Abstract
Transplantation of organs between genetically different individuals of the same species causes a T cell–mediated immune response that, if left unchecked, results in rejection and graft destruction. The potency of the alloimmune response is determined by the antigenic disparity that usually exists between donors and recipients and by intragraft expression of proinflammatory cytokines in the early period after transplantation. Studies in animal models have identified many molecules that, when targeted, inhibit T-cell activation. In addition, some of these studies have shown that certain immunologic interventions induce transplantation tolerance, a state in which the allograft is specifically accepted without the need for chronic immunosuppression. Tolerance is an important aspect of liver transplantation, because livers have a unique microenvironment that promotes tolerance rather than immunity. In contrast to the progress achieved in inducing tolerance in animal models, patients who receive transplanted organs still require nonspecific immunosuppressant drugs. The development of calcineurin inhibitors has reduced the acute rejection rate and improved short-term, but not long-term, graft survival. However, long-term use of immunosuppressive drugs leads to nephrotoxicity and metabolic disorders, as well as manifestations of overimmunosuppression such as opportunistic infections and cancers. The status of pharmacologic immunosuppression in the clinic is therefore not ideal. We review recently developed therapeutic strategies to promote tolerance to transplanted livers and other organs and diagnostic tools that might be used to identify patients most likely to accept or reject allografts.
Keywords: Regulatory T Cells, Operational Tolerance, Co-stimulation Blockade, Immunosuppression Weaning
Over the past two decades fundamental advances in T-cell biology and in the molecular mechanisms of transplant rejection and tolerance have been identified in animal models. However, translation of these advances into the clinic has been difficult. Similarly, the development and validation on non-invasive biomarkers to personalize the use of immunosuppressive therapy remains a challenge. The aim of this review is to discuss novel therapeutic strategies and diagnostic tools that could allow for the elimination or marked reduction in long term use of immunosuppressive drugs without incurring in an increased risk of rejection.
Molecular and Cellular Basis of Graft Rejection or Acceptance
Mechanisms of Rejection
After transplantation of liver or other organs, antibody-mediated, hyperacute vasculitic rejection can occur in individuals with preformed antibodies against the donor’s major histocompatibility complex (MHC) class I– encoded antigens. Under most other circumstances, acute allograft rejection is initiated by the large number of recipient T cells that recognize donor alloantigens (mostly those encoded by the highly polymorphic MHC).1,2 Transplantation of MHC histoincompatible tissues therefore elicits a strong, cytopathic, T cell– dependent immune response to donor tissues. In this T cell– dependent pathway to rejection, donor alloantigens are processed by specialized antigen-presenting cells (APCs). Donor MHC molecules are internalized by donor and recipient APCs; following intracellular processing, MHC peptide fragments are presented to the recipient’s T cells3 (Figure 1). Antigen presentation involves engagement of these peptide antigenic fragments within a groove on the MHC molecules of the APC surface. Acute cellular rejection is the best-characterized graft-specific form of immune rejection. Clinically apparent acute cellular rejection is defined by an often-sudden deterioration in allograft function; biopsy analysis of the transplanted tissue shows infiltration by host T cells and other mononuclear leukocytes and signs that these infiltrating cells have damaged the graft.
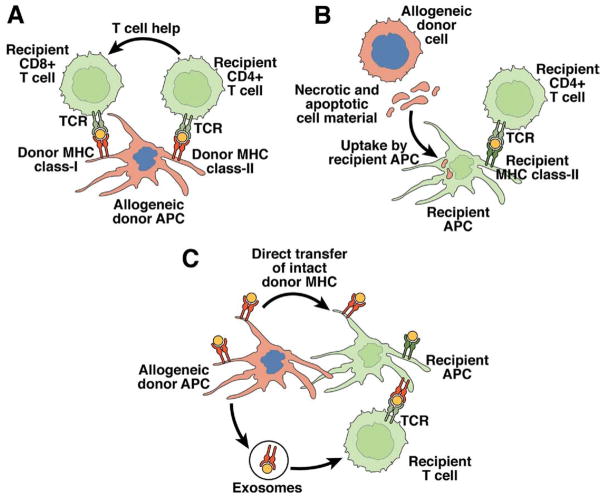
Figure 1.
Pathways of alloantigen presentation. Three nonmutually exclusive pathways of allorecognition have been described. (A) In the direct pathway, recipient T cells recognize intact allogeneic MHC molecules on the surface of donor APCs. The direct pathway is responsible for the large proportion of T cells that have reactivity against alloantigens due to cross-reactivity of the T-cell receptor (TCR) with self and foreign MHC molecules. (B) In the indirect pathway, recipient APCs trafficking through the allograft phagocytose allogeneic material shed by donor cells (mostly peptides derived from allogeneic MHC molecules) and present it to recipient T cells on recipient MHC molecules. (C) In the semidirect pathway, recipient APCs acquire intact MHC molecules following direct contact with donor APCs and/or through fusion with donor APC-derived exosomes. These chimeric recipient APCs stimulate recipient T cells through direct and indirect pathways. Modified from Afzali et al.2
Despite routine use of immunosuppressive therapy, acute rejection is not rare. CD4+ and CD8+ T cells each participate in acute cellular rejection, although the rejection response is mediated primarily by CD4+ T cells. Despite the importance of CD4+ T cells in rejection, many activated CD8+ (cytotoxic) T cells infiltrate the transplant at the time of rejection,4 along with other mononuclear leukocytes; their exact role is not clear. B-cell infiltration indicates severe acute rejection of transplanted kidneys.5 Cells of the innate immune system, such as natural killer (NK) cells, are also present in allografts during rejection. NK cells can recognize alloantigens because they constitutively express inhibitory receptors that are specific for self-MHC class I antigens (the “missing self” theory); their role in the rejection of bone marrow transplants has been recognized for many years. In the field of solid organ transplantation, however, there is only recent experimental evidence for the participation of this cell type in rejection and in tolerance.6
Humoral Rejection Chronic Allograft Failure
The production of anti-donor MHC class I and class II antibodies is also associated with acute and chronic graft damage, usually in the form of transplant vasculopathy. Antibodies can injure the graft by activating complement and mononuclear cells with Fc receptors that recognize the heavy chain of antibody. Fc receptor–expressing leukocytes can thereby be activated by antibody-coated donor cells. Anti-donor antibody can also directly inhibit signaling cascades within endothelial cells.7 Antibody-mediated rejection is frequently observed following kidney, heart, or lung transplantation, whereas liver allografts appear to be resilient to the development of antibody-mediated rejection. Over time, most transplants manifest insidious and inexorable dysfunction. Although this process was formerly called chronic rejection, it is not clear that donor-specific immune processes (ie, rejection) are the sole or even primary cause in many circumstances.8,9 Pathology analysis often reveals fibrosis and atrophy in the absence of infiltration by T cells and other mononuclear leukocytes. Potential additional causes for chronic allograft failure include viral infection, recurrence of the original disease, and drug toxicity. Recurrent infection with hepatitis C virus (HCV) is a major cause of morbidity and loss of function among the large population of liver transplant recipients with HCV-induced hepatitis.10
Memory T-Cell Responses as a Barrier to Tolerance
Following T-cell activation and proliferation, homeostasis of the adaptive immune system is restored by cell death, via “neglect,” of most antigen-specific T cells. A small number of T cells, however, survive and become long-lasting memory cells that ensure protective immunity against pathogens.11 Most resting memory T cells are characterized by high expression levels of CD45RO (CD44 in mice), CD2, and CD11a.12–16 Upon reexposure to donor antigen, donor-reactive memory cells respond faster and more powerfully than naive T cells, thereby directly or indirectly producing cytolytic effects on the transplanted tissue.17,18 Based on their homing properties, memory T cells can be separated into “central” and “effector” cells.19 Central memory T cells recirculate through the spleen and lymph nodes by means of their expression of the homing receptors CD62L and CCR7. In contrast, effector memory T cells down-regulate CD62L and CCR7 and are excluded from lymphoid tissues, migrating to peripheral tissues where they exert rapid and potent effector functions upon antigen rechallenge.19 Therefore, whereas central memory T cells are responsible for recall antigen responses, effector memory T cells survey peripheral tissues and immediately respond to invading pathogens. As a consequence of continuous exposure to foreign antigens, memory T cells accumulate with age and represent approximately 50% of the total T-cell pool in adults.
