Abstract
Background
Cutaneous human papillomaviruses (HPVs) may be associated with cutaneous epithelial lesions and non-melanoma skin cancers. No study has systematically evaluated the presence of genus beta [β]-HPV in male genital skin or external genital lesions (EGLs).
Objectives
To examine cutaneous β-HPV types detected on the surface of EGLs in men and describe their presence prior to EGL development.
Study design
A retrospective case series was conducted among 69 men with pathologically confirmed EGLs (n=72) who participated in the HPV Infection in Men Study. Archived exfoliated cells collected from the surface of each EGL and normal genital skin specimens 6–12 months preceding EGL development were tested for β-HPV DNA using a type-specific multiplex genotyping assay.
Results
β-HPV DNA was detected on 61.1% of all EGLs, with types 38 (16.7%), 5 (15.3%), and 12 (12.5%) most commonly identified. HPV prevalence differed across pathological diagnoses, with the largest number of β-HPV types detected on condylomas. Most β-HPV types were detected on normal genital skin prior to EGL development, though the prevalence was lower on EGLs compared to preceding normal genital skin.
Conclusions
EGLs and the normal genital skin of men harbor a large number of β-HPV types; however, it appears that β-HPVs are unrelated to EGL development in men. Despite evidence to support a causal role in skin carcinogenesis at UVR-exposed sites, cutaneous HPV appears unlikely to cause disease at the UVR-unexposed genitals.
Background
Human papillomaviruses (HPV) cause substantial disease in men, including non-cancerous and cancerous lesions of the external genitalia.1, 2 Condyloma, or genital warts, is a common clinical manifestation of non-oncogenic genital HPV infection.3 These benign epithelial lesions are highly infectious3 and occur most frequently among men 25–29 years of age.4 We and others have consistently shown that non-oncogenic mucosal HPV types 6/11 are detected in 75–100% of condylomas,5-9 and that half are co-infected with oncogenic mucosal HPV types, particularly HPV 16.7, 8 Penile intraepithelial neoplasia (PeIN) is a presumed precursor lesion to HPV-related penile cancer. Though rarer than cervical intraepithelial neoplasia (CIN), PeIN shares histologic similarities with CIN, presenting as low, moderate, or severe dysplasia.10 The progression of PeIN from low- to high-grade lesions is thought to be rare, though the proportion that progress to penile cancer remains unknown. Approximately 70–100% of PeIN have tested positive for mucosal HPV,11 with HPV 16 being the most common;1 however, mucosal HPV is detected in only half of penile carcinomas.11, 12
Most studies examining the role of HPV in the development of male external genital lesions (EGLs) have evaluated mucosal HPV types of genus alpha (α-HPV);5, 8, 9, 13-15 however, recent data suggest that cutaneous HPV types may also have oncogenic potential.16, 17 A subset of cutaneous HPV types in genus beta (β-HPV) have been implicated in non-melanoma skin cancers.16 It has been hypothesized that skin infected with β-HPV demonstrates increased susceptibility to ultraviolet radiation (UVR)-induced DNA damage, ultimately leading to skin carcinogenesis.17 However, little is known about the role of β-HPV types in skin carcinogenesis at UVR-unexposed anatomic sites, such as the genitals.
Objectives
The purpose of this study was to examine the distribution of cutaneous β-HPV types detected on the surface of male EGLs and describe the natural history of these infections up to 12 months prior to lesion detection.
Study design
A retrospective case series was nested within the HPV Infection in Men (HIM) Study, an ongoing prospective study of the natural history of HPV infections among men living in the United States (US; Tampa), Brazil (São Paulo), and Mexico (Cuernavaca). The HIM Study cohort consists of >4,000 men aged 18–70 years who were recruited between 2005 and 2009, reported no prior diagnosis of anogenital cancer or genital warts and no current symptoms of or treatment for a sexually transmitted infection, including HIV/AIDS. Participants were examined approximately every six months for up to four years. Additional details of the HIM Study are published elsewhere.18, 19
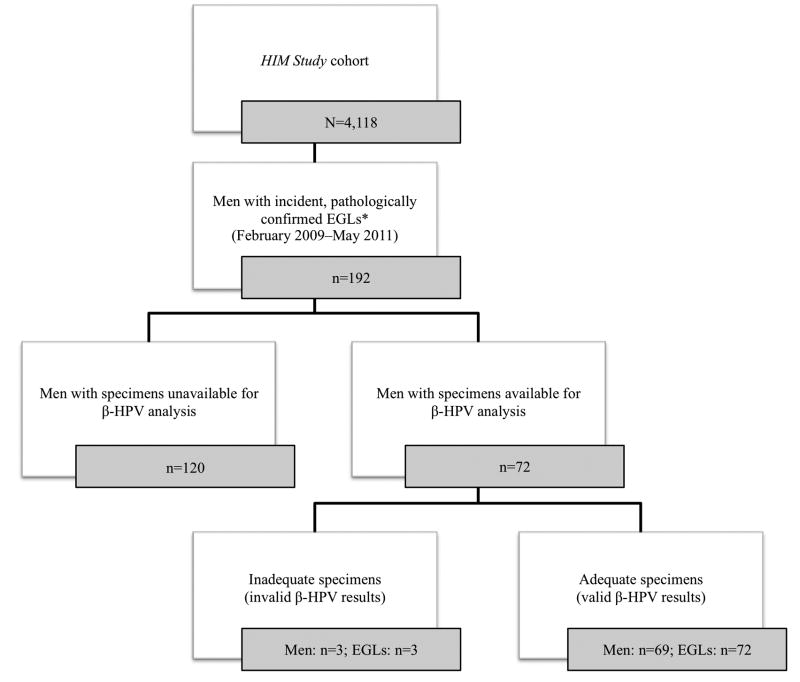
Participants were included in the current analysis if they developed an incident EGL that was detected at a follow-up visit and was pathologically confirmed between February 2009 (when lesion biopsy began in the HIM Study) and May 2011 (Figure 1). Participants provided written informed consent, and the human subjects committees of participating institutions approved all procedures.
