation and PPARγ activation increases cytochrome c oxidase 6A2 (Desvergne and Wahli, 1999; Allen et al., 2006). Activation of PPARγ also induces the expression of lipoprotein lipase and stimulates mitochondrial biogenesis (Strum et al., 2007; Benedusi et al., 2012; Morino et al., 2012). Further, PPARγ activation stabilizes existing mitochondria and prevents their dysfunction (Fuenzalida et al., 2007; Quintanilla et al., 2008). These effects may underlie the increased mitochondrial energy production observed following administration of PPARγ agonists in models of CNS insult (Hunter et al., 2007; Sauerbeck et al., 2011).
In addition to driving gene transcription, some PPAR agonists interact directly with mitochondria (Colca et al., 2004; Geldenhuys et al., 2010). These effects can occur via mitoNEET, a protein in the mitochondrial outer membrane that is essential for maximal energy production (Wiley et al., 2007). Pioglitazone binds to mitoNEET and stabilizes its conformational structure (Colca et al., 2004; Paddock et al., 2007). The mitochondrial effects of pioglitazone likely extend to other PPAR agonists such as rosiglitazone, which also binds to mitoNEET (Geldenhuys et al., 2010; Bieganski and Yarmush, 2011). Given this novel direct mitochondrial target for PPAR agonists, work has focused on creating specific ligands for mitoNEET (Geldenhuys et al., 2010, 2011; Bieganski and Yarmush, 2011). These new ligands may prove effective at targeting mitochondrial dysfunction and improving recovery similar to traditional PPAR ligands.
RXRs AS A MEANS TO TARGET PPARs
RXRs are essential for PPAR signaling. Specifically, RXRs heterodimerize with PPARs, creating ‘permissive’ signaling complexes that increase expression of PPAR target genes following ligation with either a RXR-specific agonist or a PPAR partner ligand (Mangelsdorf and Evans, 1995; Szanto et al., 2004). There are three RXR isotypes: RXRα, RXRβ and RXRγ. In the intact CNS, neurons and glia constitutively express RXRs (Schrage et al., 2006). In injury or disease, the subcellular location of RXR switches from the cytoplasm to the nucleus, suggesting transcriptional activation of RXR-containing heterodimers (Schrage et al., 2006). Known ligands for RXRs include honokiol (a naturally occurring ligand from the bark of the magnolia tree), the synthetic agonist Bexarotene (Targretin), and 9-cis retinoic acid (Qu and Tang, 2010; Kotani et al., 2010). Considering that Bexarotene is already FDA-approved and has an excellent side-effect profile, it is an optimal candidate for translational studies on neurodegenerative diseases or injuries (Lansigan and Foss, 2010).
Activation of RXR elicits a response similar to that observed after PPAR activation. For instance, RXR activation promotes an anti-inflammatory milieu by down-regulating inflammatory signaling in microglia and astrocytes (Xu and Drew, 2006). It also can initiate oligodendrocyte progenitor proliferation, differentiation and myelination (Chao et al., 2010; Nunez et al., 2010; Huang et al., 2011; Kaushik et al., 2012). Interestingly, transcripts for all RXRs are highly up-regulated in demyelinated lesions, with the RXRγ isoform being the highest of the three (Huang et al., 2011). Furthermore, 9-cis retinoic acid enhances OPC differentiation in culture and increases remyelination in cerebellar slice cultures (Huang et al., 2011). Thus, RXR activation could be therapeutic in demyelinating diseases. Additionally, their enhanced ability to readily cross the blood–brain barrier, compared with popular PPAR agonists, makes RXR agonists attractive candidates for the treatment of neurologic diseases (Cramer et al., 2012).