Patients who have not received a transplanted organ can still generate donor-reactive T cells. This can occur through immunization by direct exposure to alloantigens via pregnancy or blood transfusion.20 Furthermore, donor-reactive memory T cells can be generated in the absence of alloantigen exposure, through heterologous immunity, wherein an antigen-specific immune response, often directed against environmental pathogens, affects the response to an unrelated antigen through cross-reactivity of the T-cell receptor.21 Some memory T cells are therefore primed by an antigenic pathogen-derived peptide and cross-react with allogeneic (often MHC-derived) peptides presented by self or donor MHC molecules. Following transplantation, alloreactive naïve T cells can acquire a memory phenotype and generate a substantial pool of donor-reactive memory T cells, even when the recipient is under immunosuppressive therapy. Furthermore, the use of antibodies that deplete host T cells can amplify this phenomenon by inducing homeostatic T-cell proliferation in response to lymphopenia.22
Because of their capacity to rapidly generate effector immune responses upon rechallenge, memory T cells appear to be particularly efficient at mediating allograft rejection.23,24 In addition, memory T cells are less sensitive than naïve T cells to many immunosuppressive strategies. Compared with conventional T cells, memory T cells are less sensitive to T cell– depleting antibodies25 and to therapeutics that block CD28 and CD154 co-stimulatory signals26,27 or inhibit mammalian target of rapamycin.28 The effects of memory T cells in the allograft response have been well delineated in animal models of allograft tolerance wherein generation of memory T cells by presensitization, heterologous immunity, or homeostatic proliferation prevents the graft-protecting effects of most tolerizing therapeutic strategies.22,27,29,30 In contrast to human recipients, inbred mice that live in the protected environments of transplantation laboratories do not usually contain substantial numbers of memory T cells. This is one of the reasons that may explain the difficulties of translating into the clinic (or into nonhuman primate models, who resemble humans in terms of the size of their memory T-cell pool) the results of protocols capable of creating allograft tolerance in rodent models. Given the lower efficacy of conventional immunosuppressive drugs in the neutralization of previously activated or memory lymphocytes, it is not surprising that memory T cells also exert harmful effects in clinical transplantation. In kidney transplantation, for instance, the proportion of circulating donor-reactive memory T cells, either before31 or after32 transplantation, correlates with the incidence of rejection and graft dysfunction.
Inflammation in T-Cell Commitment and Regulatory Function
Newly engrafted organs are subject to intense inflammation. The accrued injury to the transplant, caused by donor disease, organ procurement, cold preservation in nonphysiological fluids, surgical trauma, and reperfusion injury, leads to release of proinflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor α, and IL-1β. The characteristics of the inflammatory environment in which donor-reactive CD4+ T cells recognize donor antigens determine the lineage commitment of these cells. Thus, depending on the cytokines present when antigen activation occurs, naïve CD4+ helper T cells can acquire a variety of cytopathic and/or immuno-regulatory phenotypes (Figure 1).33 When CD4+ T cells are activated in the presence of IL-12 (usually produced by activated, mature dendritic cells), they become tissue-destructive, interferon gamma–producing T-helper (Th) 1 cells. In contrast, CD4+ T cells that are activated in the presence of IL-4 differentiate into Th2 cells, which produce IL-4 and IL-5. In the absence of proinflammatory cytokines, transforming growth factor (TGF)-β induces expression of Foxp3 and differentiation of CD4+ T cells into regulatory T cells (Tregs). In contrast, expression of TGF-β with IL-6 or IL-21 prevents development of the transplant-protective Tregs; instead, the antigen-reactive CD4+ T cells become IL-17–producing T cells (Th17), which are highly cytopathic.33–36
Until recently, it was believed that antigen-activated helper T cells became terminally differentiated Th1 or Th2 cells that had opposite effects (Th1-dependent cytopathic rejection or Th2-dependent cytoprotective effects; Figure 2). However, the Th1 vs Th2 paradigm is incorrect because Th1 and Th2 can each mediate graft rejection,37,38 whereas regulatory T cells, rather than Th2 cells, are the key inhibitors of cytopathic, allospecific immune responses.39 – 41 Recent discoveries also revealed that, instead of being terminally differentiated, Th17 and Tregs have remarkable plasticity and are closely interlinked.42 Thus, Tregs can differentiate into IL-17–producing cells in the presence of IL-2 and IL-1β,43 whereas in the presence of IL-27, Th17-producing cells also produce IL-10, an immunosuppressive cytokine that prevents them from functioning as destructive effector cells.44
Figure 2.
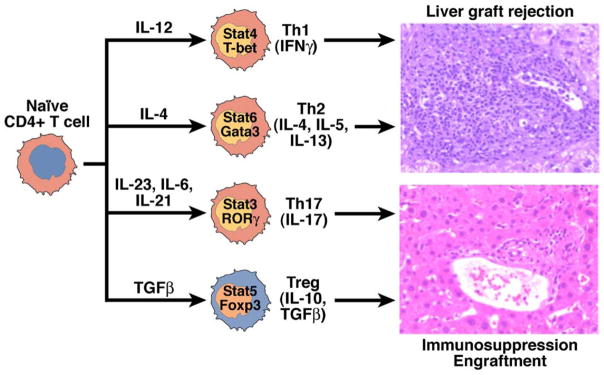
Lineage commitment of naïve CD4+ T cells. Upon activation by cognate antigens and costimulatory signals, recipient naïve CD4+ T cells acquire graft-destructive or graft-protective phenotypes, depending on the local cytokine microenvironment in which T-cell activation occurs. In the presence of the proinflammatory cytokines IL-12 or IL-4, CD4+ T cells become Th1 or Th2 cells, respectively. The presence of TGF-β and the absence of proinflammatory cytokines turns donor-activated CD4+ T cells into tissue-protective Foxp3+ Treg cells and prevents T cells from becoming tissue-destructive Th17 or Th1 cells. In contrast, if TGF-β is present with IL-6 or IL-21, the generation of Tregs is blocked and CD4+T cells acquire the Th17 phenotype. Each of these subsets expresses specific transcription factors and combinations of cytokines.
The current paradigm is that the outcome of transplant recipients, rejection or graft acceptance, is determined by the relative balance between cytopathic Th1 and Th17 CD4+ T cells versus rejection-blocking, cyto-protective regulatory T cells; this balance depends on the level of inflammation in the microenvironment in which T-cell activation takes place (Figure 2). Although the specific role of Th17 cells in allograft rejection is under investigation, events that block T-cell commitment to the graft-protective Treg phenotype prevent the development of transplant tolerance.45 The presence of Th17 cells in the allograft might be a biomarker of detrimental tissue inflammation rather than part of a mechanism that mediates graft destruction. In the peritransplant period, the production of proinflammatory cytokines such as IL-6, tumor necrosis factor (TNF)-α, TGF-β, IL-12, and interferon gamma promote the acquisition of cytodestructive Th1 and Th17, and not FOXP3+ immunoregulatory T-cell cytoprotective phenotypes, by donor reactive T cells. This induces the generation of graft-destructive lymphocyte populations and simultaneously blocks the development and suppressive function of Tregs.45,46 Thus, reagents that prevent inflammation in the graft and draining lymph nodes might help prevent graft rejection and promote tolerance.47,48
Deletion and Regulation in Allograft Tolerance
Acquired tolerance to allogeneic cells was first described more than 50 years ago in neonate mice49; many strategies have since been developed to induce tolerance to allogeneic organs in rodents. In these systems, tolerance is usually defined by a functioning graft without histologic signs of rejection in the absence of immunosuppression and in an immunocompetent host that can accept a second graft from the same donor but reject a graft from a different third-party donor.50
Central tolerance to self-antigens results primarily from apoptotic deletion of autoreactive T cells during intrathymic T-cell development; this process does not completely or permanently delete autoreactive T cells. In transplantation animal models, multi-lineage, donor-recipient–mixed hematopoietic chimerism recapitulates many of the features of central tolerance, deleting most or all alloreactive T cells and enabling drug-free tolerance to the transplanted tissue. In the absence of permanent deletion of donor-reactive cytopathic T cells through central tolerance, immunity can still tend toward tolerance through an increase in the ratio of CD4+FOXP3+ Tregs to effector T cells, which enables Tregs to permanently inhibit the actions of donor-reactive effector T cells (peripheral tolerance). Rejection or tolerance therefore depends on the balance between donor-reactive cytopathic effector and cytoprotective Tregs.
