Abstract
This study investigated the hypoglycemic effect of the Padina arborescens extract in STZ-induced diabetic mice. Freeze-dried Padina arborescens were extracted with 80% methanol and concentrated for use in this study. The hypoglycemic effect was determined by inhibitory activities against α-glucosidase and α-amylase as well as the alleviation of postprandial blood glucose level. Padina arborescens extracts showed higher inhibitory activities than acarbose, a positive control against α-glucosidase and α-amylase. The IC50 values of Padina arborescens extracts against α-glucosidase and α-amylase were 0.26 and 0.23 mg/mL, respectively, which evidenced as more effective than observed with acarbose. The increase of postprandial blood glucose levels were significantly suppressed in the Padina arborescens extract administered group than the control group in the streptozotocin induced diabetic mice. Furthermore, the area under the curve (AUC) was significantly lowered via Padina arborescens extract administration in diabetic mice (p < 0.05). These results indicated that the Padina arborescens extract might be used as an inhibitor of α-glucosidase and α-amylase and delay absorption of dietary carbohydrates.
Keywords: Padina arborescens, α-glucosidase, α-amylase, postprandial hyperglycemia, diabetic mice
INTRODUCTION
Diabetes mellitus is the most serious, chronic metabolic disorder and is characterized by high blood glucose levels (1). Postprandial hyperglycemia plays an important role in the development of type 2 diabetes and its complications, including micro-vascular and macro-vascular diseases (2). Therefore, controlling hyperglycemia is the most important factor for reducing risks of diabetic complications (3).
One therapeutic approach to decrease postprandial hyperglycemia is to retard absorption of glucose through inhibition of carbohydrate hydrolyzing enzymes, e.g., α-glucosidase and α-amylase, in the digestive organs (4–7). Mammalian starch digestion primarily occurs in the small intestine through the action of α-amylase, yielding both linear maltose and branched isomaltose oligosaccharides, which are further hydrolyzed by α-glucosidases to release glucose (8). The powerful synthetic α-glucosidase and α-amylase inhibitors, such as acarbose, miglitol, and voglibose, function directly by reducing the sharp increases in glucose levels that occur immediately after food uptake (4,9,10); however, the continuous use of these synthetic agents should be limited due to induced side effects such as flatulence, abdominal discomfort, vomiting, diarrhea (11), and hepatotoxicity (12). Therefore, numerous studies have been performed to identify more effective and safer inhibitors of carbohydrate enzymes from natural materials.
Marine algae are known to generate an abundance of bioactive compounds with great potential in the pharmaceuticals, food, and biomedical industries. In particular, brown algae display a variety of biological activities, including antioxidant (13), anti-inflammatory (14), anti-coagulant (15) and anti-hyperlipidemic properties (16). Padina arborescens, a type of brown algae, is popular in Korea and Japan as a food ingredient and marine herb. Padina arborescens contains biologically active compounds such as bromophenols (17). However, the hypoglycemic effect of Padina arborescens has yet to be studied.
Thus, in this study, we attempted to determine whether or not Padina arborescens inhibits α-glucosidase and α-amylase activities and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice.
MATERIALS AND METHODS
Materials
The brown algae, Padina arborescens (Phylum Ochro-phyta, Class Phaeophyceae, Order Dictyotales, Family Dictyotaceae), were collected along the coast of Jeju Island, Korea. The samples were washed three times with tap water to remove the salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water and maintained in a medical refrigerator at −20°C. Thereafter, the frozen samples were lyophilized and homogenized with a grinder prior to extraction. Padina arborescens were extracted with ten volumes of 80% methanol for 12 hr three times at room temperature. The filtrate was then evaporated at 40°C to obtain the methanol extract. The extract was thoroughly dried for complete removal of solvent and stored in a deep freezer (−80°C).