Figure 1.
Numbers of men and newly acquired, pathologically confirmed external genital lesions (EGLs) available for analysis among men enrolled in the HPV Infection in Men Study.
*Sample sizes reflect pathologically distinct EGLs, and not necessarily all EGLs detected.
Specimen collection and processing
At each visit, participants completed a risk factor questionnaire and underwent a clinical examination. Visual inspection of the external genital skin was conducted and exfoliated cells were collected from the normal genital skin. Using Dacron swabs prewetted with sterile saline, the penile head, penile shaft, and scrotum were sampled, combined into one specimen, and archived.19 If an EGL was identified, exfoliated cells from the surface of the lesion were collected prior to the normal genital skin sampling and archived separately.
Visually distinct EGLs were biopsied and subjected to pathological evaluation. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were processed in the US (tissue blocks from Brazil and Mexico were shipped to the US). Fifteen 4-micron paraffin sections were cut from each block. Briefly, the two outer sections were discarded, four sections were used to prepare two separate slides, and an additional nine sections were collected in an Eppendorf tube for DNA retrieval. Slides were stained with hematoxylin and eosin and were evaluated by two independent pathologists for the presence of inflammatory, infectious, pre-neoplastic, or neoplastic conditions. A pathology panel was convened to provide final adjudication for discordant interpretations and quality control for 10% of all specimens.
DNA extraction and HPV genotyping
All genital swab specimens underwent DNA extraction using the QIAamp Media MDx Kit (Qiagen, Gaithersburg, MD). Specimens were tested for the presence of mucosal HPV by PCR and genotyped using Linear Array (Roche Molecular Diagnostics, Alameda, CA),20, 21 which detects 37 α-HPV types including 6, 11, 16, and 18.22 Archived surface of EGL specimens and normal genital skin specimens from two visits prior to lesion detection (six and 12 months prior) were also tested for the presence of β-HPV using a type-specific multiplex genotyping (TS-MPG) assay (IARC, Lyon, France), which combines multiplex PC23-25 with a bead-based Luminex technology.26, 27 The TS-MPG assay detects 25 β-HPV types (species β1: HPV types 5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93; species β2: HPV types 9, 15, 17, 22, 23, 37, 38, 80; species β3: HPV types 49, 75, 76; species β4: HPV 92; species β5: HPV 96). Two primers for the amplification of β-globin were added to provide a positive control for the quality of template DNA.28
Statistical analysis
Pathological diagnoses of EGLs were categorized as condyloma, probable condyloma, PeIN, and other. Probable condylomas included those suggestive but not diagnostic of HPV or condyloma, such as benign squamous keratosis. Other EGLs included various HPV-unrelated skin conditions, such as seborrheic keratosis and skin tags. PeIN included grades I–III.
Among newly detected, pathologically confirmed, and pathologically distinct EGLs, β-HPV prevalence was estimated for grouped (any β-HPV type and species-specific types) and genotype-specific infections. The classification of any β-HPV type was defined as a positive test result for ≥1 of the 25 β-HPV genotypes listed above. Prevalence estimates were stratified by EGL pathology and examined among lesions that tested negative or positive for common causative mucosal α-HPV types (6, 11, 16, 18), with groups compared using the Monte Carlo estimation of exact Pearson chi-square tests. Additional analyses were conducted to examine whether β-HPV DNA-positive lesions also had β-HPV DNA of the same genotype detected on the normal genital skin 6-12 months prior to lesion detection. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
As of May 2011, 192 men had developed incident, pathologically confirmed EGLs within the HIM Study (Figure 1). Of these, 72 men had available archived specimens from the surface of lesions and normal genital skin specimens prior to lesion detection, both of which were evaluated for the presence of β-HPV DNA. With respect to baseline sociodemographic characteristics, men with available specimens were comparable to men with unavailable specimens. Three lesions from three men had inadequate specimens for β-HPV genotyping.
The final case series included 69 men who were the first to have developed incident EGLs (n=72) confirmed by pathology (Table 1). At biopsy, men ranged in age from 20 to 60 years with a median of 31 years. Half of men (50.7%) were white, half (47.0%) were single or never married, one-third (34.9%) had some college education, and one-fourth (25.8%) reported being current smokers. A large proportion of men (43.9%) reported having ≥20 lifetime female sex partners, and 10.6% reported having ≥1 recent male anal sex partner. Among the 72 distinct EGLs detected, 28 were condyloma, 18 were probable condyloma, six were PeIN, and 20 were other HPV-unrelated diagnoses.
