Abstract
We investigated the effect of various cooking methods (boiling, steaming, stir-frying, and roasting) and three cooking times (5, 10, and 15 min) on the antioxidant properties of red pepper. Raw and cooked peppers were measured for proximate composition, ascorbic acid (AsA) content, total carotenoid content (TCC), total polyphenol content (TP), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activities. Results showed that the proximate composition, AsA content, TCC, TP, and antioxidant activities were significantly (p<0.05) affected by the cooking procedure; the loss rate varied among individual compounds. Boiling and steaming significantly reduced AsA content (24.3~66.5%), TP (13.9~ 54.9%), and antioxidant activity (21.7~60.5%) in red pepper, while stir-frying and roasting slightly reduced AsA content (2.7~25.9%), TP (1.8~4.9%), and antioxidant activity (4.9~17.9%). The highest loss was observed after boiling, followed by steaming, roasting, and stir-frying. Stir-frying and roasting better preserved AsA content, TCC, TP, and antioxidant activity. In conclusion, dry-heat cooking methods such as stir-frying and roasting may be preferred to retain the nutrient compositions and antioxidant properties of red pepper.
Keywords: cooking, red pepper, ascorbic acid, antioxidant activity
INTRODUCTION
Peppers (Capsicum spp.), which are grown worldwide, including Korea, are used extensively as a natural food colorant and seasoning agent due to their attractive color, flavor, and taste (1). Pepper has a high nutritive value and has long been recognized as an excellent source of vitamin C. Moreover, the vitamin C, carotenoids, poly-phenols, and other phytochemicals in pepper are powerful antioxidants that destroy free radicals (2,3). The levels of these compounds in pepper depend on many factors, including cultivar, maturity, growing conditions, and climate (4,5). Numerous studies have examined peppers mainly to evaluate the chemical composition and/or antioxidant activities of various cultivars (5–7) and the effect of drying methods on physicochemical properties (8–10).
Peppers are consumed in fresh or powder form. Peppers are also cooked with vegetables and commonly used to make paste, pickles, and sauce. In Asian cuisine, peppers are boiled or stir-fried with other foods and vegetables. Cooking processes, which are usually performed to increase the palatability and improve the edibility of food (11), can alter the physical characteristics and chemical compositions of the food. A common perception is that thermally cooked foods have lower nutritional value than fresh foods because of the decline of vitamin C and loss of particular physiochemical characteristics (12- 15). In contrast, recent reports suggest that cooking increases antioxidant activities by liberating antioxidant compounds from insoluble portions of foods (16,17). Taken together, studies on the effect of cooking processes on antioxidant compounds such as polyphenols, carotenoids, and vitamin C in fruits and vegetables are inconclusive (1,14,18).
Many studies have investigated the effect of various cooking processes on the levels of antioxidant compounds and antioxidant activities in fruits and vegetables (15,16,19–21). However, very little information is available in the literature regarding the effect of cooking methods and cooking times on antioxidant properties in red pepper. This study was undertaken to investigate the effect of different cooking methods (boiling, steaming, stir-frying, and roasting) and cooking times (5, 10, and 15 min) on the antioxidant properties of red pepper.
MATERIALS AND METHODS
Plant materials and reagents
Fresh red peppers were obtained from the local market in Suwon, South Korea, in February 2012. The experiments were performed immediately after procurement. L-ascorbic acid, β-carotene, gallic acid, Folin-Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azi-no-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and potassium persulfate were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Sample preparation and cooking processes
General
The peppers were rinsed in water and dried on paper towels. The stem and seeds were removed, and edible parts were collected. These portions were cut in almost equally shaped small pieces (2 × 2 cm). One section was retained as raw and the others were cooked. The four cooking methods commonly used in Korean cuisine, boiling, steaming, stir-frying, and roasting, and three cooking times, 5, 10, and 15 min, were employed. All cooking experiments were performed in triplicate, each using 200 g of pepper.
Boiling
One liter of water was heated to boil in stainless steel pots. The pots were covered to prevent water loss. Two hundred grams of cut peppers were added to the boiling water and cooked for 5, 10, and 15 min. After boiling, the cooked peppers were drained for 1 min by using a wire mesh strainer and then freeze-dried.