Inhibition assay for α-glucosidase activity in vitro
The α-glucosidase inhibitory assay was done by the chromogenic method developed by Watanabe et al. using a readily available yeast enzyme (18). Briefly, yeast α-glucosidase (0.7 U, Sigma, St. Louis, MO, USA) was dissolved in 100 mM phosphate buffer (pH 7.0) containing 2 g/L bovine serum albumin and 0.2 g/L NaN3 and used as the enzyme solution. 5 mM p-nitrophenyl-α-D-gluco-pyranoside in the same buffer (pH 7.0) was used as a substrate solution. The 50 μL of enzyme solution and 10 μL of sample dissolved in dimethylsulfoxide at a 5 mg/mL concentration were mixed in a well, and absorbance at 405 nm was measured using a microplate reader. After incubation for 5 min, substrate solution (50 μL) was added and incubated for another 5 min at room temperature. The increase in absorbance from zero time was measured. Inhibitory activity was expressed as 100 minus relative absorbance difference (%) of test compounds to absorbance change of the control where test solution was replaced by carrier solvent. The measurements were performed in triplicate and the IC50 value, i.e., the concentration of the extracts that results in 50% inhibition of maximal activity, was determined.
Inhibition assay for α-amylase activity in vitro
The α-amylase inhibitory activity was assayed in the same way (18) as described previously for α-glucosidase except porcine pancreatic amylase (100 U, Sigma) and blocked p-nitrophenyl-α-D-maltopentoglycoside (Sigma) were used as the enzyme and substrate, respectively.
Measurement of cytotoxicity
Cell viability was assessed by measuring the supravital dye neutral red uptake (19). Human umbilical vein endo-thelial cells (HUVECs) were seeded at the concentration of 2 × 104 cells/mL in 96-well plate and pre-incubated in humidified atmosphere containing 5% CO2 at 37°C for 24 hr. The cells were then treated with various concentrations (0.1, 0.5, 1, and 2 mg/mL) of the Padina arborescens extract (PAE), and further incubated for 20 hr. Thereafter, the medium was carefully removed from each well, and replaced with 0.5 mL of fresh medium containing 1.14 mM neutral red. After 3 hr of incubation, the medium was removed and the cells were washed twice with phosphate buffered saline (PBS, pH 7.4). The incorporated neutral red was released from the cells by incubation in the presence of 1 mL of the cell lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM dithiothreital, and Triton X-100 (1%, v/v)] containing acetic acid (1%, v/v) and ethanol (50%, v/v) at room temperature for 15 min. The cell lysis products were centrifuged to measure the dye taken up and absorbance of supernatant was measured spectrophotometrically at 540 nm.
Experimental animals
Four-week old male mice (ICR, Orient, Inc., Seoul, Korea) were kept under a 12 hr light/12 hr dark cycle with controlled room temperature (n=42). The animals were maintained with pelleted food, while tap water was ad libitum. After an adjustment period of 2 weeks, diabetes was induced by intraperitoneal injection of STZ (60 mg/kg) freshly dissolved in a citrate buffer (0.1 M, pH 4.5) for the fasted (18 hr) animals. After seven days, tail bleeds were performed and animals with a blood glucose concentration above 300 mg/dL were considered to be diabetic. The mice were administered orally soluble starch (2 g/kg BW) alone (control) or with PAE (300 mg/kg BW) or acarbose (100 mg/kg BW) dissolved in 0.2 mL water.
Measurement of blood glucose level
Both normal mice and STZ-induced diabetic mice fasted overnight were randomly divided into three groups of 7 mice. Fasted animals were deprived of food for at least 12 hr but allowed free access to water. After overnight fasting, the mice were orally administered either soluble starch (2 g/kg body weight) alone (control) or starch with PAE (300 mg/kg body weight). Blood samples were taken from the tail vein at 0, 30, 60, and 120 min. Blood glucose was measured using a glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Areas under the curve (AUC) were calculated using the trapezoidal rule (20).
Data statistical analysis
The data were represented as mean ± SD. The statistical analysis was performed using SAS software. The Student’s t-test was used for comparisons between control and sample groups. The values were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple range tests.
