Abstract
Thirty-two different volatile oils were identified from Allomyrina dichotoma (A. dichotoma) larvae by gas chromatography/mass spectrometry (GC/MS). The major volatile components were 2,2,4-trimethyl-3-carboxyisopropyl pentanoic acid isobutyl ester (5.83%), phenol,2,6-bis(a,a-dimethyl ethyl)-4-(1-methyl-1-phenylethyl) (5.72%), heptacosane (5.49%) and phenol,2,4-bis(1-methyl-1-phenylethyl) (5.47%). The composition of the fatty acids in A. dichotoma larvae was also determined by gas chromatography (GC) and fourteen constituents were identified. Oleic acid (19.13%) was the most abundant fatty acid followed by palmitic acid (12.52%), palmitoleic acid (3.71%) and linoleic acid (2.08%) in 100 g of A. dichotoma larvae on a dry weight basis. The quantity of unsaturated fatty acids (64.00%) were higher than that of saturated ones (36.00%). The predominant fatty acids in A. dichotoma consist of monounsaturated fatty acid (MUFA, 57.70%) such as oleic acid, myristoleic acid and palmitoleic acid, followed by saturated fatty acids (36.00%) and polyunsaturated fatty acids (PUFA, 6.50%). In particular, the presence of essential fatty acids, such as linoleic (5.30%) and linolenic acid (0.40%) give A. dichotoma larvae considerable nutritional and functional value and it may be a useful source for food and/or industrial utilization.
Keywords: Allomyrina dichotoma, fatty acids, GC, GC/MS, SDE, volatile oils
INTRODUCTION
Allomyrina dichotoma (A. dichotoma) is a species of rhinoceros beetle and lives most of its life subterranean (1). A. dichotoma is one of many precious oriental insects found in China, Japan, Taiwan and Korea and widely used in traditional medicine as treatments for many diseases, such as the liver and diabetes mellitus (2–4).
As demonstrated by several studies, A. dichotoma possesses various biological functions including antineoplastic, anticytotoxic and antioxidant effects (1,5,6). Lectin isolated from A. dichotoma has been shown to possess an immunomodulating property through cytokine production (7). Coleoptericin A and B, purified proteins derived from A. dichotoma, were proven to have antibacterial activity against Staphylococcus aureus and Bacillus subtilis(4). These two proteins also exhibited anti-inflammatory effects by inhibiting tumor necrosis factor-α (TNF-α) production (8).
Preliminary analyses suggest that A. dichotoma larvae contains significant amounts of unsaturated fatty acids. However, only limited studies on nutritional characteristics, profiles or functions of lipid soluble components in A. dichotoma have been reported. Therefore, in the present study, we have analyzed both the composition of the essential oils and fatty acids of A. dichotoma larvae by GC and GC/MS analysis.
MATERIALS AND METHODS
Sample preparation
The freeze-dried powder of A. dichotoma larvae was provided by World Way Corp. at Chungnam, Korea. Fatty acid composition was determined using gas chromatography (GC) of fatty acid methyl esters (FAME). FAME was prepared according to the method of van-Wijngaarden with minor modifications (9). Briefly, approximately 3 g of sample was weighed into a screw-capped test tube and 5 mL of tetrahydrofuran (THF) was added. The mixture was added to 30 mL of 1 N KOH in ethanol, refluxed at 85°C for 90 min. The sample was then cooled and acidified with HCl to pH 3. Distilled water and di-ethyl ether were added, mixed and the ether layer was discarded while the aqueous layer was acidified with concentrated HCl. The aqueous layer was backwashed with additional ether and then transferred to a new Kimax tube. The ditheyl ether was removed using a sand bath. About 40 mL of methanol and 0.5 mL of H2SO4 was added and the mixture was refluxed for 3 hr. After cooling, water and diethyl ether were added and the FAME extracted into the ether. The diethyl ether was removed using a stream of N2, and the methyl esters were dissolved in methylene chloride for GC analysis (10).
Proximate composition
Proximate composition of A. dichotoma larvae was determined by AOAC methods (11). Crude protein content was calculated by converting the nitrogen content, determined by Kjeldahl’s method (6.25×N). Crude fat was determined by Soxhlet system. Ash content was determined by dry ashing in a furnace oven at 550°C for 5 hr. The moisture content was determined by drying sample in an oven at 105°C until constant weight was obtained. All analyses were done in triplicate.