Steaming
Two hundred grams of cut peppers were placed on a tray in a stainless steel steam cooker, which was covered with a lid, and steamed over 95°C water for 5, 10, and 15 min under atmospheric pressure. After steaming, the cooked peppers were drained for 1 min by using a wire mesh strainer and then freeze-dried.
Stir-frying
Two grams of soybean oil was placed in a frying pan (30 cm in diameter), which was heated at “high” on a hot plate (GKST 300Z, THIELMANN, Seoul, Korea) for 1 min. Two hundred grams of cut peppers were then placed in the pan, and the heating was reduced to “medium”. Peppers were stirred for 5, 10, and 15 min. Next, the cooked peppers were cooled for 10 min and then freeze-dried.
Roasting
Two hundred grams of cut peppers were placed in a classical home oven (GOR-4A11C, TONG-YANG/MAGIC, Seoul, Korea) and roasted at 190°C for 5, 10, and 15 min. Next, the cooked peppers were cooled for 10 min and then freeze-dried.
Proximate composition
The standard method of AOAC (22) was used for determining crude protein, fat, and ash content. Crude protein content was calculated by converting the nitrogen content determined by Kjeldahl’s method. Crude fat content was obtained using the Soxhelt method. Crude ash content was determined by dry ashing in a furnace at 550°C.
Determination of ascorbic acid (AsA) content
The AsA content in samples was determined using the method by Zhuang et al. (5) with slight modifications. The samples (1 g) were homogenized in the dark for 2 min with 100 mL of 5% metaphosphoric acid. The homogenate was then centrifuged at 15,000 rpm for 5 min at 4°C. The supernatant obtained was filtered through a 0.45 μm membrane filter for high performance liquid chromatography (HPLC) analysis. HPLC analysis was performed using Agilent Technologies 1200 series HPLC systems (Palo Alto, CA, USA) with a Mightysil RP-18 GP column (4.6 × 250 mm, 5 μm, Kanto Chemical, Tokyo, Japan) at 40°C. The mobile phase was 0.1% tri-fluoroacetic acid. The flow rate was 0.6 mL/min, and L-ascorbic acid was detected at 254 nm.
Determination of total carotenoids content (TCC)
The total carotenoid analysis was performed according to the Zhuang and Hamauzu method (15). Consecutive extractions of carotenoids from 0.5 g samples were performed with acetone and petroleum ether (1:1, v/v) by using an Ultra Turrax homogenizer (T25, IKA Labor-technik Co., Staufen, Germany) until no more color was extracted. The upper phase was collected and combined with crude extracts after being washed several times with water. The extracts were made up to a known volume with petroleum ether. TCC was determined by recording the absorbance at 450 nm with a spectrophotometer (UV- 1650PC, Shimadzu, Kyoto, Japan). The TCC was expressed as micrograms of β-carotene equivalents per 100 g fresh weight.
Extraction of samples for total polyphenol (TP) content, DPPH and ABTS radical scavenging activity
Powdered samples (2 g) were extracted with 100 mL of 80% ethanol-water solution (v/v). The contents were sonicated at room temperature for 30 min in an ultrasonic bath (frequency, 40 Hz; power, 300 W; SD-350H; Seong Dong, Seoul, Korea) and then filtered using a Whatman No. 4 filter paper. The residue was re-extracted as described above. The combined extracts were concentrated using a rotary vacuum evaporator at 40°C. The extracts were diluted to a final volume of 100 mL with 80% ethanol-water solution and stored at -70°C until analysis.
Determination of total polyphenol content
The TP content in the extract was determined using the Folin-Ciocalteu method (23). Standard solution or extract (0.2 mL) was mixed with 2 mL of 2% Na2CO3 solution and 0.1 mL of 50% Folin-Ciocalteu reagent. After 30 min, the absorbance was read at 750 nm, and TP was calculated from a calibration curve that was obtained using gallic acid as the standard. The results were expressed as milligrams of gallic acid equivalents per 100 g fresh weight. All extracts were analyzed in triplicate.