RESULTS AND DISCUSSION
Inhibitory effect of PAE on α-glucosidase and α-amylase in vitro
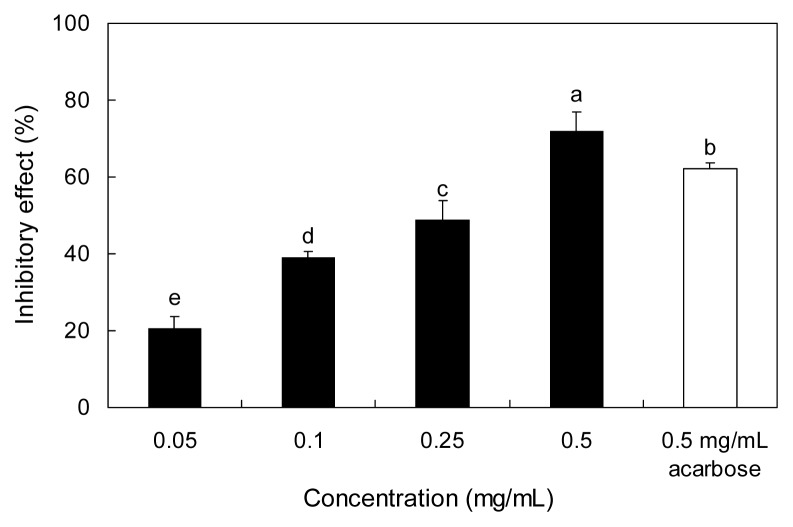
The inhibitory effect of Padina arborescens extract (PAE) against yeast α-glucosidase is shown in Fig. 1. PAE inhibited α-glucosidase activity in a dose-dependent manner by 20.55, 38.95, 49.01, and 71.93% at the concentrations of 0.05, 0.1, 0.25, and 0.5 mg/mL, respectively. Acarbose, an α-glucosidase inhibitor used as an oral hypoglycemic agent, inhibited the enzyme activity by 62.03% at a concentration of 0.5 mg/mL. The α-glucosidase inhibitory activity of PAE was higher than that of acarbose at the same concentration (0.5 mg/mL).
Fig. 1.
Inhibitory activity of PAE on α-glucosidase. Each value is expressed as mean ± SD in triplicate experiments. a–eValues with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as the positive control was 0.5 mg/mL. PAE: Padina arborescens extract.
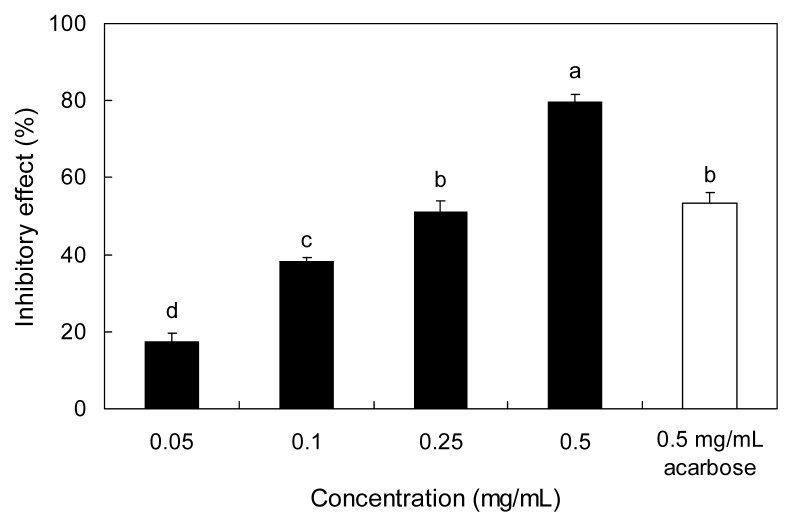
The inhibitory effect of PAE against α-amylase is shown in Fig. 2. The PAE inhibited α-amylase by 17.57, 38.22, 51.35, and 79.73% at concentrations of 0.05, 0.1, 0.25, and 0.5 mg/mL, respectively. The IC50 values of PAE against α-glucosidase and α-amylase were 0.26 and 0.23 mg/mL, respectively, which were evidenced as stronger inhibitory effects than was observed with acarbose (Table 1).
Fig. 2.
Inhibitory activity of PAE on α-amylase. Each value is expressed as mean ± SD in triplicate experiments. a–dValues with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as the positive control was 0.5 mg/mL. PAE: Padina arborescens extract.
Table 1.