Table 1. Characteristics of 69 Men in the HPV Infection in Men Study at the Time of a Newly Detected, Pathologically Confirmed External Genital Lesion.
| Characteristic | N (%) |
|---|---|
| Country of residence | |
| United States | 25 (36.2) |
| Brazil | 25 (36.2) |
| Mexico | 19 (27.5) |
| Age, years | |
| Median (range) | 31.1 (20.1–60.0) |
| Mean (SD) | 32.6 (8.8) |
| 18–30 | 34 (49.3) |
| 31–44 | 28 (40.6) |
| 45–73 | 7 (10.1) |
| Race | |
| White | 35 (50.7) |
| Black | 15 (21.7) |
| Asian/Pacific Islander | 0 (0) |
| American Indian/Alaskan Native | 0 (0) |
| Mixed | 18 (26.1) |
| Missing data | 1 (1.5) |
| Marital status | |
| Single or never married | 31 (47.0) |
| Married | 15 (22.7) |
| Cohabitating | 16 (24.2) |
| Divorced/separated/widowed | 4 (6.1) |
| Education, years | |
| <12 | 8 (12.1) |
| 12 | 15 (22.7) |
| 13–15 | 23 (34.9) |
| 16 | 14 (21.2) |
| ≥17 | 5 (7.6) |
| Missing data | 1 (1.5) |
| Circumcision | |
| No | 41 (59.4) |
| Yes | 28 (40.6) |
| Current smoker | |
| No | 49 (74.2) |
| Yes | 17 (25.8) |
| Sexual orientation | |
| MSW | 58 (84.1) |
| MSM | 4 (5.8) |
| MSWM | 4 (5.8) |
| Missing data | 3 (4.3) |
| Lifetime female sex partners | |
| 0–1 | 10 (15.2) |
| 2–9 | 11 (16.7) |
| 10–19 | 13 (19.7) |
| 20–49 | 20 (30.3) |
| ≥50 | 9 (13.6) |
| Missing data | 3 (4.6) |
| Female sex partners in past 3–6 months | |
| 0 | 7 (11.1) |
| 1 | 30 (47.6) |
| 2 | 9 (14.3) |
| ≥3 | 16 (25.4) |
| Missing data | 1 (1.6) |
| Male anal sex partners in past 3 months | |
| 0 | 59 (89.4) |
| 1 | 3 (4.6) |
| 2 | 1 (1.5) |
| ≥3 | 3 (4.6) |
Data are n (%).
MSW: men who have sex with women; MSM: men who have sex with men; MSWM: men who have sex with women and men.
Overall, 44 EGLs (61.1%) tested DNA-positive for one or more β-HPV types (Table 2). Twenty-two different β-HPV types were detected, with HPV 38 (n=12; 16.7%) being the most common, followed by HPV 5 (n=11; 15.3%), HPV 12 (n=9; 12.5%), and HPV 17 (n=8; 11.1%). Genotype diversity and distribution varied by country, with Brazil having the highest overall prevalence (Brazil: 80.0%; Mexico: 60.0%; US: 44.4%) and Mexico having the least number of types (data not shown). Multiple infections were common, with 27 β-HPV DNA-positive EGLs (61.4%) containing >2 types.
Table 2. Beta (β)-HPV Type Distribution Among Newly Detected, Pathologically Confirmed External Genital Lesions (n=72), by Pathological Diagnosis.
| HPV species/type | Total n=72 | Pathological Diagnosis N (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Condyloma n=28 | Probable condylomaa n=18 | PeIN n=6 | Otherb n=20 | ||
|
|
|
||||
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Any β-HPV type | 44 (61.1) | 22 (78.6) | 6 (33.3) | 2 (33.3) | 14 (70.0) |
| β1 | |||||
| Any β1 | 35 (48.6) | 18 (64.3) | 5 (27.8) | 2 (33.3) | 10 (50.0) |
| HPV 5 | 11 (15.3) | 6 (21.4) | 1 (5.6) | 0 (0) | 4 (20.0) |
| HPV 8 | 6 (8.3) | 5 (17.9) | 0 (0) | 0 (0) | 1 (5.0) |
| HPV 12 | 9 (12.5) | 3 (10.7) | 1 (5.6) | 0 (0) | 5 (25.0) |
| HPV 14 | 7 (9.7) | 5 (17.9) | 2 (11.1) | 0 (0) | 0 (0) |
| HPV 19 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV 20 | 3 (4.2) | 0 (0) | 2 (11.1) | 0 (0) | 1 (5.0) |
| HPV 21 | 6 (8.3) | 2 (7.1) | 0 (0) | 2 (33.3) | 2 (10.0) |