GC analysis
The samples were analyzed on a Hewlett-Packard 6890 Series GC (Hewlett-Packard Co., Wilmington, DE, USA), equipped with a flame ionization detector (FID), along with an SP-2560 column (100 m×0.25 mm, 0.2 μm). Helium was used as the carrier gas at a flow rate of 1 mL/min. The detector temperature was 260°C. The injector was set at 260°C in a split ratio of 50:1. One μL of each sample was injected. The column temperature, after an initial isothermal period of 5 min at 140°C, was increased to 240°C at a rate of 3°C/min and maintained at this temperature for 10 min.
Simultaneous distillation extraction (SDE) of volatile oils
Ten gram sample and 1 L of deionized water was placed in a 2 L round bottom flask and connected to Likens-Nickerson apparatus as described by Schultz et al. (12). Sample was extracted with 100 mL of redistilled n-pentane : diethyl ether (1:1, v/v). Extraction was carried out for 4 hr after the distilled water in the sample flask started to boil. The extract was dried over Na2SO4 overnight at −4°C and concentrated to 0.5 mL using a rotary vacuum evaporator (EYELA, N-1100, Tokyo, Japan).
GC/MS analysis
Analysis of samples was performed using a HP-5MS capillary column (30 m×0.25 mm, 0.25 μm, Agilent technologies Inc., Santa Clara, CA, USA) in a GC/MS (5975C, Agilent technologies Inc). Sample was injected into the column and ran using split mode (split ratio= 10:1). The helium carrier gas was programmed to maintain a constant flow rate of 1 mL/min. Oven was initially 80°C for 3 min, then finally raised to 300°C at 4°C/min.
Identification of fatty acids and volatile compounds
Fatty acids were identified by a reference standard mixture FAME (Supelco, Belle fonte, PA, USA) analyzed under the same operating conditions as those employed for FAME of the samples. Qualitative analysis of volatile compounds was carried out by identification of compounds from the mass spectrum library. The spectrum of compounds agreed with that present in the mass spectrum library of NIST 11.
RESULTS AND DISCUSSION
Proximate analysis in A. dichotoma larvae
The results of proximate analysis are shown in Table 1. A. dichotoma larvae contained 38.17±0.48% crude protein, 32.72±0.76% crude fat, 4.14±0.04% crude ash, 22.73±0.42% carbohydrates and 2.25±0.01% moisture.
Table 1.
Proximate composition (%) of Allomyrina dichotoma larvae
| Composition | % |
|---|---|
| Crude protein | 38.17±0.48 |
| Crude fat | 32.72±0.76 |
| Crude ash | 4.14±0.04 |
| Carbohydrate | 22.73±0.42 |
| Moisture | 2.25±0.01 |
Data are expressed as mean±SD (n=3) on a dry weight basis.
Fatty acids composition of A. dichotoma larvae
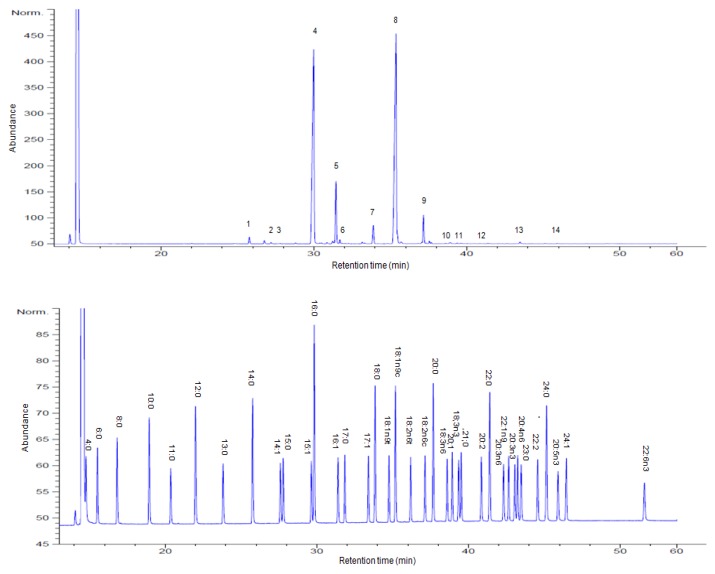
The chromatograms shown in Fig. 1 correspond to standard fatty acid methyl ester (A) and fatty acids in A. dichotoma larvae (B). The peak assignation is shown in Table 2. Fourteen different fatty acids were identified; the yield of total fatty acids in A. dichotoma larvae was 39.87% on dry weight basis. Fatty acid data, to be useful for nutritional value, were shown as grams of each fatty acid per 100 grams of A. dichotoma larvae, on dry weight basis. Fatty acid composition was dominated by oleic acid (19.13 g/100 g of sample) and palmitic acid (12.52 g/100 g of sample) followed by palmitoleic acid (3.71 g/100 g of sample) and linoleic acid (2.08 g/100 g of sample). Oleic acid (C18:1), palmitic acid (C16:0), palmitoleic acid (C16:1) and linoleic acid (C18:2) combined for more than 90% of total fatty acids in A. dichotoma larvae. The predominant fatty acid in A. dichotoma was mono-unsaturated fatty acid (MUFA, 57.7%), such as myristoleic acid, palmitoleic acid and oleic acid, followed by saturated fatty acid (SFA, 36%) and polyunsaturated fatty acid (PUFA, 6.5%). In particular, the presence of essential fatty acids in total fatty acids such as linoleic (5.3%) and linolenic (0.4%) bestow A. dichotoma larvae with considerable nutritional value. Our result showed several odd-chain fatty acids such as pentadecanoic acid (C15:0), heptadecanoic acid (C17:0) and tricosanoic acid (C23:0), all of which are rarely detected in analysis of insect fatty acids (13,14). The results give novel information of A. dichotoma larvae regarding its nutritional value and shows its possible utilization for food and/or industrial applications.