IC50 values of inhibitory effects of PAE on α-glucosidase and α-amylase
| Sample | IC50 (mg/mL) | |
|---|---|---|
|
| ||
| α-Glucosidase | α-Amylase | |
| Acarbose | 0.34 ± 0.02 | 0.45 ± 0.04 |
| PAE | 0.26 ± 0.05 | 0.23 ± 0.03* |
IC50 value is the concentration of sample required for 50% inhibition. Each value is expressed as mean ± SD (n=3). Significantly different from control at *p<0.05.
PAE: Padina arborescens extract.
The treatment goal of diabetic patients is to maintain near normal levels of glycemic control, both in the fasting and postprandial states. α-Amylase is a key digestive enzyme responsible for hydrolyzing starch to maltose, which further breaks down into glucose prior to absorption in the small intestine (21). Inhibition of α-amylase could delay carbohydrate digestion and prolong overall carbohydrate digestion time, causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise (22). On the other hand, α-glucosidase is a membrane-bound enzyme, located at the epithelium of the small intestine, which catalyses the cleavage of glucose from disaccharides and oligosaccharides (23). Inhibition of α-glucosidase and α-amylase can significantly decrease the postprandial increase of blood glucose after a mixed carbohydrate diet and therefore be an important strategy in the management of postprandial blood glucose level in type 2 diabetic and borderline patients. Thus, effective and nontoxic inhibitors of α-glucosidase and α-amylase have long been sought.
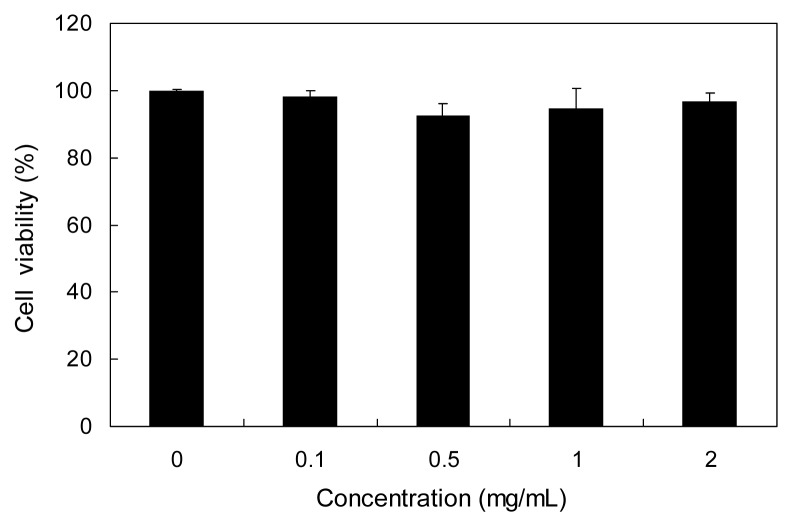
In this study, we investigated the inhibitory effect of PAE against α-glucosidase and α-amylase to elucidate the possible use of PAE as an anti-hyperglycemic agent. PAE evidenced higher inhibitory activities against both α-glucosidase and α-amylase than that of the commercial carbohydrate digestive enzyme inhibitor, acarbose, which did not exert any cytotoxicity (Fig. 3).
Fig. 3.
Cytotoxic effect of PAE in HUVECs. HUVECs were treated with various concentrations (0.1, 0.5, 1, and 2 mg/mL) of PAE for 20 hr, and cell viability was measured via the neutral red assay. Each value is expressed as mean ± SD in triplicate experiments.