Moreover, due to the promiscuous nature of RXR heterodimer activation, PPAR signaling pathways may be initiated through the use of RXR agonists. Thus, RXR agonists could benefit any disease in which PPAR activation has proven effective. This promiscuity also creates unique challenges and opportunities. Since these signaling cascades may be differentially activated based on the binding specificity and affinity of various ligands to the receptor, RXR activation may not mimic the spectrum of changes that occur when PPAR-specific agonists are used to activate the heterodimer complex. Currently, it is not known which heterodimeric partner RXR exerts its beneficial effects through. Thus, a level of precision concerning RXR signaling is missing. Given the similarity of actions between PPAR and RXR activation, RXR activation may be exerting its effects by concurrently activating multiple PPAR pathways. Also, studies have shown that PPAR and RXR agonists, when used together to simultaneously activate the heterodimer complex, have synergistic effects allowing for maximal stimulation and expanding possible treatment paradigms (Papi et al., 2009; Yamanaka et al., 2012).
NON-TRADITIONAL ACTIONS OF PPARs
PPAR agonists can influence pathological processes through mechanisms that are independent of their classical PPAR receptors. For example, when given at extremely low doses (0.5 and 1 mg/kg), far below those needed to activate PPARγ receptors, pioglitazone still attenuates inflammatory signaling by reducing TNF-α, iNOS, and IL-1β (Thal et al., 2011). Indeed, co-administration of a PPARγ antagonist does not prevent the anti-inflammatory effects of low-dose pioglitazone, confirming a PPARγ receptor-independent mechanism (Thal et al., 2011). Similarly, although pioglitazone reduces tissue loss and cognitive impairment after TBI by PPARγ activation, this drug reduces microglial activation via a PPARγ-independent mechanism (Sauerbeck et al., 2011). A different PPARγ agonist, rosiglitazone, has similar anti-inflammatory effects after TBI, yet its effects depend on PPARγ activation (Yi et al., 2008). The ability to reduce inflammation and the different receptor dependency of pioglitazone and rosiglitazone is likely explained by immune cells expressing both PPARγ and PPARδ receptors and each receptor having different thresholds for activation by rosiglitazone (Sakamoto et al., 2000; Gordon and Martinez, 2010). Additionally, the PPARγ-independent actions of these agonists likely result from their ability to directly target mitochondria and also activate other PPAR receptors (Sakamoto et al., 2000; Colca et al., 2004; Paddock et al., 2007; Orasanu et al., 2008; Geldenhuys et al., 2011). Evidence of receptor-dependent and independent effects, especially within the same animal, provides strong support for the diverse nature of the beneficial effects of PPAR agonists.
CONCLUSIONS
The beneficial effects of PPAR activation have been independently reproduced in many rodent models of traumatic injury and neurodegenerative disease and there are several potential mechanisms through which PPAR activation promotes CNS repair and functional recovery. Activation of PPARs can reduce inflammation and confer neuroprotection, in part through their ability to minimize cell death and reduce mitochondrial dysfunction. PPAR activation may also enhance axonal growth and remyelination. Through non-genomic mechanisms, PPAR agonists may have analgesic effects. Since the pathophysiology of traumatic CNS injury and neurodegeneration is dynamic, the timing of PPAR activation likely needs to be tailored to meet the specific characteristics of the disease in question. Still, the broad effects on overlapping mechanisms of neurologic injury make these drugs very promising therapeutics for treating traumatic injuries to the brain or spinal cord as well as various neurodegenerative diseases.
