Abstract
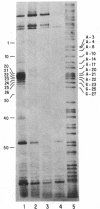
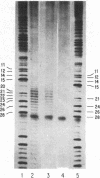
The nuclease activity of 1,10-phenanthroline-copper ion can be targeted to specific DNA sequences by attachment of the ligand to the 5' end of complementary deoxyoligonucleotides via a phosphoramidate linkage. To synthesize the adduct, the phosphorimidazolide of the deoxyoligonucleotide is prepared using a water-soluble carbodiimide and is then coupled to 5-glycylamido-1,10-phenanthroline. After hybridization to the target DNA, sequence-specific cleavage is observed upon the addition of cupric ion and 3-mercaptopropionic acid. Two methods of assaying the cutting of the operator sequence of the lac operon have been employed using the oligonucleotide 5'-AATTGTTATCCGCTCACAATT-3' representing sequence positions 21-1 of the template strand. In the first, the single-stranded DNA of the phage M13mp8 was the target, and cuts were detected using a primer-extension assay. In the second, the substrate was an EcoRI fragment 3' labeled in the nontemplate strand. After denaturation and reannealing to the oligonucleotide-1,10-phenanthroline adduct, cupric ion and 3-mercaptopropionic acid were added, and the products were analyzed directly on a sequencing gel. With the phenanthroline moiety attached to position 21 of the oligonucleotide carrier, cutting was observed at positions 20-25 using both assays.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright I. L., Hertzberg R. P., Dervan P. B., Elgin S. C. Cleavage of chromatin with methidiumpropyl-EDTA . iron(II). Proc Natl Acad Sci U S A. 1983 Jun;80(11):3213–3217. doi: 10.1073/pnas.80.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aurora V., Stern A. M., Sigman D. S. Inhibition of E. coli DNA polymerase I by 1,10-phenanthroline. Biochem Biophys Res Commun. 1977 Sep 9;78(1):170–176. doi: 10.1016/0006-291x(77)91236-0. [DOI] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Rapid "footprinting" on supercoiled DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3078–3081. doi: 10.1073/pnas.82.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Jen-Jacobson L., Kurpiewski M., Lesser D., Grable J., Boyer H. W., Rosenberg J. M., Greene P. J. Coordinate ion pair formation between EcoRI endonuclease and DNA. J Biol Chem. 1983 Dec 10;258(23):14638–14646. [PubMed] [Google Scholar]
- Jessee B., Gargiulo G., Razvi F., Worcel A. Analogous cleavage of DNA by micrococcal nuclease and a 1-10-phenanthroline-cuprous complex. Nucleic Acids Res. 1982 Oct 11;10(19):5823–5834. doi: 10.1093/nar/10.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessee B., Gargiulo G., Razvi F., Worcel A. Analogous cleavage of DNA by micrococcal nuclease and a 1-10-phenanthroline-cuprous complex. Nucleic Acids Res. 1982 Oct 11;10(19):5823–5834. doi: 10.1093/nar/10.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. Binding of an antitumor drug to DNA, Netropsin and C-G-C-G-A-A-T-T-BrC-G-C-G. J Mol Biol. 1985 Jun 25;183(4):553–563. doi: 10.1016/0022-2836(85)90171-8. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Regnier F. E., Weith H. L. Separation of synthetic oligonucleotides on columns of microparticulate silica coated with crosslinked polyethylene imine. Anal Biochem. 1983 Aug;133(1):85–93. doi: 10.1016/0003-2697(83)90225-7. [DOI] [PubMed] [Google Scholar]
- Marshall L. E., Graham D. R., Reich K. A., Sigman D. S. Cleavage of deoxyribonucleic acid by the 1,10-phenanthroline-cuprous complex. Hydrogen peroxide requirement and primary and secondary structure specificity. Biochemistry. 1981 Jan 20;20(2):244–250. doi: 10.1021/bi00505a003. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pope L. E., Sigman D. S. Secondary structure specificity of the nuclease activity of the 1,10-phenanthroline-copper complex. Proc Natl Acad Sci U S A. 1984 Jan;81(1):3–7. doi: 10.1073/pnas.81.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Reich K. A., Graham D. R., Sigman D. S. Products of DNA cleavage by the 1,10-phenanthroline-copper complex. Inhibitors of Escherichia coli DNA polymerase I. J Biol Chem. 1982 Oct 25;257(20):12121–12128. [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman D. S., Graham D. R., D'Aurora V., Stern A. M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline . cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Dec 25;254(24):12269–12272. [PubMed] [Google Scholar]
- Sigman D. S., Spassky A., Rimsky S., Buc H. Conformational analysis of lac promoters using the nuclease activity of 1,10-phenanthroline-copper ion. Biopolymers. 1985 Jan;24(1):183–197. doi: 10.1002/bip.360240115. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Wahl G. M., Creighton D. J. Models for metalloenzymes. Zinc ion catalyzed phosphorylation of 1,10-phenanthroline-2-carbinol by adenosine triphosphate. Biochemistry. 1972 Jun 6;11(12):2236–2242. doi: 10.1021/bi00762a005. [DOI] [PubMed] [Google Scholar]
- Spassky A., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion. Conformational analysis and footprinting of the lac operon. Biochemistry. 1985 Dec 31;24(27):8050–8056. doi: 10.1021/bi00348a032. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]