Effect of PAE on blood glucose level in vivo
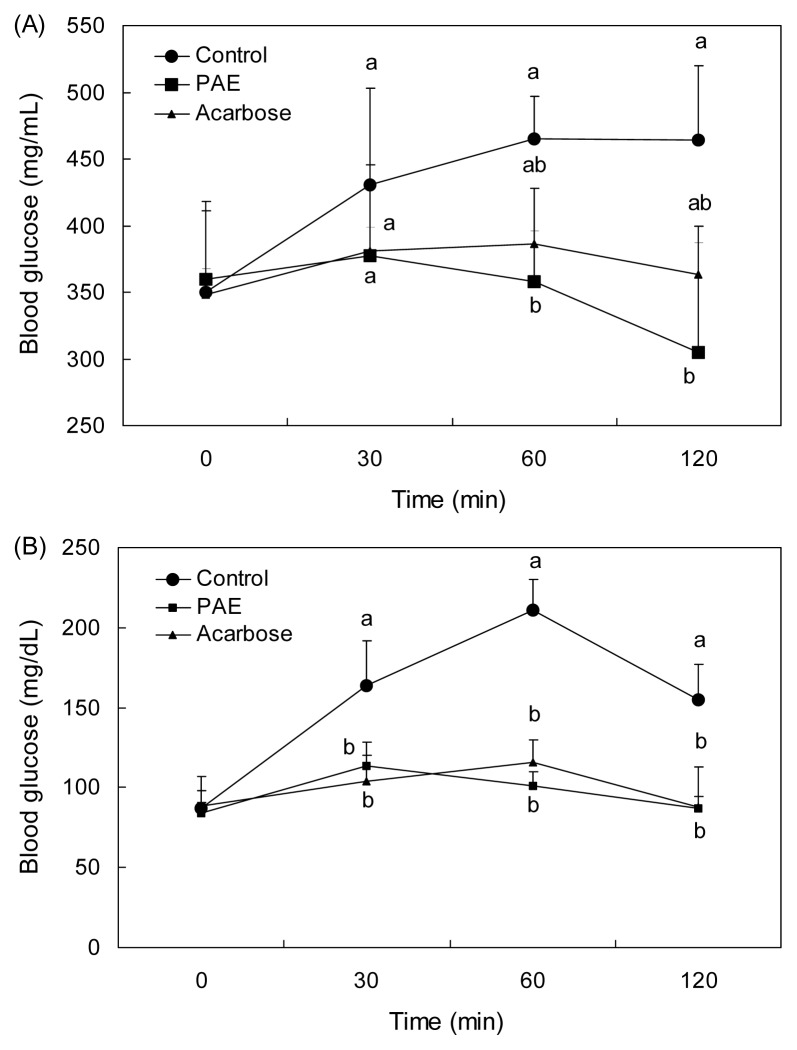
The effect of PAE on blood glucose level after a meal was investigated in STZ-induced diabetic and normal mice. Postprandial blood glucose levels of the PAE administered mice were lower than those in mice from the control group (Fig. 4A). The blood glucose level of the control group increased up to 465 mg/dL at 60 min after a meal, and decreased thereafter. However, the increases in postprandial blood glucose levels were significantly suppressed (p<0.05) when the mice were fed after the administration of PAE (377.33, 358.33, and 304.5 mg/dL at 30, 60, and 120 min, respectively). Consumption of PAE significantly decreased blood glucose levels more than acarbose (381.32, 386, and 363.68 mg/dL at 30, 60, and 120 min, respectively). The postprandial blood glucose level was also significantly decreased when the normal mice were orally administered starch with PAE (Fig. 4B). In normal mice, PAE significantly suppressed (p<0.05) the postprandial hyperglycemia caused by starch. The PAE administration group (699.5 ± 148.1 mg·hr/dL) had a lower area under the curve (AUC) glucose response than the control group (883.5 ± 103.6 mg·hr/dL) in diabetic mice (Table 2). Controlling not only fasting, but also postprandial hyperglycemia is important in achieving tight control of blood glucose levels, which is the major target of diabetic therapy (24). Furthermore, postprandial hyperglycemia has been shown to increase the production of free radicals, which induce vasoconstriction and stimulate prothrombotic pathways leading to an increased risk of cardiovascular disease, the major cause of premature death among type 2 diabetic patients (25). Thus, we determined the anti-postprandial hyperglycemic effect of PAE in streptozotocin-induced diabetic and normal mice after consumption of starch. The increases in postprandial blood glucose levels were suppressed significantly in both streptozotocin-induced diabetic and normal mice when treated with PAE. These results demonstrate that PAE may delay the absorption of dietary carbohydrates, resulting in the suppression of an increase in postprandial blood glucose level. Inoue et al. (26) reported that the medication, which flattens peak of postprandial blood glucose, reduces the AUC of the blood glucose response curve. In this study, PAE was shown to reduce both the blood glucose level at the peak time point and the AUC.