DPPH radical scavenging activity
The DPPH radical scavenging activity of the extracts was evaluated using the scavenging activity of the stable DPPH free radical, which was measured according to the method of Tepe et al. (23) with some modifications. Aliquots of 1 mL of 0.2 mM DPPH methanolic solution were mixed with 50 μL of the samples. The mixture was shaken vigorously and then kept at room temperature for 30 min in the dark. The absorbance was measured at 520 nm by using a spectrophotometer (UV- 1650PC, Shimadzu, Kyoto, Japan). The DPPH radical scavenging activity was expressed as milligrams of ascorbic acid (AA) equivalents per 100 g of fresh weight (mg AA eq/100 g). The AA equivalent antioxidant activity was calculated as (ΔA/ΔAAA) × CAA, where ΔA is the change in absorbance after the addition of the extract, ΔAAA is the change in absorbance after the addition of AA standard solution, and CAA is the concentration of the AA standard solution. All samples were analyzed in triplicate.
ABTS radical scavenging activity
The ABTS radical cation-scavenging activity of the extracts was measured according to the method described by Re et al. (24) and Hwang et al. (25) with some modifications. The ABTS radical cation was generated by adding 7 mM ABTS to 2.45 mM potassium persulfate solution and leaving the mixture to stand overnight in the dark at room temperature. The ABTS radical cation solution was diluted with distilled water to obtain an absorbance of 1.0 at 735 nm. A 1 mL aliquot of diluted ABTS radical cation solution was added to 50 μL of the sample or distilled water. The absorbance at 735 nm was determined using a spectrophotometer (UV-1650PC, Shimadzu) after 30 min. The ABTS radical cation scavenging activity was expressed as milligrams of AA equivalents per 100 g of fresh weight (mg AA eq/100 g). The AA equivalent antioxidant activity was calculated as (ΔA/ΔAAA) × CAA, where ΔA is the change in absorbance after the addition of the extract, ΔAAA is the change in absorbance after the addition of AA standard solution, and CAA is the concentration of the AA standard solution. All samples were analyzed in triplicate.
Statistical analysis
The results were reported as mean ± standard deviation (SD) values. The significant differences among the means were determined with one way analysis of variance (ANOVA) by using SPSS version 12 (SPSS Institute, Chicago, IL, USA) at a significance level of 0.05. Pearson’s correlation test was used to assess correlations between the means.
RESULTS AND DISCUSSION
Effect of cooking methods on proximate composition
The proximate compositions of raw and cooked red pepper are presented in Table 1. The proximate composition of red pepper was significantly (p<0.05) affected by boiling and steaming and unaffected by stir-frying. The protein, fat, and ash contents in raw red pepper were 1.63, 0.61, and 1.12 g/100 g, respectively. The mean concentrations of proximate compositions of red pepper found in this study are similar to those shown in previous publications (26,27). Boiling and steaming significantly (p<0.05) decreased the protein (1.01~1.33 g/100 g and 1.40~1.56 g/100 g, respectively), fat (0.49~0.56 g/100 g and 0.57~0.61 g/100 g, respectively), and ash (0.42~ 0.65 g/100 g and 0.93~1.05 g/100 g, respectively) contents, depending on the time of processing; these decreases may be caused by diffusion of the contents into cooking water (28). The fat content significantly (p< 0.05) increased during stir-frying due to addition of the oil during frying. Thus, stir-frying had minimal effects on the nutrient composition of red pepper, followed by roasting, steaming, and boiling.
Table 1.
Effects of different cooking methods and cooking times on the proximate composition of red pepper
| Cooking methods | Protein | Fat | Ash | |
|---|---|---|---|---|
|
| ||||
| g/100 g fresh weight basis | ||||
| Raw | 1.63 ± 0.05f1) | 0.61 ± 0.06a | 1.12 ± 0.05e | |
|
| ||||
| Boiling | 5 min | 1.33 ± 0.02c | 0.56 ± 0.06ab | 0.65 ± 0.03b |
| 10 min | 1.18 ± 0.06b | 0.50 ± 0.04a | 0.54 ± 0.06b | |
| 15 min | 1.01 ± 0.11a | 0.49 ± 0.05a | 0.42 ± 0.05a | |
|
| ||||
| Steaming | 5 min | 1.56 ± 0.07ef | 0.61 ± 0.02ab | 1.05 ± 0.05cde |
| 10 min | 1.44 ± 0.04cde | 0.58 ± 0.04ab | 0.96 ± 0.04cd | |
| 15 min | 1.40 ± 0.07cd | 0.57 ± 0.03ab | 0.93 ± 0.03c | |
|
| ||||
| Stir-frying | 5 min | 1.62 ± 0.05f | 2.20 ± 0.02c | 1.15 ± 0.05e |
| 10 min | 1.63 ± 0.02f | 2.21 ± 0.06c | 1.13 ± 0.04e | |
| 15 min | 1.62 ± 0.07f | 2.17 ± 0.09c | 1.15 ± 0.07e | |
|
| ||||
| Roasting | 5 min | 1.55 ± 0.07ef | 0.60 ± 0.04ab | 1.11 ± 0.06e |
| 10 min | 1.53 ± 0.04def | 0.62 ± 0.08ab | 1.10 ± 0.09e | |
| 15 min | 1.47 ± 0.06cde | 0.63 ± 0.06ab | 1.08 ± 0.07de | |
Values with different superscripts in a column indicate significant difference (p<0.05) by Duncan’s multiple range test.