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8:329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleshin S, Grabeklis S, Hanck T, Sergeeva M, Reiser G. Peroxisome proliferator-activated receptor (PPAR)-gamma positively controls and PPARalpha negatively controls cyclooxygenase-2 expression in rat brain astrocytes through a convergence on PPARbeta/delta via mutual control of PPAR expression levels. Mol Pharmacol. 2009;76:414–424. doi: 10.1124/mol.109.056010. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Moloudi R, Arababadi MK, Shamsizadeh A, Javanmardi K. Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain Res. 2009;1271:121–127. doi: 10.1016/j.brainres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Allen T, Zhang F, Moodie SA, Clemens LE, Smith A, Gregoire F, Bell A, Muscat GE, Gustafson TA. Halofenate is a selective peroxisome proliferator-activated receptor gamma modulator with antidiabetic activity. Diabetes. 2006;55:2523–2533. doi: 10.2337/db06-0618. [DOI] [PubMed] [Google Scholar]
- Almad A, Lash AT, Wei P, Lovett-Racke AE, McTigue DM. The PPAR alpha agonist gemfibrozil is an ineffective treatment for spinal cord injured mice. Exp Neurol. 2011;232:309–317. doi: 10.1016/j.expneurol.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, McTigue DM. Chronic expression of PPAR-delta by oligodendrocyte lineage cells in the injured rat spinal cord. J Comp Neurol. 2010;518:785–799. doi: 10.1002/cne.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonietta Ajmone-Cat M, Lavinia Salvatori M, De Simone R, Mancini M, Biagioni S, Bernardo A, Cacci E, Minghetti L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J Neurosci Res. 2012;90:575–587. doi: 10.1002/jnr.22783. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Martin RH, Palesch Y, Cotton D, Yusuf S, Sacco R, Diener HC, Toni D, Estol C, Roberts R. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke. 2009;40:3541–3546. doi: 10.1161/STROKEAHA.109.555623. [DOI] [PubMed] [Google Scholar]
- Benedusi V, Martorana F, Brambilla L, Maggi A, Rossi D. The peroxisome proliferator-activated receptor gamma (PPARgamma) controls natural protective mechanisms against lipid peroxidation in amyotrophic lateral sclerosis. J Biol Chem. 2012;287:35899–35911. doi: 10.1074/jbc.M112.366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Tolon RM, Castillo AI, Ruiz-Valdepenas L, Martinez-Orgado JA, Fernandez-Sanchez FJ, Vazquez C, Cravatt BF, Romero J. beta-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-alpha, PPAR-gamma and TRPV1, but not CB(1) or CB(2) receptors. Br J Pharmacol. 2012;166:1474–1489. doi: 10.1111/j.1476-5381.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Bianchi D, Magnaghi V, Minghetti L. Peroxisome proliferator-activated receptor-gamma agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J Neuropathol Exp Neurol. 2009;68:797–808. doi: 10.1097/NEN.0b013e3181aba2c1. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388:7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Bieganski RM, Yarmush ML. Novel ligands that target the mitochondrial membrane protein mitoNEET. J Mol Graph Model. 2011;29:965–973. doi: 10.1016/j.jmgm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trends Endocrinol Metab. 2003;14:20–27. doi: 10.1016/s1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60:263–273. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Bogaert E, d’Ydewalle C, Van Den Bosch L. Amyotrophic lateral sclerosis and excitotoxicity: from pathological mechanism to therapeutic target. CNS Neurol Disord Drug Targets. 2010;9:297–304. doi: 10.2174/187152710791292576. [DOI] [PubMed] [Google Scholar]
- Borland MK, Trimmer PA, Rubinstein JD, Keeney PM, Mohanakumar K, Liu L, Bennett JP., Jr Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson's disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Mol Neurodegener. 2008;3:21. doi: 10.1186/1750-1326-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougarne N, Paumelle R, Caron S, Hennuyer N, Mansouri R, Gervois P, Staels B, Haegeman G, De Bosscher K. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci USA. 2009;106:7397–7402. doi: 10.1073/pnas.0806742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlel MA, Brozek J, Derudas B, Zawadzki C, Jude B, Staels B, Chinetti-Gbaguidi G. Unlike PPARgamma, PPARalpha or PPARbeta/delta activation does not promote human monocyte differentiation toward alternative macrophages. Biochem Biophys Res Commun. 2009;386:459–462. doi: 10.1016/j.bbrc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LK, Liao PC, Ho CL, Wang EI, Chuang CC, Chiu HW, Hung LB, Hua KF. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-kappaB pathway to reduce LPS-induced TNFalpha and NO expression. J Agric Food Chem. 2010;58:3472–3478. doi: 10.1021/jf904207m. [DOI] [PubMed] [Google Scholar]
- Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Beziaud T, Plotkine M, Marchand-Leroux C. Combination therapy with fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, and simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, on experimental traumatic brain injury. J Pharmacol Exp Ther. 2008;326:966–974. doi: 10.1124/jpet.108.140368. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24:1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain. 2008;9:639–649. doi: 10.1016/j.jpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- Colton C., Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley TR, O’Sullivan J, Blau C, Deighan BF, Jones R, Kerskens C, Richardson JC, Virley D, Upton N, Lynch MA. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiol Aging. 2012;33:162–175. doi: 10.1016/j.neurobiolaging.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- David S., Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- De Nuccio C, Bernardo A, De Simone R, Mancuso E, Magnaghi V, Visentin S, Minghetti L. Peroxisome proliferator-activated receptor gamma agonists accelerate oligodendrocyte maturation and influence mitochondrial functions and oscillatory Ca2+ waves. J Neuropathol Exp Neurol. 2011;70:900–912. doi: 10.1097/NEN.0b013e3182309ab1. [DOI] [PubMed] [Google Scholar]
- Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci. 2010;30:963–972. doi: 10.1523/JNEUROSCI.5045-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Dengler R, Heneka MT, Meye T, Zierz S, Kassubek J, Fischer W, Steiner F, Lindauer E, Otto M, Dreyhaupt J, Grehl T, Hermann A, Winkler AS, Bogdahn U, Benecke R, Schrank B, Wessig C, Grosskreutz J, Ludolph AC. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE. 2012;7:e37885. doi: 10.1371/journal.pone.0037885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer's transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer's disease mouse model. Biochem Biophys Res Commun. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- Faden AI, O’Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA. Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improves outcome after brain trauma. Exp Neurol. 2001;167:435–444. doi: 10.1006/exnr.2000.7577. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Loverme J, Clarke W, Hargreaves KM, Piomelli D, Taylor BK. Rapid pain modulation with nuclear receptor ligands. Brain Res Rev. 2009;60:114–124. doi: 10.1016/j.brainresrev.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, Landreth GE, Pershadsingh HA, Weinberg G, Heneka MT. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Fuenzalida KM, Aguilera MC, Piderit DG, Ramos PC, Contador D, Quinones V, Rigotti A, Bronfman FC, Bronfman M. Peroxisome proliferator-activated receptor gamma is a novel target of the nerve growth factor signaling pathway in PC12 cells. J Biol Chem. 2005;280:9604–9609. doi: 10.1074/jbc.M409447200. [DOI] [PubMed] [Google Scholar]
- Gallardo-Soler A, Gomez-Nieto C, Campo ML, Marathe C, Tontonoz P, Castrillo A, Corraliza I. Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol Endocrinol. 2008;22:1394–1402. doi: 10.1210/me.2007-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldenhuys WJ, Funk MO, Awale PS, Lin L, Carroll RT. A novel binding assay identifies high affinity ligands to the rosiglitazone binding site of mitoNEET. Bioorg Med Chem Lett. 2011;21:5498–5501. doi: 10.1016/j.bmcl.2011.06.111. [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Funk MO, Barnes KF, Carroll RT. Structure-based design of a thiazolidinedione which targets the mitochondrial protein mitoNEET. Bioorg Med Chem Lett. 2010;20:819–823. doi: 10.1016/j.bmcl.2009.12.088. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Cannavo G, Muia C, Bramanti P, Cuzzocrea S. Role of endogenous ligands for the peroxisome proliferators activated receptors alpha in the secondary damage in experimental spinal cord trauma. Exp Neurol. 2005;194:267–278. doi: 10.1016/j.expneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Giri S, Rattan R, Singh AK, Singh I. The 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB-p300 pathway independent of peroxisome proliferator-activated receptor gamma. J Immunol. 2004;173:5196–5208. doi: 10.4049/jimmunol.173.8.5196. [DOI] [PubMed] [Google Scholar]
- Giulian D, Robertson C. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Ann Neurol. 1990;27:33–42. doi: 10.1002/ana.410270107. [DOI] [PubMed] [Google Scholar]