Fig. 1.
Gas chromatogram of standard fatty acids (A) and fatty acids of Allomyrina dichotoma larvae (B). Four major compounds are indicated as 4, palmitic acid; 5, palmitoleic acid; 8, oleic acid; 9, linoleic acid. For peaks numbers, see Table 2.
Table 2.
Fatty acid composition of Allomyrina dichotoma larvae
| No. | Fatty acid | Mol. formula | Retention time | Amount (g/100 g of sample) |
|---|---|---|---|---|
| 1 | Myristic acid | C14:0 | 25.78 | 0.45 |
| 2 | Myristoleic acid | C14:1 | 27.61 | 0.13 |
| 3 | Pentadecanoic acid | C15:0 | 27.80 | 0.13 |
| 4 | Palmitic acid | C16:0 | 29.99 | 12.52 |
| 5 | Palmitoleic acid | C16:1 | 31.45 | 3.71 |
| 6 | Heptadecanoic acid | C17:0 | 31.87 | 0.13 |
| 7 | Stearic acid | C18:0 | 33.89 | 0.91 |
| 8 | Oleic acid | C18:1 | 35.37 | 19.13 |
| 9 | Linoleic acid | C18:2 | 37.17 | 2.08 |
| 10 | γ-Linolenic acid | C18:3 | 38.62 | 0.12 |
| 11 | α-Linolenic acid | C18:3 | 39.37 | 0.15 |
| 12 | cis-11,14-Eicosadienoic acid | C20:2 | 40.85 | 0.11 |
| 13 | Tricosanoic acid | C23:0 | 43.48 | 0.20 |
| 14 | cis-5,8,11,14,17-Eicosapentaenoic acid | C20:5 | 45.91 | 0.10 |
Volatile constituents of A. dichotoma larvae
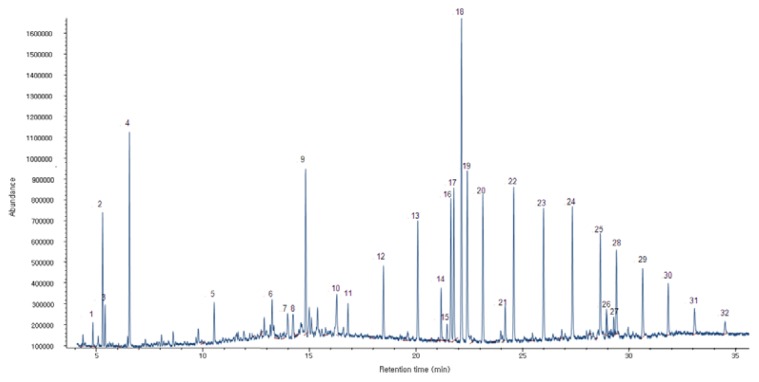
The results of volatile compounds in A. dichotoma larvae analyzed by GC/MS were exhibited in Fig. 2. Thirty two volatile compounds belonging to chemical classes of acids (2), alcohols (5), esters (1), hydrocarbons (16), terpenes (2) and others (6) were tentatively determined (Table 3, 4). Hydrocarbons were detected as the dominant group due to the highest proportion (50.46%). The major compounds belonging to hydrocarbons were heptacosane (5.49%), hexacosane (5.43%), tetracosane (5.20 %), octacosane (4.88%), pentacosane (4.87%) and heneicosane (4.41%).