Effect of cooking methods on ascorbic acid content
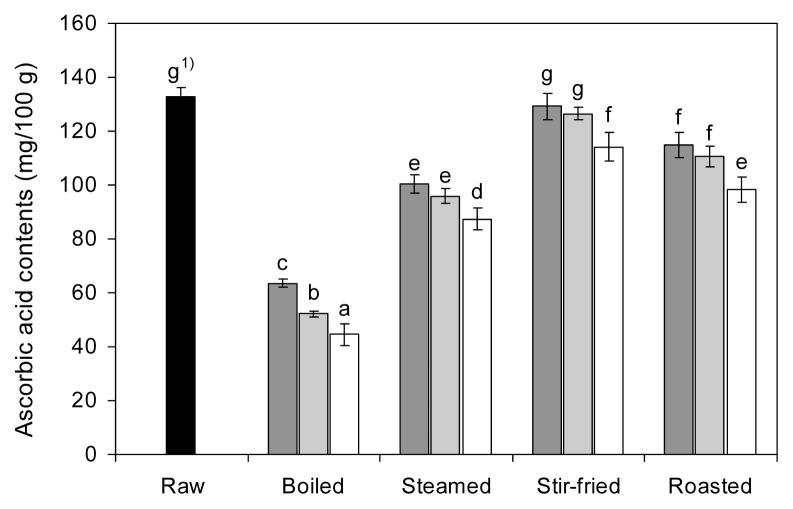
The AsA levels in raw and cooked red peppers are presented in Fig. 1 and are expressed on a fresh weight basis. The AsA content of raw red pepper was 132.72 mg/100 g. All cooking methods lead to a significant loss (p<0.05) in the amount of AsA compared with fresh red pepper. As cooking time increased, the AsA content of boiled, steamed, stir-fried, and roasted red pepper decreased, with a range of 55.80~75.87, 88.51~98.66, 105.82~119.95, and 103.46~109 mg/100 g, respectively. For boiling, steaming, stir-frying, and roasting, the mean AsA amount lost after cooking for 15 min were 66.5%, 34.2%, 14.0%, and 25.9%, respectively. The result of the effect of four different cooking methods indicated that the highest reduction was noted after boiling, followed by steaming, roasting, and stir-frying. The results show that moist-heat cooking methods (boiling and steaming) result in high losses, while dry-heat cooking methods (stir-frying and roasting) result in only small losses. Previous studies have shown that cooking reduces AsA content in fruits and vegetables, including red pepper (15,18,29); therefore, as a commonly perceived idea, AsA is destroyed during cooking because it is unstable at high temperature. Chuah et al. (18) reported that the AsA levels in peppers decreased during cooking procedures such as boiling, microwave cooking, and stir- frying. Significant reductions were documented for boiling particularly due to the diffusion of AsA into cooking water. Somsub et al. (29), Lešková et al. (30), and Masri-zal et al. (31) reported higher AsA retention values in foods processed by stir-frying and microwave cooking than in those processed by boiling or blanching. Various studies report that cooking reduces AsA content in food, including sweet chestnuts (12), potatoes (19), tropical leafy vegetables (20), selected Thai vegetables (29), and broccoli and sweet peppers (32). The amount of cooking-related loss of AsA depends on several factors, including cooking method, heating temperature, cooking time, enzymatic oxidation during preparation, and surface area exposed to water and oxygen (18,30).
Fig. 1.
Effects of different cooking methods and cooking times on the ascorbic acid contents of red pepper (■ 5 min cooking;
 10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Dun-can’s multiple range tests.
10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Dun-can’s multiple range tests.