| HPV 24 | 5 (6.9) | 2 (7.1) | 1 (5.6) | 0 (0) | 2 (10.0) |
| HPV 25 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV 36 | 5 (6.9) | 3 (10.7) | 0 (0) | 0 (0) | 2 (10.0) |
| HPV 47 | 4 (5.6) | 3 (10.7) | 0 (0) | 0 (0) | 1 (5.0) |
| HPV 93 | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 1 (5.0) |
| β2 | |||||
| Any β2 | 25 (34.7) | 13 (46.4) | 4 (22.2) | 2 (33.3) | 6 (30.0) |
| HPV 9 | 6 (8.3) | 2 (7.1) | 0 (0) | 1 (16.7) | 3 (15.0) |
| HPV 15 | 2 (2.8) | 2 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| HPV 17 | 8 (11.1) | 3 (10.7) | 2 (11.1) | 1 (16.7) | 2 (10.0) |
| HPV 22 | 7 (9.7) | 4 (14.3) | 0 (0) | 0 (0) | 3 (15.0) |
| HPV 23 | 5 (6.9) | 4 (14.3) | 0 (0) | 0 (0) | 1 (5.0) |
| HPV 37 | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 1 (5.0) |
| HPV 38 | 12 (16.7) | 6 (21.4) | 2 (11.1) | 1 (16.7) | 3 (15.0) |
| HPV 80 | 6 (8.3) | 3 (10.7) | 1 (5.6) | 1 (16.7) | 1 (5) |
| β3 | |||||
| Any β3 | 3 (4.2) | 1 (3.6) | 0 (0) | 0 (0) | 2 (10.0) |
| HPV 49 | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
| HPV 75 | 3 (4.2) | 1 (3.6) | 0 (0) | 0 (0) | 2 (10.0) |
| HPV 76 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| β4 | |||||
| HPV 92 | 1 (1.4) | 1 (3.6) | 0 (0) | 0 (0) | 0 (0) |
| β5 | |||||
| HPV 96 | 5 (6.9) | 2 (7.1) | 0 (0) | 0 (0) | 3 (15.0) |
| Multiple β-HPV infections | |||||
| β-HPV negative | 28 (38.9) | 6 (21.4) | 12 (66.7) | 4 (66.7) | 6 (30.0) |
| 1 type | 17 (23.6) | 7 (25.0) | 3 (16.7) | 0 (0) | 7 (35.0) |
| 2 types | 13 (18.1) | 8 (28.6) | 1 (5.6) | 1 (16.7) | 3 (15.0) |
| ≥3 types | 14 (19.4) | 7 (25.0) | 2 (11.1) | 1 (16.7) | 4 (20.0) |
PeIN: Penile intraepithelial neoplasia (I, II, or III).
Includes lesions suggestive but not diagnostic of HPV or condyloma.
Includes various HPV-unrelated skin conditions, such as seborrheic keratosis and skin tags.
The greatest β-HPV prevalence and type diversity were observed among condylomas (Table 2), with 22 (78.6%) containing β-HPV DNA. Of the 18 types detected, HPV 5 and 38 (n=6; 21.4% each) were the most common, followed by HPV 8 and 14 (n=5; 17.9% each). Only six (33.3%) of the 18 probable condylomas were β-HPV DNA-positive. Among six cases of PeIN, two (33.3%) were β-HPV DNA-positive. Five types were detected on PeIN, with HPV 21 (n=2; 33.3%) being the most common.
To assess whether the presence of known causative α-HPV types was associated with β-HPV detection, β-HPV prevalence was further stratified by pathological diagnosis and presence of α-HPV types 6/11 or 16/18. Few cases of condyloma (n=6; 21.4%) were negative for both α-HPV 6 and 11 (Table 3). β-HPV prevalence was highest among α-HPV 6/11 DNA-positive condylomas for all types except β-HPV 21, 38, 80, and 96, which had a higher prevalence in α-HPV 6/11 DNA-negative condylomas. Half of PeIN (n=3; 50.0%) were negative for both α-HPV 16 and 18. A greater number of β-HPV types were present among α-HPV 16/18 DNA-positive PeIN. β-HPV 17 was present in HPV 16/18 DNA-negative PeIN (n=1; 33.3%) but was absent in all HPV 16/18 DNA-positive PeIN.
Table 3. Beta (β)-HPV Type Distribution Among Newly Detected, Pathologically Confirmed Condyloma and PeIN (n=34), by Mucosal Alpha (α)-HPV Status.