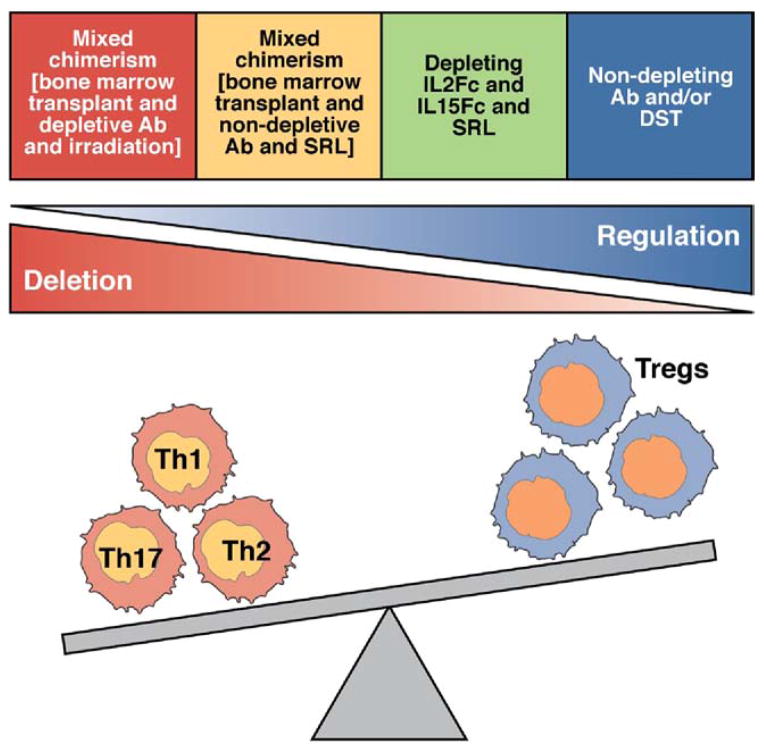
We propose that peripheral tolerance to MHC-mismatched grafts is achieved via the complementary mechanisms of deletion and regulation51 (Figure 3). This model posits that induction of tolerance initially requires the depletion of the large number of alloreactive T cells. Depletion and immunoregulation appear to function separately, in chronological order, to create peripheral tolerance. For instance, costimulation blockade initially depletes alloreactive effector cytopathic T cells; tolerance cannot be induced in mice whose T cells are resistant to apoptosis.52,53 Infiltration of the allograft by effector, but not Tregs, is reduced in tolerant hosts54 (Figure 4). Once the effector T cells are depleted, tolerance is, at least at first, quasi-stable and is maintained via the action of Tregs, which restrain nondeleted alloreactive cells and new alloreactive thymic emigrants. In essence, mechanisms that deplete donor-reactive effector T cells lower the threshold for effective regulatory T-cell action. In animal models, a complex array of costimulatory signals regulates both rejection and tolerance. These pathways can have either stimulatory or inhibitory effects and can regulate the activation of naïve, memory, and regulatory T cells. Reagents that target costimulatory pathways could promote transplantation tolerance (Supplementary Table 1). In patients, attempts to achieve stable multi-lineage mixed donor-recipient hematopoietic chimerism have not been as successful as in rodents. Protocols adapted from research in mice have nonetheless allowed some patients to achieve long-term drug-free survival after kidney transplantation (see the following text).
Figure 3.
Pool size model of the allograft response. The ultimate outcome of graft rejection or tolerance depends on the relative balance between rejection-prone effector T cells and rejection-blocking, immunosuppressive Tregs. In one model, on activation, CD4+T cells became terminally differentiated Th1 or Th2 cells; Th1 cells mediate allograft rejection, whereas Th2 cells protect allografts from tissue-destructive Th1 cells. Th2 cells are most prominent in grafts of tolerant hosts. However, allograft rejection still occurs in the absence of Th1 cells, and the recent discovery of 2 additional types of CD4+ T cells (Tregs and Th17) that have plasticity has invalidated the Th1 and Th2 model. In a new model, CD4+ Tregs, rather than Th2 cells, protect foreign tissues from the destructive effects of cytopathic T cells. Tolerogenic therapeutic strategies differ in their capacity to directly delete effector T cells and/or promote the function and number of Tregs.
Figure 4.
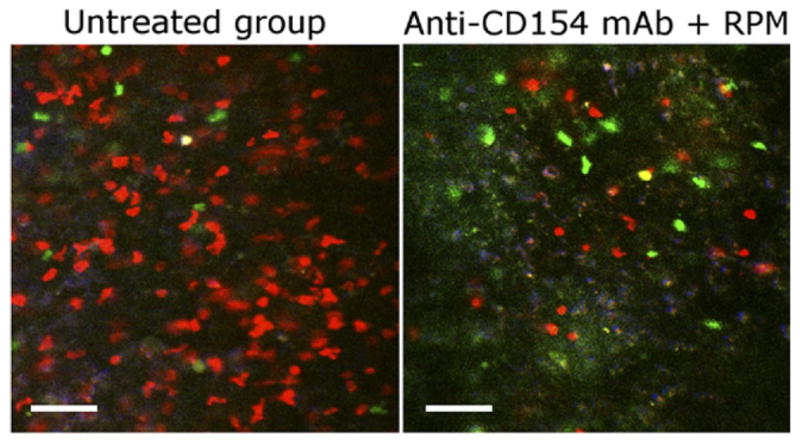
Tolerogenic agents modulate the type of T cells that infiltrate the allograft. The fate of the allograft, either rejection or tolerance, depends on the functional balance of alloreactive graft-protecting Tregs to alloreactive graft-destroying T effector cells. In the absence of a favorable change in this balance, Tregs are unable to restrain effector T cells from rejecting the graft. However, in response to tolerogenic agents such as anti-CD154 and rapamycin (RPM), the magnitude of allograft infiltration by effector T cells decreases, whereas the number of natural or induced regulatory T cells (nTregs/iTregs) is preserved or increased. As a consequence, the proportion of allograft-infiltrating Treg to T effector cells is increased in tolerized hosts. The representative intravital microscopy image shows the infiltration of color-coded T cells within the allograft 1 week after transplantation in mice following islet transplantation. Infiltrating nTregs, green; iTregs, yellow; T effectors, red. Modified from Fan et al.54
Immunity and Tolerance in the Liver
The liver exhibits a unique immunologic micro-environment and responds differently than other organs to rejection and immune-mediated injuries. This micro-environment depends on the liver anatomy; the liver has portal and arterial blood supplies that circulate blood to the fenestrated sinusoidal capillary system from the intestinal and systemic circulation. Portal venous blood is rich in food-derived antigens and in bacterial degradation products such as endotoxin. The continuous exposure of liver cells to these molecules leads to “endotoxin tolerance.”55 A consequence of this process is that antigen presentation within the liver, which is mediated by a variety of cell types such as dendritic cells, Kupffer cells, sinusoidal endothelial cells, and hepatocytes, leads to tolerization of T cells rather than T cell–mediated immunity.56 These mechanisms are likely to be responsible for the spontaneous acceptance of liver allografts observed in animal models and selected patients.57 Following infection, however, tolerance can be reversed and result in T-cell immunity against microorganisms such as hepato-tropic viruses. Innate immune cells such as NK and NKT cells, which are present in greater numbers in the liver than in other organs, contribute to these pathogen-induced immune responses. Given the difficulties in generating efficient adaptive immune responses within the liver, the role of innate immune mechanisms in the induction of defensive and antimicrobial responses is probably greater than in other tissues.56
The mechanisms responsible for the switch from tolerance induction to immunogenicity, however, have not been clarified; this is an important area of study in the field of liver immunology.55 The question of why, in patients with recurrent HCV infection, HCV-specific T-cell clones, some of which are presumed to be alloreactive, do not usually induce rejection despite heavy infiltration of the graft also remains to be answered. Some HCV-positive recipients develop spontaneous tolerance and can completely discontinue immunosuppressive therapy.58 Another aspect of recurrent HCV infection that is incompletely understood is the relative role of anti-HCV T-cell responses, restricted by donor versus recipient HLA, in containing the viral infection and preventing liver damage.