Fig. 2.
GC/MS Chromatogram of volatile compounds in Allomyrina dichotoma larvae.
Table 3.
Relative content of functional groups of volatile oils in Allomyrina dichotoma larvae
| Functional groups | Relative peak area percentage (%) | Number of compounds |
|---|---|---|
| Acids | 6.95 | 2 |
| Alcohols | 17.04 | 5 |
| Esters | 2.43 | 1 |
| Hydrocarbons | 50.46 | 16 |
| Terpenes | 1.78 | 2 |
| Miscellaneous (including unknowns) | 21.34 | 6 |
|
| ||
| Total | 100 | 32 |
Table 4.
Volatile compounds of Allomyrina dichotoma larvae
| No. | Compound name | Retention time | Relative peak area% |
|---|---|---|---|
| 1 | 4-(1,2-Dimethyl-cyclopent-2-enyl)-butan-2-one | 4.34 | 0.66 |
| 2 | Phenol,2,5-bis(1,1-dimethylethyl) | 4.80 | 3.37 |
| 3 | Butylated hydroxytoluene | 4.91 | 1.03 |
| 4 | Pentanoic acid,2,2,4-trimethyl-3-carboxy isopropyl, isobutyl ester | 6.06 | 5.83 |
| 5 | 2,4-Diphenyl-4-methyl-2(E)-pentene | 10.03 | 1.14 |
| 6 | Hexadecanoic acid, ethyl ester | 12.75 | 1.12 |
| 7 | Phenol,2-(1,1-dimethylethyl)-4-(1-methyl-1-phenylethyl)- | 13.48 | 1.35 |
| 8 | 10,18-Bisnorabieta-8,11,13-triene | 13.73 | 1.31 |
| 9 | Phenol,2,6-bis(1,1-dimethylethyl)-4-(1-methyl-1-phenylethyl)- | 14.33 | 5.72 |
| 10 | Ethyl oleate | 15.79 | 2.43 |
| 11 | Heptadecane | 16.32 | 1.04 |
| 12 | Eicosane | 17.98 | 2.46 |
| 13 | Heneicosane | 19.59 | 4.41 |
| 14 | 1,21-Docosadiene | 20.69 | 1.61 |
| 15 | 2,4-Diphenyl-4-methyl-1-pentene | 20.96 | 0.64 |
| 16 | Pentacosane | 21.14 | 4.87 |
| 17 | 2,4-bis(1-methyl-1-phenylethyl)-phenol | 21.28 | 5.47 |
| 18 | Unknown | 21.64 | 11.74 |
| 19 | Unknown | 21.91 | 5.92 |
| 20 | Hexacosane | 22.64 | 5.43 |
| 21 | 1,13-Tetradecadiene | 23.69 | 1.34 |
| 22 | Heptacosane | 24.09 | 5.49 |
| 23 | Tetracosane | 25.49 | 5.20 |
| 24 | Octacosane | 26.85 | 4.88 |
| 25 | Triacontane | 28.16 | 3.69 |
| 26 | Phenol,2,4,6-tris(1-methyl-1-phenylethyl)- | 28.46 | 1.13 |
| 27 | 1-Allyl-5-bromo-6-hydroxypyridazin-6-one | 29.30 | 0.68 |
| 28 | Tetratriacontane | 29.43 | 3.33 |
| 29 | Hexadecane, 1-iodo- | 30.66 | 2.79 |
| 30 | Tetratetracontane | 31.85 | 1.98 |
| 31 | Octadecane | 33.09 | 1.32 |
| 32 | Eicosane, 2-methyl- | 34.52 | 0.69 |
The alcohol group (17.04%) was characterized as the second major chemical group. The major constituents of alcohols were phenol,2,6-bis(1,1-dimethylethyl)-4-(1- methyl-1-phenylethyl) (5.72%), phenol,2,4-bis(1-methyl-phenylethyl) (5.47%), phenol,2,5-bis(1,1-dimethyletyl) (3.37%) and phenol,2-(1,1-dimethylethyl)-4-(1-methyl- 1-phenylethyl) (1.35%). Acids (6.95%) include pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl (5.83%) and hexadecanoic acid, ethyl ester (1.12%). The main compounds of esters (2.43%), and terpenes (1.78%) were ethyl oleate (2.43%) and 2,4-diphenyl-4-methyl-2(E)-pentene (1.14%), respectively.