Effect of cooking methods on total carotenoid content
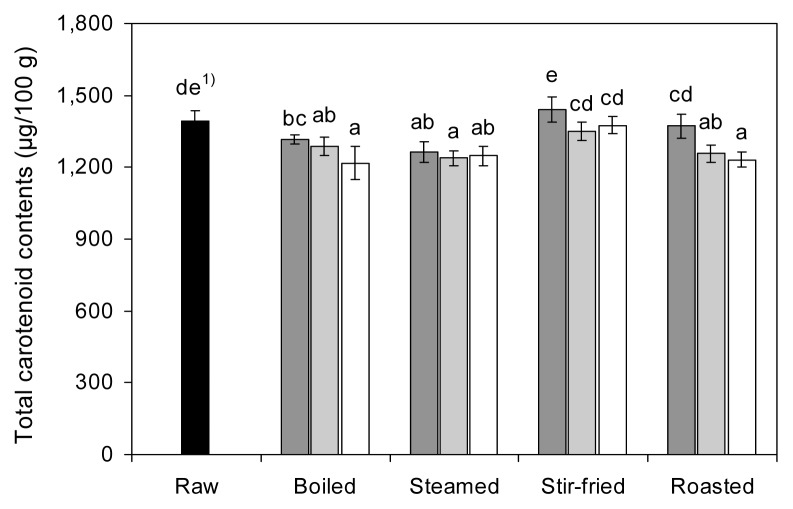
The effect of cooking on TCC in red pepper is shown in Fig. 2. The TCC in raw red pepper, expressed as micrograms of β-carotene equivalent per 100 g of fresh weight, was 1,394.08 μg/100 g. Our results showed that the TCC in red pepper was significantly reduced (p< 0.05) by boiling, steaming, and roasting, and not significantly affected by stir-frying, compared to that in raw red pepper. As the cooking time increased, the TCC in boiled and roasted red peppers significantly decreased (p<0.05), with a range of 1,217.25~1,317.86 and 1,232.58~1,372.06 μg/100 g, respectively. The TCC in red pepper significantly decreased (p<0.05) to 1,239.58 ~1,264.86 μg/100 g during steaming, however there were no significant differences among steamed and raw red peppers. After stir-frying for 5, 10, and 15 min, the TCC in red pepper ranged from 1,351.75~1,441.21 μg/100 g. Although TCC was slightly increased after frying for 5 min, the data was not significantly different from control. In this study, stir-fry cooking had minimal effects on the TCC in red pepper as compared to other cooking methods.
Fig. 2.
Effects of different cooking methods and cooking times on the total carotenoid contents of red pepper (■ 5 min cooking;
 10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
These results are similar to those of Chuah et al. (18), who reported that TCC in peppers was reduced by 3.2~ 36.0% and 11.6~40.9% by microwave heating and boiling, respectively. In addition, Ornelas-Paz et al. (1) found a 3~53% and 2~46% loss of TCC in pungent and non- pungent peppers during boiling and grilling, respectively. Similar observations were reported by Zhang and Hamauzu (15) and Kao et al. (33). In contrast, several authors observed that cooking may increase TCC because of its better extractability from heat treatment or dehydration of the food matrix (18,34,35). Thermal lability of carotenoids may be influenced by cooking conditions, food type, and the nature of the food matrix.