| HPV species/type | Condyloma n=28 | PeIN n=6 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| α HPV 6/11- n=6a | α-HPV 6/11+ n=22b | Pc | α-HPV 16/18- n=3a | α-HPV 16/18+ n=3b | Pc | |
|
|
|
|||||
| N (%) | N (%) | N (%) | N (%) | |||
| Any β-HPV type | 5 (83.3) | 17 (77.3) | 1.000 | 1 (33.3) | 1 (33.3) | 1.000 |
| β1 | ||||||
| Any β1 | 2 (33.3) | 16 (72.7) | 0.153 | 1 (33.3) | 1 (33.3) | 1.000 |
| HPV 5 | 1 (16.7) | 5 (22.7) | 1.000 | 0 (0) | 0 (0) | NA |
| HPV 8 | 0 (0) | 5 (22.7) | 0.314 | 0 (0) | 0 (0) | NA |
| HPV 12 | 0 (0) | 3 (13.6) | 0.574 | 0 (0) | 0 (0) | NA |
| HPV 14 | 1 (16.7) | 4 (18.2) | 1.000 | 0 (0) | 0 (0) | NA |
| HPV 21 | 1 (16.7) | 1 (4.5) | 0.388 | 1 (33.3) | 1 (33.3) | 1.000 |
| HPV 24 | 0 (0) | 2 (9.1) | 1.000 | 0 (0) | 0 (0) | NA |
| HPV 36 | 0 (0) | 3 (13.6) | 0.579 | 0 (0) | 0 (0) | NA |
| HPV 47 | 0 (0) | 3 (13.6) | 0.578 | 0 (0) | 0 (0) | NA |
| β2 | ||||||
| Any β2 | 2 (33.3) | 11 (50) | 0.653 | 1 (33.3) | 1 (33.3) | 1.000 |
| HPV 9 | 0 (0) | 2 (9.1) | 1.000 | 0 (0) | 1 (33.3) | 1.000 |
| HPV 15 | 0 (0) | 2 (9.1) | 1.000 | 0 (0) | 0 (0) | NA |
| HPV 17 | 0 (0) | 3 (13.6) | 0.578 | 1 (33.3) | 0 (0) | 1.000 |
| HPV 22 | 0 (0) | 4 (18.2) | 0.550 | 0 (0) | 0 (0) | NA |
| HPV 23 | 0 (0) | 4 (18.2) | 0.553 | 0 (0) | 0 (0) | NA |
| HPV 38 | 2 (33.3) | 4 (18.2) | 0.576 | 0 (0) | 1 (33.3) | 1.000 |
| HPV 80 | 1 (16.7) | 2 (9.1) | 1.000 | 0 (0) | 1 (33.3) | 1.000 |
| β3 | ||||||
| Any β3 | 0 (0) | 1 (4.5) | 1.000 | 0 (0) | 0 (0) | NA |
| HPV 75 | 0 (0) | 1 (4.5) | 1.000 | 0 (0) | 0 (0) | NA |
| β4 | ||||||
| HPV 92 | 0 (0) | 1 (4.5) | 1.000 | 0 (0) | 0 (0) | NA |
| β5 | ||||||
| HPV 96 | 1 (16.7) | 1 (4.5) | 0.387 | 0 (0) | 0 (0) | NA |
| Multiple β-HPV infections | ||||||
| β-HPV negative | 1 (16.7) | 5 (22.7) | NA | 2 (66.7) | 2 (66.7) | NA |
| 1 type | 3 (50.0) | 4 (18.2) | NA | 0 (0) | 0 (0) | NA |
| 2 types | 2 (33.3) | 6 (27.3) | NA | 1 (33.3) | 0 (0) | NA |
| ≥3 types | 0 (0) | 7 (31.8) | NA | 0 (0) | 1 (33.3) | NA |
PeIN: Penile intraepithelial neoplasia (I, II, or III); NA: Not available.
Negative for both α-HPV types.
Positive for one or both α-HPV types.
Exact Pearson chi-square P value using Monte Carlo estimation.
β-HPVs were repeatedly detected 6–12 months prior to EGL development (Table 4). For all EGLs that tested positive for β-HPV types 15, 36, 37, 75, or 92, DNA of the same genotype was detected on normal genital skin 6–12 months prior to lesion development. Lesions positive for β-HPV types 38 (91.7%), 5 (81.8%), and 23, 24, and 96 (80.0% each) also demonstrated high levels of detection prior to lesion development. Similar trends were seen among condyloma and PeIN (data not shown). Generally, it was uncommon for β-HPV types to be detected for the first time on the EGL.
Table 4. Proportion of beta (β)-HPV DNA-positive external genital lesions (EGL) (n=72) with HPV of the same genotype detected, and not detected, on normal genital skin 6–12 months prior to lesion detection.
| HPV species/type | β-HPV detected on normal genital skin 6–12 months prior to lesiona | β-HPV not detected on normal genital skin 6–12 months prior to lesionb |
|---|---|---|
|
| ||
| N (%) | N (%) | |
| β1 | ||
| HPV 5 | 9 (81.8) | 2 (18.2) |
| HPV 8 | 4 (66.7) | 2 (33.3) |
| HPV 12 | 6 (66.7) | 3 (33.3) |
| HPV 14 | 4 (57.1) | 3 (42.9) |
| HPV 20 | 2 (66.7) | 1 (33.3) |
| HPV 21 | 3 (50.0) | 3 (50.0) |
| HPV 24 | 4 (80.0) | 1 (20.0) |
| HPV 36 | 5 (100) | 0 (0) |
| HPV 47 | 2 (50.0) | 2 (50.0) |
| HPV 93 | 0 (0) | 1 (100) |
| β2 | ||
| HPV 9 | 3 (50.0) | 3 (50.0) |
| HPV 15 | 2 (100) | 0 (0) |
| HPV 17 | 4 (50.0) | 4 (50.0) |
| HPV 22 | 5 (71.4) | 2 (28.6) |
| HPV 23 | 4 (80.0) | 1 (20.0) |
| HPV 37 | 1 (100) | 0 (0) |
| HPV 38 | 11 (91.7) | 1 (8.3) |
| HPV 80 | 4 (66.7) | 2 (33.3) |
| β3 | ||
| HPV 49 | 0 (0) | 1 (100) |
| HPV 75 | 3 (100) | 0 (0) |
| β4 | ||
| HPV 92 | 1 (100) | 0 (0) |
| β5 | ||
| HPV 96 | 4 (80.0) | 1 (20.0) |
Proportion of β-HPV DNA-positive lesions that were β-HPV DNA-positive for the same genotype on normal genital skin 6–12 months prior to lesion detection.