- Gocke AR, Hussain RZ, Yang Y, Peng H, Weiner J, Ben LH, Drew PD, Stuve O, Lovett-Racke AE, Racke MK. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-alpha agonists in autoimmune disease. J Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamagi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Granneman J, Skoff R, Yang X. Member of the peroxisome proliferator-activated receptor family of transcription factors is differentially expressed by oligodendrocytes. J Neurosci Res. 1998;51:563–573. doi: 10.1002/(SICI)1097-4547(19980301)51:5<563::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Gulick T, Cresci S, Caira T, Moore DD, Kelly DP. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci USA. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- Hardy RJ, Friedrich VL., Jr Oligodendrocyte progenitors are generated throughout the embryonic mouse brain, but differentiate in restricted foci. Development. 1996;122:2059–2069. doi: 10.1242/dev.122.7.2059. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Payappilly JB, Xu H, Bennett SA, Ratan RR. 15-Deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) protects neurons from oxidative death via an Nrf2 astrocyte-specific mechanism independent of PPARgamma. J Neurochem. 2013;124:536–547. doi: 10.1111/jnc.12107. [DOI] [PubMed] [Google Scholar]
- Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010;202(383):e381–387. doi: 10.1016/j.ajog.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Hinzman M, Thomas TC, Burmeister JJ, Quintero JE, Huettl P, Pomerleau F, Gerhardt GA, Lifshitz J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J Neurotrauma. 2010;27:889–899. doi: 10.1089/neu.2009.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinzman JM, Thomas TC, Quintero JE, Gerhardt GA, Lifshitz J. Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J Neurotrauma. 2012;29:1197–1208. doi: 10.1089/neu.2011.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Xin Y, HaiQin W, GuiLian Z, Ru Z, ShuQin Z, HuQing W, Li Y, Yun D. The PPARgamma agonist rosiglitazone prevents cognitive impairment by inhibiting astrocyte activation and oxidative stress following pilocarpine-induced status epilepticus. Neurol Sci. 2012;33:559–566. doi: 10.1007/s10072-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Evercooren AB, Chambon P, Ffrench-Constant C, Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Jana M, Mondal S, Gonzalez FJ, Pahan K. Gemfibrozil, a lipid-lowering drug, increases myelin genes in human oligodendrocytes via peroxisome proliferator-activated receptor-beta. J Biol Chem. 2012;287:34134–34148. doi: 10.1074/jbc.M112.398552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HB, Wang XM, Qiu LL, Liu XY, Shen JC, Ji Q, Yang JJ. Spinal neuroimmune activation inhibited by repeated administration of pioglitazone in rats after L5 spinal nerve transection. Neurosci Lett. 2013;543:130–135. doi: 10.1016/j.neulet.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wan J, Ke LQ, Lu QG, Tong NW. Activation of PPARdelta promotes mitochondrial energy metabolism and decreases basal insulin secretion in palmitate-treated beta-cells. Mol Cell Biochem. 2010;343:249–256. doi: 10.1007/s11010-010-0520-8. [DOI] [PubMed] [Google Scholar]
- Jung KM, Park KS, Oh JH, Jung SY, Yang KH, Song YS, Son DJ, Park YH, Yun YP, Lee MK, Oh KW, Hong JT. Activation of p38 mitogen-activated protein kinase and activator protein-1 during the promotion of neurite extension of PC-12 cells by 15-deoxy-delta12,14-prostaglandin J2. Mol Pharmacol. 2003;63:607–616. doi: 10.1124/mol.63.3.607. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kaiser CC, Shukla DK, Stebbins GT, Skias DD, Jeffery DR, Stefoski D, Katsamakis G, Feinstein DL. A pilot test of pioglitazone as an add-on in patients with relapsing remitting multiple sclerosis. J Neuroimmunol. 2009;211:124–130. doi: 10.1016/j.jneuroim.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kalinin S, Richardson JC, Feinstein DL. A PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2009;6:431–437. doi: 10.2174/156720509789207949. [DOI] [PubMed] [Google Scholar]
- Kalman B, Lublin FD, Alder H. Impairment of central and peripheral myelin in mitochondrial diseases. Mult Scler. 1997;2:267–278. doi: 10.1177/135245859700200602. [DOI] [PubMed] [Google Scholar]
- Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, Bright JJ. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;130:572–588. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakasabai S, Pestereva E, Chearwae W, Gupta SK, Ansari S, Bright JJ. PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes. PLoS ONE. 2012;7:e50500. doi: 10.1371/journal.pone.0050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Mukhopadhyay R, Kumawat KL, Gupta M, Basu A. Therapeutic targeting of Kruppel-like factor 4 abrogates microglial activation. J Neuroinflammation. 2012;9:57. doi: 10.1186/1742-2094-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J Neurochem. 2005;94:1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Pocernich CB, Waldmeier PC, Maragos WF. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J Neurotrauma. 2007;24:895–908. doi: 10.1089/neu.2006.0122. [DOI] [PubMed] [Google Scholar]