Effect of cooking methods on total polyphenol content
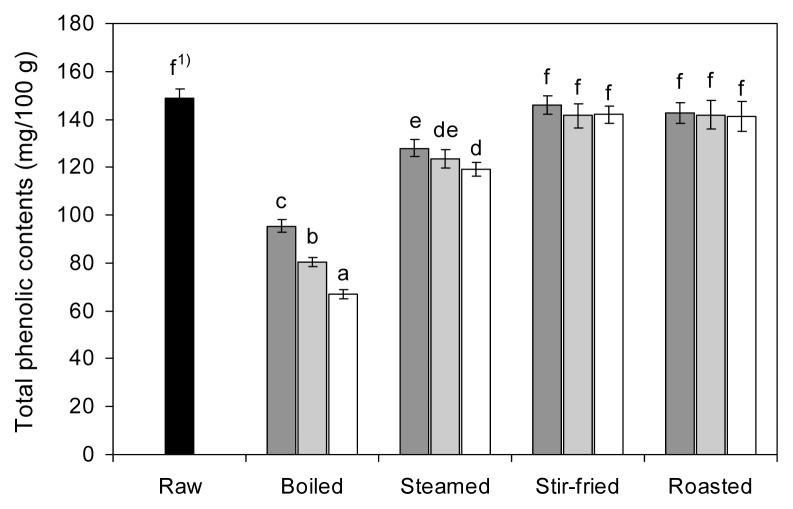
The TP in raw and cooked red peppers, expressed as milligrams of gallic acid equivalent per 100 g of fresh weight, is presented in Fig. 3. The TP in raw red pepper was 148.66 mg/100 g. For boiling, steaming, stir-frying, and roasting, TP decreased by 66.99~95.47, 119.10~ 127.99, 141.54~145.93, and 141.32~142.61 mg/100 g, respectively. Stir-frying and roasting did not significantly affect the TP levels in red pepper compared with raw pepper, whereas boiling and steaming significantly decreased (p<0.05) TP levels. The highest reduction was observed after boiling (35.8~54.9%), followed by steaming (13.9~19.9%), roasting (4.1~4.9%), and stir-frying (1.8~4.8%). These results are confirmed by Chuah et al. (18), who found that boiling significantly reduced the TP levels in colored peppers, while stir-fry and microwave cooking had no impact on TP levels. Our results are also confirmed by the findings of earlier studies by Francisco et al. (13), Zhang and Hamauzu (15), and Ismail et al. (36), who reported that cooking vegetables significantly decreased TP levels. Reduced TP in boiled or steamed foods has been attributed to the dissolution of phenolic compounds into the cooking water (5,18). The loss of phenolic compounds also depends on the processing time and food size. In contrast, Ornelas-paz et al. (1), Adefegha and Oboh (11), Turkmen et al. (16), and Dewanto et al. (17) found that cooking increased TP levels in some vegetables due to the disruption of cell walls, which liberated soluble phenolic compounds from insoluble ester bonds.
Fig. 3.
Effects of different cooking methods and cooking times on the total polyphenol contents of red pepper (■ 5 min cooking;
 10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
Effect of cooking methods on antioxidant activity
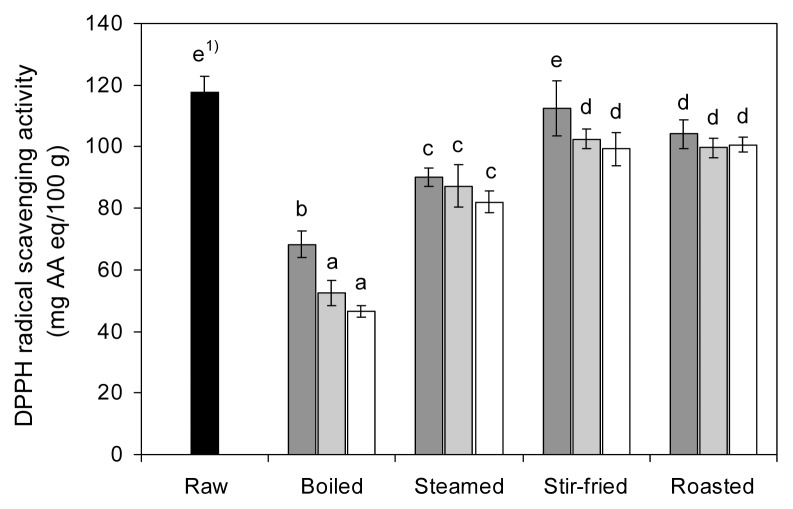
The DPPH radical scavenging activity of raw and cooked red peppers, expressed as milligrams of AA equivalent per 100 g of fresh weight, is presented in Fig. 4. The DPPH radical scavenging activity of red pepper extract was significantly reduced (p<0.05) after cooking. The DPPH radical scavenging activity of raw red pepper was 117.82 mg AA eq/100 g and after cooking, decreased by 46.56~68.29, 82.10~90.10, 99.25~ 112.44, and 99.68~104.15 mg AA eq/100 g for boiling, steaming, stir-frying, and roasting, respectively. The DPPH radical scavenging activity was reduced by 42.0~60.5% of its initial capacities after boiling, followed by steaming (23.5~30.3%), roasting (11.6~15.4%), and stir-frying (4.6~15.8%).