In this study, we determined the profile of fatty acids and volatile constituents of A. dichotoma larvae. The idea of eating insects is not well received due to their appearance. However, many insects are rich sources of proteins, lipids, minerals and vitamins (15). The present study clearly suggests, for the first time, the compositions of fatty acids and volatile oils in A. dichotoma larvae. The overall result gives novel information on the property of A. dichotoma larvae as a good source of oil and suggests a potential source of oil for nutritional and medicinal purposes.
ACKNOWLEDGMENTS
This work was supported by the Agenda Program funded by Rural Development Administration (2012- PJ008969).
REFERENCES
- 1.Suh HJ, Kim SR, Lee KS, Park S, Kang SC. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: insecta) larvae. J Photochem Photobiol B: Biol. 2010;99:67–73. doi: 10.1016/j.jphotobiol.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Taketa K, Ichikawa E, Umetsu K, Suzuki T. Allomyrina dichotoma lectin-nonreactive α-fetoprotein in hepatocellular carcinoma and other tumors: Comparison with Ricinus communis agglutinin-1. Cancer Lett. 1986;31:325–331. doi: 10.1016/0304-3835(86)90155-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee KJ, Lee JB. Protective effect of Allomyrina dichotoma larva extract on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Korean J Environ Biol. 2009;27:230–236. [Google Scholar]
- 4.Sagisaka A, Miyanoshita A, Ishibashi J, Yamakawa M. Purification, characterization and gene expression of glycine and proline-rich antibacterial protein family from larvae of beetle, Allomyrina dichotoma. Insect Mol Biol. 2001;10:293–302. doi: 10.1046/j.0962-1075.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa K, Umetsu K, Shinzawa H, Yuasa I, Maruyama K, Ohkura T, Yamashita K, Suzuki T. Determination of carbohydrate-deficient transferring separated by lectin affinity chromatography for detecting chronic alcohol abuse. FEBS Lett. 1999;458:112–116. doi: 10.1016/s0014-5793(99)01137-0. [DOI] [PubMed] [Google Scholar]
- 6.Kim DS, Huh J, You GC, Chae SC, Lee OS, Lee HB, Lee JB, Kim JS. Allomyrina dichotoma larva extracts protect streptozotocin-induced oxidative cytotoxicity. J Environ Toxicol. 2007;22:349–355. [Google Scholar]
- 7.Jeune KH, Jung MY, Choi SJ, Lee JW, Park WH, Cho SH, Lee SH, Chung SR. Immunomodulating effect of the lectin from Allomyrina dichotoma. Korean J Pharmacogn. 2001;32:31–38. [Google Scholar]
- 8.Koyama Y, Motobu M, Hikosaka K, Yamada M, Nakamura K, Saido-Sakanaka H, Asaoka A, Yamakawa M, Sekikawa K, Kitani H, Shimura K, Nakai Y, Hirota Y. Protective effects of antimicrobial peptides derived from the beetle Allomyrina dichotoma defensin on endotoxic shock in mice. Int Immunopharmacol. 2006;6:234–240. doi: 10.1016/j.intimp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Choo WS, Birch J, Dufour JP. Physicochemical and quality characteristics of cold-pressed flaxseed oils. J Food Comp Anal. 2007;20:202–211. [Google Scholar]
- 10.Woodbury SE, Evershed RP. Detection of vegetable oil adulteration using gas chromatography combustion/isotope ratio mass spectrometry. Anal Chem. 1995;67:2685–2690. [Google Scholar]
- 11.AOAC. Official methods of analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC, USA: 1990. p. 69.p. 70.p. 79. [Google Scholar]
- 12.Schultz TH, Flath RA, Mon TR, Eggling SB, Teranishi R. Isolation of volatile components from a model system. J Agric Food Chem. 1977;25:446–449. [Google Scholar]
- 13.Slover HT, Lanza E. Quantitative analysis of food fatty acids by capillary gas chromatography. J Am Oil Chem Soc. 1979;56:933–943. [Google Scholar]
- 14.Howard RW, Stanley-Samuelson DW. Phospholipid fatty acid composition and arachidonic acid metabolism in selected tissues of adult Tenebrio molitor (Coleoptera: Tenebrionidae) Ann Entomol Soc Am. 1990;83:975–981. [Google Scholar]
- 15.Aguilar-Miranda ED, Lopez MG, Escamilla-Santana C, Barba de la Rosa AP. Characteristics of maize flour tortilla supplemented with ground Tenebrio molitor larvae. J Agric Food Chem. 2002;50:192–195. doi: 10.1021/jf010691y. [DOI] [PubMed] [Google Scholar]