- Kotani H, Tanabe H, Mizukami H, Makishima M, Inoue M. Identification of a naturally occurring rexinoid, honokiol, that activates the retinoid X receptor. J Nat Prod. 2010;73:1332–1336. doi: 10.1021/np100120c. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Hanyu H, Sakurai H, Takada Y, Onuma T, Iwamoto T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer's disease. Geriatr Gerontol Int. 2012;12:207–214. doi: 10.1111/j.1447-0594.2011.00746.x. [DOI] [PubMed] [Google Scholar]
- Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273–286. doi: 10.2165/11532190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lee JH, Woo JH, Woo SU, Kim KS, Park SM, Joe EH, Jou I. The 15-deoxy-delta 12,14-prostaglandin J2 suppresses monocyte chemoattractant protein-1 expression in IFN-gamma-stimulated astrocytes through induction of MAPK phosphatase-1. J Immunol. 2008;181:8642–8649. doi: 10.4049/jimmunol.181.12.8642. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Leisewitz AV, Urrutia CR, Martinez GR, Loyola G, Bronfman M. A PPARs cross-talk concertedly commits C6 glioma cells to oligodendrocytes and induces enzymes involved in myelin synthesis. J Cell Physiol. 2008;217:367–376. doi: 10.1002/jcp.21509. [DOI] [PubMed] [Google Scholar]
- Lim SY, Jang JH, Na HK, Lu SC, Rahman I, Surh YJ. 15-Deoxy-Delta12,14-prostaglandin J(2) protects against nitrosative PC12 cell death through up-regulation of intracellular glutathione synthesis. J Biol Chem. 2004;279:46263–46270. doi: 10.1074/jbc.M406555200. [DOI] [PubMed] [Google Scholar]
- Lunnon K, Ibrahim Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M, Furney S, Saleem M, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Coppola G, Geschwind D, Simmons A, Lovestone S, Dobson R, Hodges A. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer's disease blood. J Alzheimers Dis. 2012;30:685–710. doi: 10.3233/JAD-2012-111592. [DOI] [PubMed] [Google Scholar]
- Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. 2004;5:412–417. doi: 10.1038/sj.embor.7400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci. 2008;108:341–347. doi: 10.1254/jphs.08207fp. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J Neurosci. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- McTigue DM. Potential therapeutic targets for PPARgamma after spinal cord injury. PPAR Res. 2008;2008:517162. doi: 10.1155/2008/517162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Cherubini A, Beal MF, Cecchetti R, Chionne F, Polidori MC, Romano G, Senin U. Altered mitochondrial membrane fluidity in AD brain. Neurosci Lett. 1996;207:129–132. doi: 10.1016/0304-3940(96)12509-x. [DOI] [PubMed] [Google Scholar]
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Meng B, Zhang Q, Huang C, Zhang HT, Tang T, Yang HL. Effects of a single dose of methylprednisolone versus three doses of rosiglitazone on nerve growth factor levels after spinal cord injury. J Int Med Res. 2011;39:805–814. doi: 10.1177/147323001103900313. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rattazzi L, Rosa AC, Fantozzi R. PPARgamma stimulation promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells. Neurosci Lett. 2009;454:134–138. doi: 10.1016/j.neulet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Sono S, Choi CS, Samuel VT, Lin A, Gallo A, Zhao H, Kashiwagi A, Goldberg IJ, Wang H, Eckel RH, Maegawa H, Shulman GI. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes. 2012;61:877–887. doi: 10.2337/db11-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Muthian G, Barak Y, Evans RM, Bright JJ. Peroxisome proliferator-activated receptor-gamma-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J Immunol. 2003;171:5743–5750. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino M, Iwabuchi K, Kikuchi S, Ato M, Morohashi T, Ogata A, Tashiro K, Onoe K. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma. J Neuroimmunol. 2001;116:40–48. doi: 10.1016/s0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Nunez V, Alameda D, Rico D, Mota R, Gonzalo P, Cedenilla M, Fischer T, Bosca L, Glass CK, Arroyo AG, Ricote M. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc Natl Acad Sci USA. 2010;107:10626–10631. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES, Plutzky J. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol. 2008;52:869–881. doi: 10.1016/j.jacc.