Fig. 4.
Effects of different cooking methods and cooking times on the DPPH radical scavenging activity of red pepper (■ 5 min cooking;
 10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
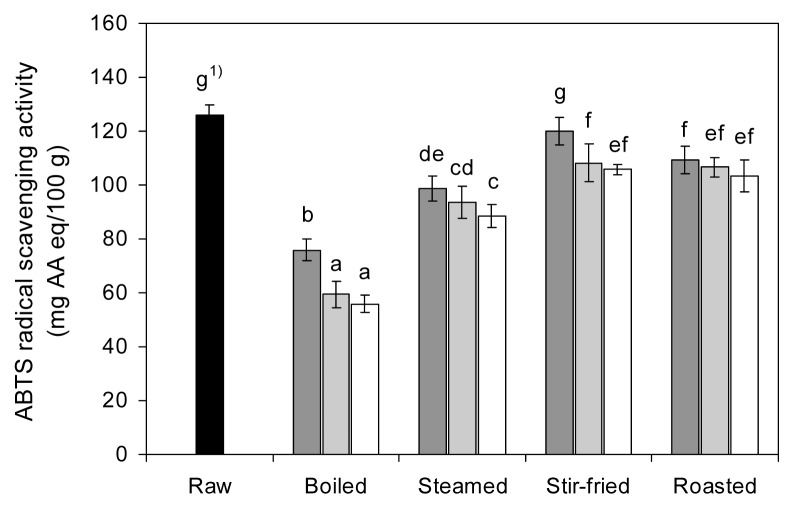
The ABTS radical scavenging activity of raw and cooked red pepper, expressed as milligrams of AA equivalent per 100 g of fresh weight, is presented in Fig. 5. The ABTS radical scavenging activity of raw red pepper was 126.08 mg AA eq/100 g and decreased significantly (p<0.05) after cooking. The ABTS radical scavenging activity was reduced by 39.8~55.7% of its initial capacities after boiling, followed by steaming (21.7~29.8%), roasting (13.2~17.9%), and stir-frying (4.9~16.1%). Boiling and steaming caused a higher reduction in the antioxidant capacity of red pepper than stir-frying and roasting.
Fig. 5.
Effects of different cooking methods and cooking times on the ABTS radical scavenging activity of red pepper (■ 5 min cooking;
 10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
10 min cooking; □ 15 min cooking). 1)Values with different letters on the bars are significantly different (p<0.05) by Duncan’s multiple range tests.
A significant correlation was observed among AsA, TP, and antioxidant activities of cooked red peppers (data not shown). A positive correlation was observed between the AsA or TP and DPPH (for AsA vs. DPPH radical, R = 0.976, p<0.01; for TP vs. DPPH radical, R = 0.991, p<0.01) and ABTS radical scavenging activity (for AsA vs. ABTS radical, R = 0.981, p<0.01; for TP vs. ABTS radical, R = 0.985, p<0.01) of cooked red pepper. However, no significant correlation was observed between TCC and antioxidant activity of cooked red pepper.
Cooking factors, including method, temperature, cooking time, and portion size, strongly affect the antioxidant activity of food. Many reports indicate that thermal treatment affects the antioxidant activity of various food samples. For example, the antioxidant capacities in tomatoes (17) and other selected vegetables (16,37) were enhanced after thermal treatment, possibly because the antioxidant compounds were liberated from insoluble portions of food, or that novel compounds, such as Maillard reaction products, possess antioxidant capacities (25,38). In contrast, antioxidant capacities were reported to decrease after thermal treatment of carrots, onions, white cabbage (37), and other vegetables (36,39). Also, significant reductions (23~36%) in DPPH radical scavenging activities have been reported for colored peppers after boiling for 5~30 min (18). According to Sikora et al. (21), aquathermal processing of vegetables causes a large decrease in antioxidant activity due to the loss of vitamin C and polyphenols, which dissolve in water.
CONCLUSION
In our study, the four different cooking processes (boiling, steaming, stir-frying, and roasting) changed the proximate composition, AsA content, TCC, TP, and anti-oxidant activities of red peppers. Boiling and steaming significantly decreased the AsA content, TP, and anti-oxidant levels compared with the other cooking methods. The contents of red peppers decreased with prolonged cooking times. Moreover, antioxidant activities were less dependent on the AsA content and TP levels of red peppers. Stir-frying and roasting preserved the nutrient compositions, antioxidant components, such as AsA content, TCC, and TP levels, and antioxidant activities in red peppers than boiling and steaming. These results may contribute to consumers’ knowledge when choosing cooking practices that retain the nutritional quality of peppers. However, this research has a limitation, in that only red pepper was evaluated. Therefore, further study using other foods is needed to validate matrix effects.