Clinical Transplantation Immunology
Status of Immunosuppressive Therapy
The introduction of calcineurin inhibitor–type immunosuppressive drugs in the 1980s substantially reduced acute graft rejection and improved rates of early engraftment.59 Despite progress in experimental immunology, therapeutic regimens have not substantially evolved over the past 20 years; we continue to rely on nonspecific immunosuppressive drugs. Immunosuppressants (small molecules or biologics) used in transplantation have been developed for their ability to inhibit T-cell responses by directly depleting T cells, interfering with lymphocyte traffic, or targeting molecules that transduce the signals required for T-cell activation (Figure 5 and Supplementary Table 3). Immunosuppressive drugs are combined to achieve potent and safe effects in the immediate posttransplantation period; doses are gradually decreased thereafter. This dose reduction is based on the concept that as graft inflammation subsides and donor-derived APCs are cleared, the transplanted organ progressively adapts to the host, decreasing in immunogenicity and risk of rejection.60 The immunosuppressive drugs most frequently given to patients in the early posttransplantation period include a calcineurin inhibitor, azathioprine or mycophenolate mofetil, and corticosteroids; patients then receive maintenance therapy with lower doses of the same combination (with or without discontinuation of corticosteroids). Short-term induction therapy with a biologic (an antibody or immunoglobulin fusion protein) is often added to the protocol, based on immunogenicity of the transplanted organ (livers are the least immunogenic and hearts, lungs, and intestines are the most). Immunosuppressive therapy of liver transplant recipients is therefore often less aggressive than for recipients of other tissues.
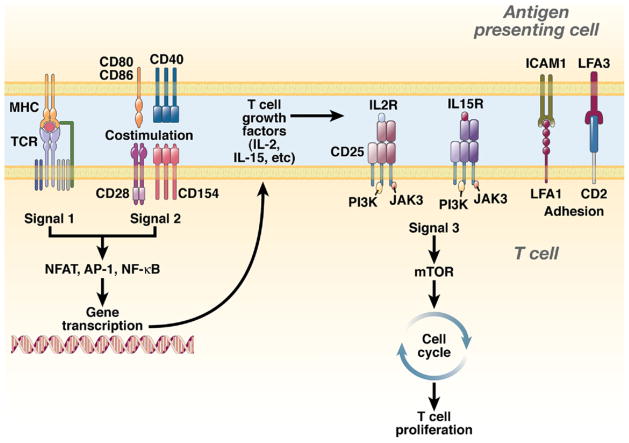
Figure 5.
Targets of immunosuppressive drugs used in solid organ transplantation, according to the 3-signal model of T-cell activation. T-cell activation is initiated by signal 1, which is delivered by the interaction between the TCR and the MHC-peptide complex presented by the APC. In transplantation, signal 1 can be delivered through the direct and/or indirect pathways of allorecognition and it defines the specificity of the alloimmune response. Signal 1 alone is not capable of generating productive T-cell responses. However, in combination with signal 2 (also known as the costimulatory signal), signal 1 triggers several intracellular signaling pathways, such as the calcium-calcineurin pathway, mitogen-activated protein (MAP) kinase pathway, and the protein kinase C/nuclear factor κB (NF-κB) pathway; together, these activate transcription factors that mediate cell survival and expression of cytokines. Several cytokines (IL-2, IL-15, IL-4, IL-7, IL-21) induce proliferation (signal 3) through the phosphoinositide 3-kinase (PI3-K) and mammalian target of rapamycin (mTOR) pathways. APCs express different costimulatory molecules; many deliver activation signals that amplify signal 1 and prevent T-cell anergy (eg, CD28-CD80/86 and CD154-CD40 pathways), whereas others inhibit T-cell activation (eg, CTLA4-CD80/86 and PD1-PDL1/PDL2 pathways).
In recent years, the main end point of immunosuppressive therapy has shifted from the prevention of acute rejection toward the preservation of long-term graft function and prevention of immunosuppression-related side effects (particularly nephrotoxicity, cancer, and cardiovascular events). This has fueled a search for new immunosuppressive drugs that can replace the toxic, broad immunosuppressive drugs such as corticosteroids and calcineurin inhibitors (Supplementary Table 2). In addition, recognition of the pathogenic role of donor-specific antibodies has prompted the development of strategies to target B cells, plasma cells, and other elements of the humoral immune response61 (Supplementary Table 2). This is particularly relevant to kidney and heart transplantation; livers, in contrast, appear to be more resilient to antibody-mediated damage. Another innovation has been the use of biologics in maintenance immunosuppression regimens. Unlike conventional small drugs designed to treat patients with immune system diseases or following transplantation, biologics only bind to immune cells, reducing or preventing toxicity to non–immune system tissues. Biologics have long half-lives and require only intermittent administration. Agents such as belatacept, which blocks T-cell costimulation, are being tested as a maintenance drug for recipients of kidney transplants62; patients given this drug do not need to receive corticosteroids or calcineurin inhibitors, which are nephrotoxic. Through reduced reliance on long-term administration of calcineurin inhibitors, renal function improves in this patient population. The use of biologics as maintenance agents is likely to increase because of the low toxicity of these immune system–specific therapeutics.
Inducing Tolerance to Transplanted Organs
Although we have learned much about T-cell biology and the molecular mechanisms of tolerance from studies in mice, translation of these advances into the clinic or even nonhuman primate models has been difficult. This is because of differences between rodents and primates (eg, the barrier of memory T-cell responses) and practical and ethical constraints associated with the implementation of immunosuppressive drug withdrawal strategies in patients.63– 65 To bring transplantation tolerance studies to the clinic, international collaborative consortia have been created, such as the Reprogramming the Immune System for the Establishment of Tolerance (RISET; www.risetfp6.org) in Europe and the Immune Tolerance Network (ITN; www.immunetolerance.org) in the United States.
The most successful interventional strategy derived from clinical studies has been the induction of tolerance in some HLA-mismatched kidney allograft recipients following induction of transient mixed chimerism using donor bone marrow transplantation and a nonmyeloablative conditioning regimen.66 This approach was based on animal studies that showed the tolerizing effects of mixed-donor hematopoietic chimerism and case reports in which bone marrow transplant recipients accepted liver or kidney grafts from the same donors in the absence of immunosuppressive therapy.67–71 Based on these studies and a trial conducted in patients with advanced multiple myeloma who received HLA-identical kidneys,72 recipients of kidney transplants were given a combination of anti-CD2 antibody (MEDI-507), cyclophosphamide, thymic irradiation, cyclosporin A, rituximab, corticosteroids, and donor bone marrow.66 Four of 5 recipients were able to discontinue immunosuppression in the first year after transplantation and maintained graft function for 3 to 6 years (one graft was lost to rejection). The intense conditioning protocol, appropriate for patients with myeloma but not all recipients, raises concerns about the widespread applicability of the regimen. A similar strategy, which used donor bone marrow and total body irradiation, has induced tolerance in recipients of kidney transplants but was only effective in HLA-identical donor-recipient combinations.73
Although long-term T-cell depletion is sufficient to induce allograft tolerance in animal models,74,75 it does not induce tolerance in patients.76,77 T-cell depletion might enable graft acceptance with reduced doses of conventional immunosuppressants, a concept proposed by Roy Calne called “prope” tolerance.78 Prope tolerance protocols have been tested in several clinical trials of kidney and liver recipients.79 – 82 Although these regimens are safe, it is unclear whether they produce better long-term outcomes than conventional immunosuppressive protocols.83
Strategies that have shown tolerogenic effects in animal models and are ready for clinical application include the combination of costimulatory blockade reagents and T-cell depletion, as well as Treg therapy. Concerns have been raised about testing these approaches in recipients of organs such as kidney or heart; an episode of acute rejection could severely affect graft survival. In these settings, most physicians are reluctant to withdraw immunosuppressive drugs in the absence of validated biomarkers of transplantation tolerance. Liver allografts, in contrast, are resilient to rejection injury and can occasionally promote spontaneous tolerance; patients who receive liver transplants might be the best population for studies of tolerance induction.