Proportion of β-HPV DNA-positive lesions that were β-HPV DNA-negative for the same genotype on normal genital skin 6–12 months prior to lesion detection.
To further examine temporality, β-HPV prevalence was compared across study visits (Figure 2). The overall prevalence of β-HPV was substantially lower on the surface of lesions than on preceding normal genital skin, with similar trends seen among condyloma. Similarly, multiple β-HPV types presented less frequently on the surface of lesions than on normal genital skin prior to lesion development.
Figure 2.
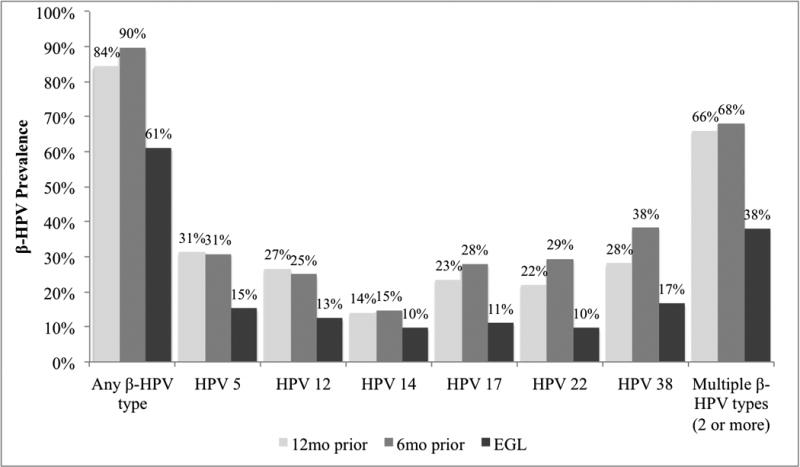
Beta (β)-HPV prevalence among newly detected, pathologically confirmed external genital lesions (EGLs) (n=72) and among normal genital skin 6 and 12 months prior to lesion detection.
Discussion
In this case series, we demonstrate that condyloma and the normal genital skin of otherwise healthy men harbor a large number of cutaneous β-HPV types. While most types detected on male EGLs were present on the normal genital skin prior to lesion development, the prevalence of β-HPV was lower among lesions compared to normal genital skin.
Few published studies have examined the presence of cutaneous HPV among male EGLs;9, 29-31 however, all have used varying methods and have focused on a single lesion type, making comparisons across studies difficult. In two studies examining penile carcinomas,29, 30,31 HPV 8 was detected in all tumors. While our study did not include men with invasive penile carcinoma, we are the first to show that β-HPV was present on 33% of pre-cancerous PeIN, though β-HPV types 5 and 8 were not detected.
According to condyloma surveillance recently conducted in Sweden, 42% of mucosal HPV DNA-negative condylomas were positive for β-HPV types 36, 49, 75, and 80,9 though the β-HPV prevalence among mucosal HPV DNA-positive condylomas was not reported. In the current study, we found a high prevalence of β-HPV DNA (77–83%), regardless of mucosal HPV 6/11 DNA-status. Among PeIN, the prevalence of β-HPV was 50% for both HPV 16/18 DNA-negative and -positive lesions, though our sample size was limited. Co-infection with mucosal and cutaneous HPV types appears to be common among EGLs, with another study reporting that co-infection with mucosal HPV 16/18 and β-HPV 8 was common among penile carcinomas in situ.31 Altogether, these findings suggest the possibility of multiple viral pathways to disease or perhaps co-transmission of mucosal and cutaneous HPV types during sexual activity.
For most β-HPV DNA-positive lesions examined in this study, DNA of the same genotype was detected on normal genital skin collected prior to lesion detection. Interestingly, the prevalence of β-HPV was substantially lower among lesions than on normal genital skin ≥6 months prior to lesion development. In a cross-sectional study of six benign and malignant cutaneous tumors, similar findings were observed, with β-HPV detected in 33% of tumor tissues and 50% of adjacent normal tissues.32 In contrast, the prevalence of β-HPV detected in or near cutaneous squamous cell carcinoma tissue has been shown to be considerably higher than in adjacent normal skin,33 supporting the hypothesis that β-HPV may be involved in the development of non-melanoma skin cancer. Furthermore, epidemiologic studies conducted among women have shown that the relative frequency of mucosal α-HPV types 16 and 18 (known to cause cervical cancer) increases significantly with increasing lesion severity.34, 35 As such, we would expect the prevalence of β-HPV to be highest in lesions if the virus was actively involved in EGL development. Given that β-HPVs are commonly detected on normal genital skin, we concur that β-HPVs may simply be part of the commensal microbiome of the cutaneous epithelium.36
To our knowledge, this is the first systematic investigation into the role of cutaneous HPV in the development of a variety of male EGLs and the first to report on the simultaneous presence of cutaneous and mucosal HPV types on the surface of both pathologically confirmed lesions and normal genital skin samples collected prior to lesion development. In this study, we employed the highly sensitive TS-MPG assay, which improved our ability to detect a large number of β-HPV infections compared with traditional PCR methods.37 However, there are limitations that must be considered. First, HPV types detected on the surface of a lesion may not represent those present within biopsy tissue.38 Moderate to high agreement between sampling methods was found for α-HPV types39 but remains unknown for β-HPV types. Second, swabbing the surface of a lesion may introduce contamination from the normal genital skin surrounding the EGL; however, this is unlikely given the lower HPV prevalence among EGLs. Third, misclassification of pathological diagnosis may have occurred, though it would have been minimized by the use of a pathology panel. To further evaluate the causal role of cutaneous HPV in the development of male EGLs, studies with a larger sample size and matched controls, in addition to a measure of HPV transcriptional activity, would be needed.
