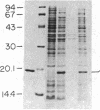
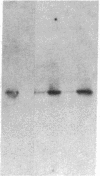
Abstract
Expression of human Cu/Zn superoxide dismutase (SOD) with activity comparable to the human erythrocyte enzyme was achieved in Escherichia coli by using a vector containing a thermoinducible lambda PL promoter and a beta-lactamase-derived ribosomal binding site. The recombinant human SOD was found in the cytosol of disrupted bacteria and represented greater than 10% of the total bacterial protein. The enzyme was purified to homogeneity by salt precipitation, gel filtration chromatography, and ion exchange chromatography. The active enzyme was obtained in high yield only when 1 mol of copper and 1 mol of zinc were incorporated into each mol of subunit during bacterial growth or by reconstitution of the apoenzyme. Human Cu/Zn SOD produced in bacteria has an apparent subunit molecular mass of 19 kDa on NaDodSO4/polyacrylamide gels. The native enzyme behaves as a dimer of 32 kDa as determined by gel filtration. Sequence analysis of the NH2 terminus revealed that the first 14 amino acids corresponded to authentic human SOD except that the NH2-terminal alanine was not acetylated. Thus, the bacterial processing system readily removes the NH2-terminal methionine residue from recombinant human SOD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra D., Martini F., Bannister J. V., Schininà M. E., Rotilio G., Bannister W. H., Bossa F. The complete amino acid sequence of human Cu/Zn superoxide dismutase. FEBS Lett. 1980 Oct 20;120(1):53–56. doi: 10.1016/0014-5793(80)81044-1. [DOI] [PubMed] [Google Scholar]
- Briggs R. G., Fee J. A. Further characterization of human erythrocyte superoxide dismutase. Biochim Biophys Acta. 1978 Nov 20;537(1):86–99. doi: 10.1016/0005-2795(78)90605-0. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Geller B. L., Winge D. R. A method for distinguishing Cu,Zn- and Mn-containing superoxide dismutases. Anal Biochem. 1983 Jan;128(1):86–92. doi: 10.1016/0003-2697(83)90348-2. [DOI] [PubMed] [Google Scholar]
- Hallewell R. A., Masiarz F. R., Najarian R. C., Puma J. P., Quiroga M. R., Randolph A., Sanchez-Pescador R., Scandella C. J., Smith B., Steimer K. S. Human Cu/Zn superoxide dismutase cDNA: isolation of clones synthesising high levels of active or inactive enzyme from an expression library. Nucleic Acids Res. 1985 Mar 25;13(6):2017–2034. doi: 10.1093/nar/13.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz J. W., Deutsch H. F. Subunit structure of human superoxide dismutase. J Biol Chem. 1972 Nov 10;247(21):7043–7050. [PubMed] [Google Scholar]
- Honigman A., Mahajna J., Altuvia S., Koby S., Teff D., Locker-Giladi H., Hyman H., Kronman C., Oppenheim A. B. Plasmid vectors designed for the analysis of transcription termination signals. Gene. 1985;36(1-2):131–141. doi: 10.1016/0378-1119(85)90077-0. [DOI] [PubMed] [Google Scholar]
- Jabusch J. R., Farb D. L., Kerschensteiner D. A., Deutsch H. F. Some sulfhydryl properties and primary structure of human erythrocyte superoxide dismutase. Biochemistry. 1980 May 27;19(11):2310–2316. doi: 10.1021/bi00552a005. [DOI] [PubMed] [Google Scholar]
- Jewett S. L., Latrenta G. S., Beck C. M. Metal-deficient copper-zinc superoxide dismutases. Arch Biochem Biophys. 1982 Apr 15;215(1):116–128. doi: 10.1016/0003-9861(82)90285-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Friedman D. J., Ayala F. J. Superoxide dismutase: an evolutionary puzzle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):824–828. doi: 10.1073/pnas.82.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieman-Hurwitz J., Dafni N., Lavie V., Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808–2811. doi: 10.1073/pnas.79.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Oberley L. W., Buettner G. R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979 Apr;39(4):1141–1149. [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasoff J. M., Ono T., Cutler R. G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980 May;77(5):2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weser U., Hartmann H. J. Preparation of pure bovine apo-erythrocuprein by gel filtration. FEBS Lett. 1971 Sep 15;17(1):78–80. doi: 10.1016/0014-5793(71)80567-7. [DOI] [PubMed] [Google Scholar]