Spontaneous Operational Tolerance
In contrast to the intentional induction of allograft tolerance, successful only in highly selected recipients following aggressive conditioning,66 “spontaneous” long-term acceptance of transplanted organs following discontinuation of conventional immunosuppression has been much more frequently observed. This “spontaneous operational tolerance” is defined as the long-term maintenance of stable graft function without a clinically significant, detrimental immune response or immune deficit.50,57,84,85 Patients who received kidney, or more frequently liver, transplants have been reported to achieve operational tolerance. In kidney transplantation, most tolerant recipients are noncompliant patients or those who discontinued immunosuppression because of severe side effects. In liver transplantation, in contrast, several trials have been conducted to assess the feasibility of purposely discontinuing all immunosuppressive drugs over under medical supervision (Supplementary Table 2).58,86 –95 This strategy was successful in almost 20% of the participants. However, 2 recent unpublished studies showed that in stable recipients of liver transplants, operational tolerance might occur more frequently than originally estimated, particularly at later time points (more than 10 years after transplantation) or in pediatric patients.96,97 In these immunosuppressive drug-weaning trials conducted in liver transplantation, rejection occurred very frequently but episodes tended to be mild and easily resolved without the need to administer high doses of immunosuppressants. This strategy has some risks, however, and requires close monitoring and frequent collection of liver biopsy samples; rejection often occurs with minimal increases in liver function test results, and transitory biochemical abnormalities can be observed in the absence of rejection.57,85 Furthermore, the histologic identification of cellular rejection during or after dose reduction can be challenging. Although portal and/or perivenular inflammation are almost always observed in patients who undergo graft rejection after withdrawal of immunosuppression, these patients often have less inflammatory bile duct damage and more lobular necroinflammatory activity than patients who reject tissues in the immediate posttransplantation period; the histologic results observed resemble hepatitis more than rejection.85,98
Success in discontinuation of immunosuppressant therapy correlates with close matching of HLA types between donor and recipient, the absence of autoimmune liver diseases, and little or no inflammatory infiltrate in the graft.86,99 The type of immunosuppressive therapy might also determine the ability to successfully withdraw immunosuppressive therapy.100 Chronic HCV infection does not preclude withdrawal of immunosuppression,101 although it complicates patient monitoring because it is difficult to differentiate cellular rejection from HCV-mediated graft inflammation by histologic analysis. Studies indicate that operational tolerance is stable, particularly by 2 years after discontinuation of immunosuppressive therapy.102 Nonetheless, in the absence of long-term follow-up studies with biopsy analyses, it is not clear whether the long-term absence of immunosuppressive drugs increases development of subclinical rejection-related histologic lesions.103
Biomarkers of Rejection or Tolerance
Biomarkers are needed to better evaluate the immune status of transplant recipients, diagnose graft rejection noninvasively, and individualize immunosuppressive therapy. Technical advances in multi-parameter flow cytometry, antigen-specific lymphocyte assays, and genome-wide analyses (proteomics, microarray, and quantitative reverse-transcription polymerase chain reaction gene expression profiling) have led to the development of powerful and easier-to-standardize immunomonitoring techniques to characterize alloimmune responses. Histologic analysis remains the best way to detect graft rejection, but collection of biopsy samples is an invasive procedure that cannot be routinely performed for surveillance purposes; it is also associated with sampling errors and interpretation biases. Molecular or cell-based biomarkers could be used to monitor immune status and detect rejection or immune events before the transplanted organ is damaged. These developments could enable individualized day-to-day patient management care.
Upon rejection, activated anti-donor cytotoxic T cells from the recipient infiltrate the graft and kill donor cells. Following the original report by Vasconcellos et al104 showing increased levels of messenger RNA transcripts derived from cytotoxic T cells in peripheral blood mono-nuclear cells (PBMCs) collected from kidney recipients undergoing graft rejection, many laboratories investigated the use of blood and urine cell transcriptional biomarkers to noninvasively detect acute rejection of kidney. Urine cell specimens are useful because they provide a representative sample of the entire kidney allograft. Many transcripts have been reported to be associated with acute rejection in kidney transplantation, detected in urine (PRF, GZB, TIM-3, PI-9, CD103, IP-10, Foxp-3, CXCR3, NKG2D)105–110 or PBMCs (PRF, GZB, FASL, CD40L, IFNγ, IL-4, IL-5, IL-6).104,111,112
Messenger RNA biomarkers of acute rejection have also been investigated in PBMCs from patients who received heart113,114 and pancreas115,116 allografts. In heart transplantation, the Cardiac Allograft Rejection Gene Expression Observation (CARGO) studies117–119 led to the development of a noninvasive, commercially available diagnostic test for acute rejection (www.allomap.com). Messenger RNA biomarkers have been investigated in bronchoalveolar fluid from patients who received lung transplants120,121 but have not been explored in liver or intestinal allograft recipients.
Cell-based functional assays are more cumbersome and difficult to standardize than molecular tests, although they have been used to assess the risk of rejection, before or after transplantation, through quantification of allospecific T cells.31,32,122,123 Once noninvasive biomarkers of rejection are identified, they must be validated in independent prospective trials. Excellent examples of attempts to develop and validate biomarkers are presented by the National Institutes of Health–sponsored Clinical Trials in Organ Transplantation (www.ctotstudies.org).
Identification of Operationally Tolerant Transplant Recipients
The development of noninvasive biomarkers of tolerance has been hampered by the lack of effective means to induce tolerance in patients. For this reason, it is important to better characterize operationally tolerant recipients; they might have a signature (such as a PBMC gene expression profile) of tolerance that could be used to identify patients in whom immunosuppressive therapy could be reduced or stopped (Figure 6). In kidney transplantation, Brouard et al124 used a custom complementary DNA array platform to compare PBMC expression profiles of tolerant organ recipients, healthy individuals, and patients undergoing chronic organ rejection. Expression levels of a set of 49 genes could discriminate patients with graft tolerance from patients with chronic rejection and from healthy individuals. The same group reported that, compared with patients undergoing chronic rejection, operationally tolerant kidney recipients had increased numbers of peripheral blood B cells and CD4+CD25+ T cells.125 In a study funded by the ITN and one by the RISET consortium, a combination of different immunomonitoring assays was used to compare large cohorts of operationally tolerant kidney recipients with healthy individuals and stable recipients of kidneys who received maintenance immunosuppression.126,127 In both studies, tolerant recipients had unique, whole-blood gene expression patterns and lymphocyte subset profiles that accurately discriminated between tolerant and nontolerant recipients. Blood samples from tolerant recipients had increased numbers of B cells and a significant over-expression of B cell–related genes. Interestingly, B cell–related transcripts were detected in urine samples collected from tolerant recipients.126 Gene expression profiling studies have also been conducted in patients who underwent liver transplantation.128 –130 In the largest published study, Martínez-Llordella et al129 analyzed blood samples from operationally tolerant and nontolerant recipients of liver grafts and identified gene expression “classifiers” that detected tolerant recipients in the training group and in an independent validation cohort. Functional analysis of the whole expression data set identified the NK cell signaling pathway as the most significant in association with tolerance. In additional flow cytometry studies, specific PBMC subsets (CD4+CD25+Foxp3 T cells, Vδ1+ γδ T cells, plasmacytoid dendritic cells) were increased in tolerant recipients of liver grafts.128,131,132 A role for Tregs in the maintenance of tolerance has also been proposed by the observation that tolerant recipients exhibited increased Foxp3 expression levels in blood88 and in liver tissue samples.133
Figure 6.