Fig. 4.
Blood glucose levels after the administration of PAE in streptozotocin-induced diabetic mice (A) and normal mice (B). Control (distilled water), PAE (300 mg/kg), and acarbose (100 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean ± SD of seven mice (n=21). a,bValues with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. PAE: Padina arborescens extract
Table 2.
Area under the curve (AUC) of postprandial glucose responses in normal and streptozotocin-induced diabetic mice
| Group1) | AUC (mg·hr/dL) | |
|---|---|---|
|
| ||
| Normal mice | Diabetic mice | |
| PAE | 196.6 ± 19.9b | 699.5 ± 148.1 |
| Acarbose | 204.7 ± 33.3b | 749.1 ± 32.8 |
| Control | 339.4 ± 23.5a | 883.5 ± 103.6 |
Padina arborescens extract (PAE, 300 mg/kg), acarbose (100 mg/kg), and control (distilled water) were co-administered orally with starch (2 g/kg). Each value is expressed as mean ± SD of 7 mice (n=42).
Values with different alphabets in a column are significantly different at p<0.05 as analyzed by Duncan’s multiple range test.
Type 2 diabetic patients have both postprandial hyperglycemia and atherogenic dyslipidemia. Postprandial hyperglycemia is also involved in a variety of metabolic disorders and other diseases, including virus-based diseases and cancer (27). Although α-glucosidase and α-amylase inhibitors are common oral hypoglycemic agents, the chronic use of these agents can lead to gastrointestinal side effects such as flatulence, vomiting, and diarrhea. Thus, marine algae are currently recognized as good candidate sources for naturally-derived anti-diabetic materials. Previously, we evaluated the effects of dieckol isolated from a brown alga, Ecklonia cava, on postprandial hyperglycemia in an in vivo test, and also assessed the prominent effects of dieckol in both streptozotocin-induced diabetic mice and normal mice (28). Nam et al. also noted that brown algae extract may have a beneficial effect on controlling postprandial glucose levels in diabetic rats (29). The finding of this study showed that PAE may prove useful as an effective natural anti-diabetic material.
In conclusion, PAE inhibited α-glucosidase and α-amylase activities followed by a diminished rise in blood glucose, resulting in a reduction in postprandial hyperglycemia. Further, PAE may delay the absorption of dietary carbohydrates in the intestine, resulting in the suppression of increased blood glucose levels after a meal. Thus, we suggested that PAE can be developed in medicinal preparations as a nutraceutical or functional food for diabetes. Also, further studies are needed to reveal which types of active constituents have anti-diabetic effects.
ACKNOWLEDGMENTS
This research was supported by Basic science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
REFERENCES
- 1.Corry DB, Tuck ML. Protection from vascular risk in diabetic by pretension. Curr Hyperten Rep. 2002;2:154–159. doi: 10.1007/s11906-000-0075-2. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD. Postprandial hyperglycemia and α-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40:S51–55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Saito N, Sakai H, Sekihara H, Yajima Y. Effect of an α-glucosidase inhibitor (voglibose), in combination with sulphonilureas, on glycaemic control in type 2 diabetes patients. J Int Med Res. 1998;26:219–232. doi: 10.1177/030006059802600501. [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycaemic control over 3 years (U.K. Prospective Diabetes study 44) Diabetes Care. 1999;22:960–964. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 6.Toeller M. α-Glucosidase inhibitiors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest. 1994;24:31–35. doi: 10.1111/j.1365-2362.1994.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 7.Clissold SP, Edwards C. A preliminary review of its pharmacodynamic and pharmacokinetics properties, and therapeutic potential. Drugs. 1988;35:214–243. doi: 10.2165/00003495-198835030-00003. [DOI] [PubMed] [Google Scholar]
- 8.Casirola DM, Ferraris RP. Alpha-glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55:832–841. doi: 10.1016/j.metabol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alpha-glucosidase inhibitor. Expert Opin Pharmacother. 1999;1:149–156. doi: 10.1517/14656566.1.1.149. [DOI] [PubMed] [Google Scholar]