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE, Jennings PA. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci USA. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Papi A, Tatenhorst L, Terwel D, Hermes M, Kummer MP, Orlandi M, Heneka MT. PPARgamma and RXRgamma ligands act synergistically as potent antineoplastic agents in vitro and in vivo glioma models. J Neurochem. 2009;109:1779–1790. doi: 10.1111/j.1471-4159.2009.06111.x. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87:130–140. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S. Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. J Pharmacol Exp Ther. 2010;333:465–477. doi: 10.1124/jpet.110.165605. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2004;17:500–506. doi: 10.1016/j.nbd.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pei L, Zhang Y, Zhang Y, Chu X, Zhang J, Wang R, Liu M, Zhu X, Yu W. Peroxisome proliferator-activated receptor gamma promotes neuroprotection by modulating cyclin D1 expression after focal cerebral ischemia. Can J Physiol Pharmacol. 2010;88:716–723. doi: 10.1139/y10-058. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Qu L, Tang X. Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol. 2010;65:201–205. doi: 10.1007/s00280-009-1140-4. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. J Biol Chem. 2008;283:25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikwar HP, Muthian G, Rajasingh J, Johnson C, Bright JJ. PPARgamma antagonists exacerbate neural antigen-specific Th1 response and experimental allergic encephalomyelitis. J Neuroimmunol. 2005;167:99–107. doi: 10.1016/j.jneuroim.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75:870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Pandya JD, McEwen ML, Pauly JR, Springer JE, Sullivan PG. Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J Neurotrauma. 2011;28:1845–1853. doi: 10.1089/neu.2011.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Makley MJ, Stone RD, Kling MA. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J Neurol Sci. 2008;275:106–112. doi: 10.1016/j.jns.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19:464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, Bronfman M. PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res. 2003;72:425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medina J, Flores JA, Tasset I, Tunez I, Valverde O, Fernandez-Espejo E. Alteration of neuropathic and visceral pain in female C57BL/6J mice lacking the PPAR-alpha gene. Psychopharmacology (Berl) 2012;222:477–488. doi: 10.1007/s00213-012-2662-8. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Grayson BE, Jones KR, Schneider AL, Woods SC, Seeley RJ, Herman JP, Ulrich-Lai YM. Physiological responses to acute psychological stress are reduced by the PPARgamma agonist rosiglitazone. Endocrinology. 2012;153:1279–1287. doi: 10.1210/en.2011-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- Saluja I, Granneman JG, Skoff RP. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia. 2001;33:191–204. [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage K, Koopmans G, Joosten EA, Mey J. Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. Eur J Neurosci. 2006;23:285–295. doi: 10.1111/j.1460-9568.2005.04534.x. [DOI] [PubMed] [Google Scholar]
- Schutz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schurmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, Kobayashi M. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology. 2008;28:387–398. doi: 10.1111/j.1440-1789.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Kaiser CC, Stebbins GT, Feinstein DL. Effects of pioglitazone on diffusion tensor imaging indices in multiple sclerosis patients. Neurosci Lett. 2010;472:153–156. doi: 10.1016/j.neulet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Ali TA, Roy NS, Vates GE, Pilcher WH, Goldman SA. Statin treatment of adult human glial progenitors induces PPAR gamma-mediated oligodendrocytic differentiation. Glia. 2008;56:954–962. doi: 10.1002/glia.20669. [DOI] [PubMed] [Google Scholar]