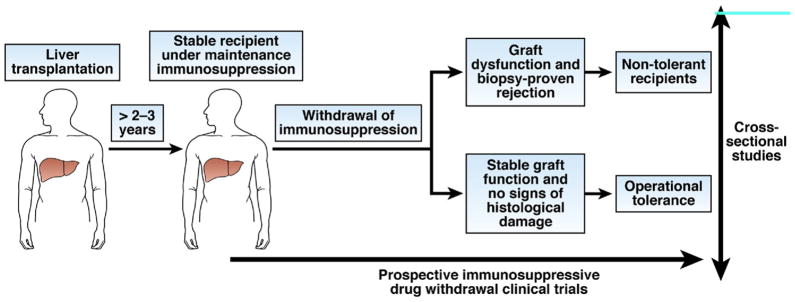
Identification of biomarkers of operational tolerance in clinical transplantation. The search for diagnostic biomarkers of operational tolerance has been conducted mainly in cross-sectional case-control studies, in which peripheral blood samples collected from recipients who became tolerant to transplanted organs after ending immunosuppressive therapy were compared with samples collected from control recipients. In liver transplantation, stable recipients under maintenance immunosuppression with the history of a previous failed attempt at drug withdrawal are used as the control group; in these recipients, the absence of tolerance has been established. Patients who failed to induce tolerance after kidney transplantation and drug withdrawal are not available, so stable recipients who receive maintenance immunosuppression or patients with chronic humoral rejection are used as the control group. In these studies, the confounding effects of pharmacologic immunosuppression are of concern; biomarker discovery trials should be designed as prospective studies in which immune monitoring analyses are performed before immunosuppressive drugs are discontinued. These types of studies have only been attempted with patients undergoing liver transplantation.
These findings indicate that peripheral blood samples and modern immunomonitoring techniques can be used to identify markers of tolerance in transplant recipients. The main limitations of these cross-sectional case-control studies are the small sample sizes due to the paucity of operationally tolerant recipients and difficulties in accounting for the confounding effects of pharmacologic immunosuppression in the control recipient group (eg, stable recipients of transplants who receive maintenance immunosuppression or recipients who undergo graft rejection). Furthermore, because most studies do not include analysis of allograft tissue material, mechanisms of tolerance in patients are hard to determine. Recently identified biomarkers of tolerance should be included in large prospective validation trials (Figure 6), but this has only been attempted in liver transplantation studies; preliminary data are promising.96,97,100
Future Directions
Administration of lymphocyte-depleting antibodies, radiation, and conventional immunosuppressive drugs induces tolerance in at least some patients, although this occurs in the absence of long-term mixed chimerism or permanent clonal deletion. This aggressive regimen might not be well suited for many recipients, but it is effective for a subset. Costimulation blockade, which induces tolerance in rodents but not nonhuman primates, has been tested in patients who underwent renal transplantation and produced interesting results; it might be useful but by itself does not induce tolerance.
A major impediment to inducing tolerance is the presence of memory T cells with antigen receptors that react or cross-react with histocompatibility antigens. Another major barrier is inflammation, which damages the graft in the immediate posttransplantation period and promotes cytopathic, not cytoprotective, immune responses.
Molecular signatures of transplant tolerance have been identified in liver and kidney recipients; progress is expected for other organs. A strategy for inducing tolerance that we favor is to administer standard therapy in the early posttransplantation period. In patients with a clinically favorable course, a decrease or complete discontinuation of immunosuppressive drugs might be attempted after the period of posttransplantation adverse inflammation has ended. Individualized assessments of patients for molecular signatures of tolerance could identify those who are the best candidates for reduction or elimination of maintenance therapy. During and after drug withdrawal, the patients could be monitored for molecular signatures that warn of impending rejection; these patients could be placed on antirejection therapy before the advent of clinical rejection.
Supplementary Material
Abbreviations used in this paper
- Ab
antibody
- APC
antigen-presenting cell
- DST
donor specific transfusion
- IL
interleukin
- IL2Fc
IL-2 fusion protein
- IL15Fc
IL-15 fusion protein
- ITN
Immune Tolerance Network
- MHC
major histocompatibility complex
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- RISET
Reprogramming the Immune System for the Establishment of Tolerance
- SRL
sirolimus
- TGF
transforming growth factor
- Th
T-helper
- Th17
IL-17–producing T cell
- Treg
regulatory T cell
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2010.10.059.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Stefanova I, Dorfman JR, Tsukamoto M, et al. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 2.Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69:545–556. doi: 10.1111/j.1399-0039.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 3.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strom TB, Tilney NL, Carpenter CB, et al. Identity and cytotoxic capacity of cells infiltrating renal allografts. N Engl J Med. 1975;292:1257–1263. doi: 10.1056/NEJM197506122922402. [DOI] [PubMed] [Google Scholar]
- 5.Sarwal M, Chua MS, Kambham N, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer A, Edtinger K, Li XC. The innate natural killer cells in transplant rejection and tolerance induction. Curr Opin Organ Transplant. 2008;13:339–343. doi: 10.1097/MOT.0b013e3283061115. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Atz ME, Reed EF. Human leukocyte antigen antibodies in chronic transplant vasculopathy-mechanisms and pathways. Curr Opin Immunol. 2009;21:557–562. doi: 10.1016/j.coi.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seetharam A, Tiriveedhi V, Mohanakumar T. Alloimmunity and autoimmunity in chronic rejection. Curr Opin Organ Transplant. 2010;15:531–536. doi: 10.1097/MOT.0b013e32833b31f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nankivell BJ, Chapman JR. Chronic allograft nephropathy: current concepts and future directions. Transplantation. 2006;81:643–654. doi: 10.1097/01.tp.0000190423.82154.01. [DOI] [PubMed] [Google Scholar]
- 10.O’Grady JG. Phenotypic expression of recurrent disease after liver transplantation. Am J Transplant. 2010;10:1149–1154. doi: 10.1111/j.1600-6143.2010.03080.x. [DOI] [PubMed] [Google Scholar]
- 11.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 12.Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45RO (UCHL1) Int Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- 13.Budd RC, Cerottini JC, Horvath C, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 14.Wallace DL, Beverley PC. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990;69:460–467. [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Hengel RL, Thaker V, Pavlick MV, et al. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Langenkamp A, Geginat J, et al. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167–171. doi: 10.1007/978-3-642-57276-0_21. [DOI] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 20.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transplant. 2004;4:846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 21.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 24.Schenk AD, Nozaki T, Rabant M, et al. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 26.Vu MD, Clarkson MR, Yagita H, et al. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176:1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 27.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 31.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 32.Bestard O, Nickel P, Cruzado JM, et al. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. 2008;19:1419–1429. doi: 10.1681/ASN.2007050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strom TB, Koulmanda M. Recently discovered T cell subsets cannot keep their commitments. J Am Soc Nephrol. 2009;20:1677–1680. doi: 10.1681/ASN.2008101027. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-) evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 37.Strom TB, Roy-Chaudhury P, Manfro R, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–693. doi: 10.1016/s0952-7915(96)80087-2. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Zand MS, Li Y, et al. On histocompatibility barriers, Th1 to Th2 immune deviation, and the nature of the allograft responses. J Immunol. 1998;161:2241–2247. [PMC free article] [PubMed] [Google Scholar]