Our findings and those of others have shown that cutaneous β-HPVs are ubiquitous in the general population24, 33 and may even be commensal organisms. While there is increasing evidence to support a causal role in skin carcinogenesis at UVR-exposed anatomic sites,26, 40, 41 it appears that cutaneous HPV infection is unlikely to cause malignant or benign disease at UVR-unexposed sites, such as the genitals. The underlying mechanism of cutaneous HPV pathogenesis, and particularly carcinogenesis, remains unclear. Future prospective studies should focus on elucidating the pathways from HPV infection to disease progression and uncover the role of UVR in the disease process.
Acknowledgments
The authors would like to thank the HIM Study Teams in the USA (Moffitt Cancer Center, Tampa, FL: HY Lin, C Gage, K Eyring, K Kennedy, K Isaacs, A Bobanic, MT O'Keefe, MR Papenfuss, W Fulp, MB Schabath, AG Nyitray), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, and Ludwig Institute for Cancer Research, São Paulo: ML Baggio, L Galan, RJ Carvalho da Silva, E Gomes, E Brito, F Cernicchiaro, R Cintra, R Cunha, R Matsuo, V Souza, B Fietzek, R Hessel, V Relvas, F Silva, J Antunes, G Ribeiro, R Bocalon, R Otero, R Terreri, S Araujo, M Ishibashi, CRT-DST/AIDS nursing team), and Mexico (Instituto Mexicano del Seguro Social and Instituto Nacional de Salud Pública, Cuernavaca: J Salmerón, M Quiterio Trenado, A Cruz Valdez, R Alvear Vásquez, O Rojas Juárez, R González Sosa, A Salgado Morales, A Rodríguez Galván, P Román Rodríguez, A Landa Vélez, M Zepeda Mendoza, G Díaz García, V Chávez Abarca, J Ruiz Sotelo, A Gutiérrez Luna, M Hernández Nevárez, G Sánchez Martínez, A Ortiz Rojas, C Barrera Flores).
Funding: Infrastructure of the HIM Study cohort was supported through a grant from the National Cancer Institute, National Institutes of Health (CA R01CA098803 to ARG). CMPC was supported through a cancer prevention fellowship (National Cancer Institute, R25T CA147832).
Abbreviations
- HPV
Human papillomavirus
- EGL
external genital lesion
- PeIN
penile intraepithelial neoplasia
- CIN
cervical intraepithelial neoplasia
- US
United States
- HIM Study
HPV Infection in Men Study
- β-HPV
beta-HPV
- α-HPV
alpha-HPV
Footnotes
Competing interests: ARG receives research funding from Merck Sharp & Dohme Corp. MHS, LLV, and ARG are consultants of Merck Sharp & Dohme Corp. for HPV vaccines. None of the other authors have conflicts of interest to report.
Ethical approval: Ethical approval was given by the University of South Florida (IRB# 102660), Ludwig Institute for Cancer Research, Centro de Referência e Treinamento em Doenças Sexualmente Transmissíveis e AIDS, and Instituto Nacional de Salud Pública de México.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anic GM, Giuliano AR. Genital HPV infection and related lesions in men. Prev Med. 2011;53(Suppl 1):S36–41. doi: 10.1016/j.ypmed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Suppl 3):S3/35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anic GM, Lee JH, Stockwell H, Rollison DE, Wu Y, Papenfuss MR, et al. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis. 2011;204:1886–92. doi: 10.1093/infdis/jir652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubin F, Pretet JL, Jacquard AC, Saunier M, Carcopino X, Jaroud F, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV) Clin Infect Dis. 2008;47:610–5. doi: 10.1086/590560. [DOI] [PubMed] [Google Scholar]
- 7.Ball SL, Winder DM, Vaughan K, Hanna N, Levy J, Sterling JC, et al. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol. 2011;83:1345–50. doi: 10.1002/jmv.22111. [DOI] [PubMed] [Google Scholar]
- 8.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–22. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturegard E, Johansson H, Ekstrom J, Hansson BG, Johnsson A, Gustafsson E, et al. Human papillomavirus typing in reporting of condyloma. Sex Transm Dis. 2013;40:123–9. doi: 10.1097/OLQ.0b013e31827aa9b3. [DOI] [PubMed] [Google Scholar]
- 10.Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol. 2004;193:35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]
- 11.Dillner J, von Krogh G, Horenblas S, Meijer CJ. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl. 2000;205:189–93. doi: 10.1080/00365590050509913. [DOI] [PubMed] [Google Scholar]
- 12.Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de Sanjose S. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 13.Cupp MR, Malek RS, Goellner JR, Smith TF, Espy MJ. The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol. 1995;154:1024–9. [PubMed] [Google Scholar]