- Simonini VM, Polak PE, Boullerne AI, Peters JM, Richardson JC, Feinstein DL. Regulation of oligodendrocyte progenitor cell maturation by PPARdelta: effects on bone morphogenetic proteins. ASN NEURO. 2010;2(1) doi: 10.1042/AN20090033. art:e00025.doi:10.1042/AN20090033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson MP. 4-Hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Miller GW, Levey AI, Greenamyre JT. Acute mitochondrial and chronic toxicological effects of 1-methyl-4-phenylpyridinium in human neuroblastoma cells. Neurotoxicology. 2002;23:569–580. doi: 10.1016/s0161-813x(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005a;161:113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Storer PD, Xu J, Chavis JA, Drew PD. Cyclopentenone prostaglandins PGA2 and 15-deoxy-delta12,14 PGJ2 suppress activation of murine microglia and astrocytes: implications for multiple sclerosis. J Neurosci Res. 2005b;80:66–74. doi: 10.1002/jnr.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C, Saunders A, Roses A. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Zariwala MG, Holness MJ. PPARs and the orchestration of metabolic fuel selection. Pharmacol Res. 2009;60:141–150. doi: 10.1016/j.phrs.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int. 2006;49:136–144. doi: 10.1016/j.neuint.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl 2):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Hasegawa-Moriyama M, Sakurai T, Inada E. The macrophage-mediated effects of the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuate tactile allodynia in the early phase of neuropathic pain development. Anesth Analg. 2011;113:398–404. doi: 10.1213/ANE.0b013e31821b220c. [DOI] [PubMed] [Google Scholar]
- Taylor BK. Spinal inhibitory neurotransmission in neuropathic pain. Curr Pain Headache Rep. 2009;13:208–214. doi: 10.1007/s11916-009-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J Neurotrauma. 2011;28:983–993. doi: 10.1089/neu.2010.1685. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Hinzman JM, Gerhardt GA, Lifshitz J. Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J Neurotrauma. 2012;29:187–200. doi: 10.1089/neu.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Liu X, Mohl B, Wright T, Moreno JA, Carbone DL, Safe S. The peroxisome proliferator-activated receptor-gamma agonist 1,1-bis(3′-indolyl)-1-(p-trifluoromethylphenyl)methane suppresses manganese-induced production of nitric oxide in astrocytes and inhibits apoptosis in cocultured PC12 cells. J Neurosci Res. 2008;86:618–629. doi: 10.1002/jnr.21524. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry. 2010;15:272–285. doi: 10.1038/mp.2009.72. 228. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55:698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- Valles SL, Dolz-Gaiton P, Gambini J, Borras C, Lloret A, Pallardo FV, Vina J. Estradiol or genistein prevent Alzheimer's disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2010;1312:138–144. doi: 10.1016/j.brainres.2009.11.044. [DOI] [PubMed] [Google Scholar]
- Verma R, Mishra V, Gupta K, Sasmal D, Raghubir R. Neuroprotection by rosiglitazone in transient focal cerebral ischemia might not be mediated by glutamate transporter-1. J Neurosci Res. 2011;89:1849–1858. doi: 10.1002/jnr.22710. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology. 2012;37:2817–2829. doi: 10.1038/npp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyshkina T, Sylvester A, Sadiq S, Bonilla E, Canter JA, Perl A, Kalman B. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin Immunol. 2008;129:31–35. doi: 10.1016/j.clim.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T. Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004;95:e45–e55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, Ding JQ, Chen SD. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20:1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, Wyse R, Wrathall JR. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia. 2012;60:281–294. doi: 10.1002/glia.21262. [DOI] [PubMed] [Google Scholar]
- Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, Berger JP. Localization of PPARdelta in murine central nervous system: expression in oligodendrocytes and neurons. Brain Res. 2003;975:10–21. doi: 10.1016/s0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997;145:565–573. doi: 10.1006/exnr.1997.6506. [DOI] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Zhang L, Cain L, McAdoo DJ. Administration of glutamate into the spinal cord at extracellular concentrations reached post-injury causes functional impairments. Neurosci Lett. 2005a;384:271–276. doi: 10.1016/j.neulet.2005.04.100. [DOI] [PubMed] [Google Scholar]
- Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuroimmunol. 2006;176:95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Xu J, Drew PD. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2006;171:135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005b;81:403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, Hassan S, Vempati P, Chen F, Qian X, Pasinetti GM. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1alpha) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6:51. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29:6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]