- 39.Hall BM, Pearce NW, Gurley KE, et al. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J Exp Med. 1990;171:141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 41.Waldmann H, Chen TC, Graca L, et al. Regulatory T cells in transplantation. Semin Immunol. 2006;18:111–119. doi: 10.1016/j.smim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell P, Afzali B, Lombardi G, et al. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14:326–331. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 43.Koenen HJ, Smeets RL, Vink PM, et al. Human CD25high Foxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 44.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Ahmed E, Wang T, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koulmanda M, Bhasin M, Hoffman L, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105:16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis EC, Mizrahi M, Toledano M, et al. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A. 2008;105:16236–16241. doi: 10.1073/pnas.0807627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billingham R, Brent L, Medawar P. Acquired immunological tolerance to foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 50.Ashton-Chess J, Giral M, Brouard S, et al. Spontaneous operational tolerance after immunosuppressive drug withdrawal in clinical renal allotransplantation. Transplantation. 2007;84:1215–1219. doi: 10.1097/01.tp.0000290683.54937.1b. [DOI] [PubMed] [Google Scholar]
- 51.Li XC, Strom TB, Turka LA, et al. T cell death and transplantation tolerance. Immunity. 2001;14:407–416. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Li XC, Zheng XX, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 53.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 54.Fan Z, Spencer JA, Lu Y, et al. In vivo tracking of ‘color-coded’ effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kern M, Popov A, Kurts C, et al. Taking off the brakes: T cell immunity in the liver. Trends Immunol. 2010;31:311–317. doi: 10.1016/j.it.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 57.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774–1780. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 58.Tisone G, Orlando G, Cardillo A, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702–709. doi: 10.1016/j.jhep.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 59.Grinyo JM, Cruzado JM. Mycophenolate mofetil and calcineurin-inhibitor reduction: recent progress. Am J Transplant. 2009;9:2447–2452. doi: 10.1111/j.1600-6143.2009.02812.x. [DOI] [PubMed] [Google Scholar]
- 60.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 61.Stegall MD, Gloor JM. Deciphering antibody-mediated rejection: new insights into mechanisms and treatment. Curr Opin Organ Transplant. 2010;15:8–10. doi: 10.1097/MOT.0b013e3283342712. [DOI] [PubMed] [Google Scholar]
- 62.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 63.Kirk AD. Meteorology and tolerance. Am J Transplant. 2006;6:645–646. doi: 10.1111/j.1600-6143.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 64.Halloran PF, Bromberg J, Kaplan B, et al. Tolerance versus immunosuppression: a perspective. Am J Transplant. 2008;8:1365–1366. doi: 10.1111/j.1600-6143.2008.02289.x. [DOI] [PubMed] [Google Scholar]
- 65.Thomson AW, Mazariegos GV, Reyes J, et al. Monitoring the patient off immunosuppression. Conceptual framework for a proposed tolerance assay study in liver transplant recipients. Transplantation. 2001;72:S13–S22. [PubMed] [Google Scholar]
- 66.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sayegh MH, Fine NA, Smith JL, et al. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 68.Helg C, Chapuis B, Bolle JF, et al. Renal transplantation without immunosuppression in a host with tolerance induced by allogeneic bone marrow transplantation. Transplantation. 1994;58:1420–1422. [PubMed] [Google Scholar]
- 69.Matthes-Martin S, Peters C, Konigsrainer A, et al. Successful stem cell transplantation following orthotopic liver transplantation from the same haploidentical family donor in a girl with hemophagocytic lymphohistiocytosis. Blood. 2000;96:3997–3999. [PubMed] [Google Scholar]
- 70.Urban CH, Deutschmann A, Kerbl R, et al. Organ tolerance following cadaveric liver transplantation for chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30:535–537. doi: 10.1038/sj.bmt.1703688. [DOI] [PubMed] [Google Scholar]
- 71.Kadry Z, Mullhaupt B, Renner EL, et al. Living donor liver transplantation and tolerance: a potential strategy in cholangiocarcinoma. Transplantation. 2003;76:1003–1006. doi: 10.1097/01.TP.0000083981.82522.13. [DOI] [PubMed] [Google Scholar]
- 72.Buhler LH, Spitzer TR, Sykes M, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 73.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 74.Knechtle SJ, Vargo D, Fechner J, et al. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation. 1997;63:1–6. doi: 10.1097/00007890-199701150-00002. [DOI] [PubMed] [Google Scholar]
- 75.Thomas JM, Neville DM, Contreras JL, et al. Preclinical studies of allograft tolerance in rhesus monkeys: a novel anti-CD3-immunotoxin given peritransplant with donor bone marrow induces operational tolerance to kidney allografts. Transplantation. 1997;64:124–135. doi: 10.1097/00007890-199707150-00022. [DOI] [PubMed] [Google Scholar]
- 76.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 77.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 78.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 79.Ciancio G, Burke GW, Gaynor JJ, et al. The use of Campath-1H as induction therapy in renal transplantation: preliminary results. Transplantation. 2004;78:426–433. doi: 10.1097/01.tp.0000128625.29654.eb. [DOI] [PubMed] [Google Scholar]
- 80.Knechtle SJ, Pascual J, Bloom DD, et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. Am J Transplant. 2009;9:1087–1098. doi: 10.1111/j.1600-6143.2009.02581.x. [DOI] [PubMed] [Google Scholar]
- 81.Shapiro R, Jordan ML, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pretreatment and low-dose postoperative immunosuppression with subsequent weaning. Ann Surg. 2003;238:520–525. doi: 10.1097/01.sla.0000089853.11184.53. discussion 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson CJ, Bradley JA, Friend PJ, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation—efficacy and safety at five years. Am J Transplant. 2005;5:1347–1353. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 83.Benitez C, Puig-Pey I, Lozano JJ, et al. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am J Transplant. 2010;10:9. doi: 10.1111/j.1600-6143.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- 84.Girlanda R, Kirk AD. Frontiers in nephrology: immune tolerance to allografts in humans. J Am Soc Nephrol. 2007;18:2242–2251. doi: 10.1681/ASN.2007020180. [DOI] [PubMed] [Google Scholar]
- 85.Demetris AJ, Lunz JG, III, Randhawa P, et al. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transpl Int. 2009;22:120–141. doi: 10.1111/j.1432-2277.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 86.Devlin J, Doherty D, Thomson L, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926–933. doi: 10.1002/hep.510270406. [DOI] [PubMed] [Google Scholar]
- 87.Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pons JA, Revilla-Nuin B, Baroja-Mazo A, et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation. 2008;86:1370–1378. doi: 10.1097/TP.0b013e318188d3e6. [DOI] [PubMed] [Google Scholar]
- 89.Pons JA, Yelamos J, Ramirez P, et al. Endothelial cell chimerism does not influence allograft tolerance in liver transplant patients after withdrawal of immunosuppression. Transplantation. 2003;75:1045–1047. doi: 10.1097/01.TP.0000058472.71775.7D. [DOI] [PubMed] [Google Scholar]
- 90.Orlando G, Manzia T, Baiocchi L, et al. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol. 2008;20:43–47. doi: 10.1016/j.trim.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Eason JD, Cohen AJ, Nair S, et al. Tolerance: is it worth the risk? Transplantation. 2005;79:1157–1159. doi: 10.1097/01.tp.0000162084.46555.10. [DOI] [PubMed] [Google Scholar]
- 92.Takatsuki M, Uemoto S, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 93.Tryphonopoulos P, Tzakis AG, Weppler D, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant. 2005;5:608–613. doi: 10.1111/j.1600-6143.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 94.Girlanda R, Rela M, Williams R, et al. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplant Proc. 2005;37:1708–1709. doi: 10.1016/j.transproceed.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 95.Assy N, Adams PC, Myers P, et al. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: role of ursodeoxycholic acid. Transplantation. 2007;83:1571–1576. doi: 10.1097/01.tp.0000266678.32250.76. [DOI] [PubMed] [Google Scholar]
- 96.Benitez C, Lozano JJ, Martínez-Llordella M, et al. Use of transcriptional biomarkers to identify liver transplant recipients who can successfully discontinue immunosuppressive therapy (abstr 213). Am J Transplant; Paper presented at: American Transplant Congress; May 20–Jun 3, 2009; Boston, MA. 2009. p. 252. [Google Scholar]
- 97.Feng S, Ekong U, Lobritto S, et al. ITN029ST: Immunosuppression withdrawal in pediatric recipients of parental living donor liver transplants: preliminary results of a pilot study (abstr 189). Am J Transplant; Paper presented at: American Transplant Congress; Boston, MA. May 30–Jun 3, 2009; 2009. p. 244. [Google Scholar]
- 98.Demetris AJ, Adeyi O, Bellamy CO, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 99.Wong T, Nouri-Aria KT, Devlin J, et al. Tolerance and latent cellular rejection in long-term liver transplant recipients. Hepatology. 1998;28:443–449. doi: 10.1002/hep.510280223. [DOI] [PubMed] [Google Scholar]