- 10.Stand E, Baumgartl HJ, Fuchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999;1:125–220. doi: 10.1046/j.1463-1326.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. J Diabetes Complicat. 1998;12:228–237. doi: 10.1016/s1056-8727(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao SH, Liao LH, Cheng PN, Wu TJ. Hepatotoxicity associated with acarbose therapy. Ann Pharmacother. 2006;40:151–154. doi: 10.1345/aph.1G336. [DOI] [PubMed] [Google Scholar]
- 13.Chandini SK, Ganesan P, Bhaskar N. In vitro anti-oxidant activities of three selected brown seaweeds of India. Food Chem. 2008;107:707–713. [Google Scholar]
- 14.Kang JY, Khan MNA, Park NH, Cho JY, Lee MC, Fujii H, Hong YK. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–190. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Fukuyama Y, Kodama M, Miura I, Kinzyo Z, Kido M, Mori H, Nakayama Y, Takahashi M. Structure of an anti-plasmin inhibitor, eckol, isolated from the brown alga Ecklonia kurome Okamura and inhibitory activities of its derivatives on plasma plasmin inhibitors. Chem Pharm Bull. 1989;37:349–353. doi: 10.1248/cpb.37.349. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Min KH, Han JS, Lee DH, Park DB, Jung WK, Park PJ, Jeon BT, Kim SK, Jeon YJ. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem Toxicol. 2012;50:575–582. doi: 10.1016/j.fct.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Chung HY, Ma WC, Ang PO, Jr, Kim JS, Chen F. Seasonal variations of bromophenols in brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegate) collected in Hong Kong. J Agric Food Chem. 2003;51:2619–2624. doi: 10.1021/jf026082n. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of alpha-glucosidase inhibitors from tochucha (Eucommia ulmoides) Biosci Biotechnol Biochem. 1997;61:177–178. doi: 10.1271/bbb.61.177. [DOI] [PubMed] [Google Scholar]
- 19.Fautz R, Husen B, Hechenberger C. Application of the neutral red assay (NR assay) to monolayer cultures of primary hepatocytes: rapid colorimetric viability determination for the unscheduled DNA synthesis test (UDS) Mutat Res. 1991;253:173–179. doi: 10.1016/0165-1161(91)90130-z. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS. Effect of Rhemanniae radix on the hyperglycemic mice induced with streptozotocin. J Korean Soc Food Sci Nutr. 2004;33:1133–1138. [Google Scholar]
- 21.Prashanth D, Samiulla DS, Padmaja R. Effect of certain plant extracts on α-amylase activity. Fitoterapia. 2001;72:179–181. doi: 10.1016/s0367-326x(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AYY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can Med Assoc J. 2005;172:213–226. doi: 10.1503/cmaj.1031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanefeld M, Schaper F. Pharmacotherapy of Diabetes: New Developments Improving Life and Prognosis for Diabetic Patients. Springer Science; New York, NY, USA: 2007. The role of alpha-glucosidase inhibitors (acarbose) pp. 143–152. [Google Scholar]
- 24.Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004;164:486–491. doi: 10.1001/archinte.164.5.486. [DOI] [PubMed] [Google Scholar]
- 25.Ceriello A, Davidson J, Hanefeld M, Leiter L, Monnier L, Owens D, Tajima N, Tuomilehto J. International Prandial Glucose Regulation Study Group. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis. 2006;16:453–456. doi: 10.1016/j.numecd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyper-proinsulinemia in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1997;36:143–151. doi: 10.1016/s0168-8227(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 27.Dennis JW, Laferte S, Waghorne C, Breitman ML, Kergel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Park MH, Heo SJ, Kang SM, Ko SC, Han JS. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozocin-induced diabetic mice. Food Chem Toxicol. 2010;48:2633–2637. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Nam JS, Lee WJ, Yoon IS, Kang MW, Jang HS, Youn JH, Kim BR, Kong HJ, Kim KH, Kim YH, Lee DS, Choi HJ. Effect of a brown algae extract on postprandial glucose control in neonatal diabetic and obese rats. J FASEB. 2007;21(845):2. [Google Scholar]