- 14.Mannweiler S, Sygulla S, Beham-Schmid C, Razmara Y, Pummer K, Regauer S. Penile carcinogenesis in a low-incidence area: a clinicopathologic and molecular analysis of 115 invasive carcinomas with special emphasis on chronic inflammatory skin diseases. Am J Surg Pathol. 2011;35:998–1006. doi: 10.1097/PAS.0b013e3182147e59. [DOI] [PubMed] [Google Scholar]
- 15.Wikstrom A, Hedblad MA, Syrjanen S. Penile intraepithelial neoplasia: histopathological evaluation, HPV typing, clinical presentation and treatment. J Eur Acad Dermatol Venereol. 2012;26:325–30. doi: 10.1111/j.1468-3083.2011.04069.x. [DOI] [PubMed] [Google Scholar]
- 16.Akgul B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol. 2006;208:165–75. doi: 10.1002/path.1893. [DOI] [PubMed] [Google Scholar]
- 17.Viarisio D, Decker KM, Aengeneyndt B, Flechtenmacher C, Gissmann L, Tommasino M. Human papillomavirus type 38 E6 and E7 act as tumour promoters during chemically induced skin carcinogenesis. J Gen Virol. 2013;94:749–52. doi: 10.1099/vir.0.048991-0. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 23.Gheit T, Billoud G, de Koning MN, Gemignani F, Forslund O, Sylla BS, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J Clin Microbiol. 2007;45:2537–44. doi: 10.1128/JCM.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2012;21:1303–13. doi: 10.1158/1055-9965.EPI-12-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollison DE, Pawlita M, Giuliano AR, Iannacone MR, Sondak VK, Messina JL, et al. Measures of cutaneous human papillomavirus infection in normal tissues as biomarkers of HPV in corresponding nonmelanoma skin cancers. Int J Cancer. 2008;123:2337–42. doi: 10.1002/ijc.23795. [DOI] [PubMed] [Google Scholar]
- 26.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomavirus infection in Basal cell carcinoma of the skin. J Invest Dermatol. 2013;133:1512–20. doi: 10.1038/jid.2012.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;44:504–12. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–91. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 29.Heideman DA, Waterboer T, Pawlita M, Delis-van Diemen P, Nindl I, Leijte JA, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25:4550–6. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 30.Humbey O, Cairey-Remonnay S, Guerrini JS, Algros MP, Mougin C, Bittard H, et al. Detection of the human papillomavirus and analysis of the TP53 polymorphism of exon 4 at codon 72 in penile squamous cell carcinomas. Eur J Cancer. 2003;39:684–90. doi: 10.1016/s0959-8049(02)00835-3. [DOI] [PubMed] [Google Scholar]
- 31.Wieland U, Jurk S, Weissenborn S, Krieg T, Pfister H, Ritzkowsky A. Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J Invest Dermatol. 2000;115:396–401. doi: 10.1046/j.1523-1747.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 32.Astori G, Lavergne D, Benton C, Hockmayr B, Egawa K, Garbe C, et al. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J Invest Dermatol. 1998;110:752–5. doi: 10.1046/j.1523-1747.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 33.Plasmeijer EI, Neale RE, Buettner PG, de Koning MN, Ter Schegget J, Quint WG, et al. Betapapillomavirus infection profiles in tissue sets from cutaneous squamous cell-carcinoma patients. Int J Cancer. 2010;126:2614–21. doi: 10.1002/ijc.24991. [DOI] [PubMed] [Google Scholar]
- 34.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–59. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 36.Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–41. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sichero L, Pierce Campbell CM, Ferreira S, Sobrinho JS, Luiza Baggio M, Galan L, et al. Broad HPV distribution in the genital region of men from the HPV infection in men (HIM) study. Virology. 2013;443:214–7. doi: 10.1016/j.virol.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins MG, Winder DM, Ball SL, Vaughan K, Sonnex C, Stanley MA, et al. Detection of specific HPV subtypes responsible for the pathogenesis of condylomata acuminata. Virology journal. 2013;10:137. doi: 10.1186/1743-422X-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anic GM, Messina JL, Stoler MH, Rollison DE, Stockwell H, Villa LL, et al. Concordance of human papillomavirus types detected on the surface and in the tissue of genital lesions in men. J Med Virol. 2013;85:1561–6. doi: 10.1002/jmv.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farzan SF, Waterboer T, Gui J, Nelson HH, Li Z, Michael KM, et al. Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: A population-based study. Int J Cancer. 2013;133:1713–20. doi: 10.1002/ijc.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannacone MR, Wang W, Stockwell HG, O'Rourke K, Giuliano AR, Sondak VK, et al. Sunlight exposure and cutaneous human papillomavirus seroreactivity in basal cell and squamous cell carcinomas of the skin. J Infect Dis. 2012;206:399–406. doi: 10.1093/infdis/jis374. [DOI] [PMC free article] [PubMed] [Google Scholar]