- 100.Benitez C, Lozano JJ, Martinez-Llordella M, et al. Use of transcriptional biomarkers to identify liver transplant recipients who can successfully discontinue immunosuppressive therapy (abstr 517) Am J Transplant. 2010;10:191. [Google Scholar]
- 101.Tisone G, Orlando G, Palmieri G, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702–709. doi: 10.1016/j.jhep.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 102.Mazariegos GV, Sindhi R, Thomson AW, et al. Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol. 2007;17:114–119. doi: 10.1016/j.trim.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 103.Yoshitomi M, Koshiba T, Haga H, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606–614. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
- 104.Vasconcellos LM, Schachter AD, Zheng XX, et al. Cytotoxic lymphocyte gene expression in peripheral blood leukocytes correlates with rejecting renal allografts. Transplantation. 1998;66:562–566. doi: 10.1097/00007890-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 105.Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344:947–954. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 106.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 107.Dadhania D, Muthukumar T, Ding R, et al. Molecular signatures of urinary cells distinguish acute rejection of renal allografts from urinary tract infection. Transplantation. 2003;75:1752–1754. doi: 10.1097/01.TP.0000063931.08861.56. [DOI] [PubMed] [Google Scholar]
- 108.Ding R, Li B, Muthukumar T, et al. CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation. 2003;75:1307–1312. doi: 10.1097/01.TP.0000064210.92444.B5. [DOI] [PubMed] [Google Scholar]
- 109.Tatapudi RR, Muthukumar T, Dadhania D, et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65:2390–2397. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 110.Seiler M, Brabcova I, Viklicky O, et al. Heightened expression of the cytotoxicity receptor NKG2D correlates with acute and chronic nephropathy after kidney transplantation. Am J Transplant. 2007;7:423–433. doi: 10.1111/j.1600-6143.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- 111.Shoker A, George D, Yang H, et al. Heightened CD40 ligand gene expression in peripheral CD4+ T cells from patients with kidney allograft rejection. Transplantation. 2000;70:497–505. doi: 10.1097/00007890-200008150-00018. [DOI] [PubMed] [Google Scholar]
- 112.Dugre FJ, Gaudreau S, Belles-Isles M, et al. Cytokine and cytotoxic molecule gene expression determined in peripheral blood mononuclear cells in the diagnosis of acute renal rejection. Transplantation. 2000;70:1074–1080. doi: 10.1097/00007890-200010150-00014. [DOI] [PubMed] [Google Scholar]
- 113.Shulzhenko N, Morgun A, Rampim GF, et al. Monitoring of intragraft and peripheral blood TIRC7 expression as a diagnostic tool for acute cardiac rejection in humans. Hum Immunol. 2001;62:342–347. doi: 10.1016/s0198-8859(01)00211-7. [DOI] [PubMed] [Google Scholar]
- 114.Schoels M, Dengler TJ, Richter R, et al. Detection of cardiac allograft rejection by real-time PCR analysis of circulating mono-nuclear cells. Clin Transplant. 2004;18:513–517. doi: 10.1111/j.1399-0012.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 115.Cashion A, Sabek O, Driscoll C, et al. Correlation of genetic markers of rejection with biopsy findings following human pancreas transplant. Clin Transplant. 2006;20:106–112. doi: 10.1111/j.1399-0012.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 116.Han D, Xu X, Baidal D, et al. Assessment of cytotoxic lymphocyte gene expression in the peripheral blood of human islet allograft recipients: elevation precedes clinical evidence of rejection. Diabetes. 2004;53:2281–2290. doi: 10.2337/diabetes.53.9.2281. [DOI] [PubMed] [Google Scholar]
- 117.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 118.Bernstein D, Williams GE, Eisen H, et al. Gene expression profiling distinguishes a molecular signature for grade 1B mild acute cellular rejection in cardiac allograft recipients. J Heart Lung Transplant. 2007;26:1270–1280. doi: 10.1016/j.healun.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 119.Mehra MR, Kobashigawa JA, Deng MC, et al. Transcriptional signals of T-cell and corticosteroid-sensitive genes are associated with future acute cellular rejection in cardiac allografts. J Heart Lung Transplant. 2007;26:1255–1263. doi: 10.1016/j.healun.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Lu BS, Yu AD, Zhu X, et al. Sequential gene expression profiling in lung transplant recipients with chronic rejection. Chest. 2006;130:847–854. doi: 10.1378/chest.130.3.847. [DOI] [PubMed] [Google Scholar]
- 121.Patil J, Lande JD, Li N, et al. Bronchoalveolar lavage cell gene expression in acute lung rejection: development of a diagnostic classifier. Transplantation. 2008;85:224–231. doi: 10.1097/TP.0b013e318160268a. [DOI] [PubMed] [Google Scholar]
- 122.Ashokkumar C, Talukdar A, Sun Q, et al. Allospecific CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant. 2009;9:179–191. doi: 10.1111/j.1600-6143.2008.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashokkumar C, Gupta A, Sun Q, et al. Allospecific CD154+ T cells identify rejection-prone recipients after pediatric small-bowel transplantation. Surgery. 2009;146:166–173. doi: 10.1016/j.surg.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 124.Brouard S, Mansfield E, Braud C, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007;104:15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 126.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martinez-Llordella M, Puig-Pey I, Orlando G, et al. Multi-parameter of immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 129.Martinez-Llordella M, Lozano JJ, Puig-Pey I, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawasaki M, Iwasaki M, Koshiba T, et al. Gene expression profile analysis of the peripheral blood mononuclear cells from tolerant living-donor liver transplant recipients. Int Surg. 2007;92:276–286. [PubMed] [Google Scholar]
- 131.Li Y, Koshiba T, Yoshizawa A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant. 2004;4:2118–2125. doi: 10.1111/j.1600-6143.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- 132.Mazariegos GV, Zahorchak AF, Reyes J, et al. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005;5:314–322. doi: 10.1111/j.1600-6143.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 133.Li Y, Zhao X, Cheng D, et al. The presence of Foxp3 expressing T cells within grafts of tolerant human liver transplant recipients. Transplantation. 2008;86:1837–1843. doi: 10.1097/TP.0b013e31818febc4. [DOI] [PubMed] [Google Scholar]
- 134.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 135.Judge TA, Wu Z, Zheng XG, et al. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162:1947–1951. [PubMed] [Google Scholar]
- 136.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 137.Ozkaynak E, Gao W, Shemmeri N, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 138.Harada H, Salama AD, Sho M, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quezada SA, Jarvinen LZ, Lind EF, et al. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 140.Domenig C, Sanchez-Fueyo A, Kurtz J, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol. 2005;175:51–60. doi: 10.4049/jimmunol.175.1.51. [DOI] [PubMed] [Google Scholar]
- 141.Sanchez-Fueyo A, Weber M, Domenig C, et al. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168:2274–2281. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 142.Yamada A, Salama AD, Sho M, et al. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- 143.Demirci G, Li XC. Novel roles of OX40 in the allograft response. Curr Opin Organ Transplant. 2008;13:26–30. doi: 10.1097/MOT.0b013e3282f3def3. [DOI] [PubMed] [Google Scholar]
- 144.Mariat C, Degauque N, Balasubramanian S, et al. Tim-1 signaling substitutes for conventional signal 1 and requires costimulation to induce T cell proliferation. J Immunol. 2009;182:1379–1385. doi: 10.4049/jimmunol.182.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Degauque N, Mariat C, Kenny J, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Degauque N, Mariat C, Kenny J, et al. Regulation of T-cell immunity by T-cell immunoglobulin and mucin domain proteins. Transplantation. 2007;84:S12–S16. doi: 10.1097/01.tp.0000269111.87719.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perez VL, Van Parijs L, Biuckians A, et al. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 148.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 149.Sandner SE, Clarkson MR, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 150.Ozkaynak E, Wang L, Goodearl A, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 151.Kurts C, Carbone FR, Krummel MF, et al. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398:341–344. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- 152.Dai Z, Li Q, Wang Y, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sanchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 154.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.