ACKNOWLEDGMENTS
This study was carried out with the support of “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ007805)”, Rural Development Administration, Republic of Korea and “2012 Postdoctoral Fellowship Program”, National Academy of Agricultural Science, Rural Development Administration, Republic of Korea.
REFERENCES
- 1.Ornelas-Paz JJ, Martínez-Burrola JM, Ruiz-Cruz S, Santana-Rodríguez V, Ibarra-Junquera V, Olivas GI, Pérez-Martí-nez JD. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010;119:1619–1625. [Google Scholar]
- 2.Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers – A review on tissue culture and trans-genesis. Biotechnol Adv. 2010;28:35–48. doi: 10.1016/j.biotechadv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Topuz A, Ozdemir F. Assessment of carotenoids. Capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J Food Compos Anal. 2007;20:596–602. [Google Scholar]
- 4.Menichini F, Tundis R, Bonesi M, Loizzo MR, Conforti F, Statti G, Cindio BD, Houghton PJ, Menichini F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009;114:553–560. [Google Scholar]
- 5.Zhuang Y, Chen L, Sun L, Cao J. Bioactive characteristics and antioxidant activities of nine peppers. J Funct Foods. 2012;4:331–338. [Google Scholar]
- 6.Deepa N, Kaur C, Singh B, Kapoor HC. Antioxidant activity in some red sweet pepper cultivars. J Food Compos Anal. 2006;19:572–578. [Google Scholar]
- 7.Ghasemnezhad G, Sherafati M, Payvast GA. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J Funct Foods. 2011;3:44– 49. [Google Scholar]
- 8.Doymaz I, Pala M. Hot-air drying characteristics of red pepper. J Food Eng. 2002;55:331–335. [Google Scholar]
- 9.Scala KD, Crapiste G. Drying kinetics and quality changes during drying of red pepper. LWT-Food Sci Tech-nol. 2008;41:789–795. [Google Scholar]
- 10.Vega-Gálvez A, Scala KD, Rodríguez K, Lemus-Mondaca R, Miranda M, López J, Perez-Won M. Effect of air-drying temperature on physico-chemical properties, an-tioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian) Food Chem. 2009;11:647–653. [Google Scholar]
- 11.Adefegha SA, Oboh G. Cooking enhances the anti-oxidant properties of some tropical green leafy vegetables. Afr J Biotechnol. 2011;10:632–639. [Google Scholar]
- 12.Barros AIRNA, Nunes FM, Gonçalves B, Bennett RN, Silvan AP. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.) Food Chem. 2011;128:165–172. doi: 10.1016/j.foodchem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Francisco M, Velasco P, Moreno DA, García-Viguera C, Cartea ME. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res Int. 2010;43:1455–1463. [Google Scholar]
- 14.Wachtel-Galor S, Wong KW, Benzie IFF. The effect of cooking on Brassica vegetables. Food Chem. 2008;110:706– 710. [Google Scholar]
- 15.Zhang DL, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88:503–509. [Google Scholar]
- 16.Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. [Google Scholar]
- 17.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 18.Chuah AM, Lee YC, Yamaguchi T, Takamura H, Yin LJ, Matoba T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008;111:20–28. [Google Scholar]
- 19.Chung HM, Lee GJ. The effects of blanching temperature and cooking methods on the changes in vitamin C of potato. Korean J Food Sci Technol. 1989;21:788–794. [Google Scholar]
- 20.Oteng-Gyang K, Mbachu JI. Changes in the ascorbic acid content of some tropical leafy vegetables during traditional cooking and local processing. Food Chem. 1987;23:9–17. [Google Scholar]
- 21.Sikora E, Cieslik E, Leszczynska T, Filipiak-Florkiewicz F, Pisulewski PM. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008;107:55–59. [Google Scholar]
- 22.AOAC. Official methods of analysis. 15th ed. Association of Official Analytical Chemists; Washington DC, USA: 1990. pp. 8–35. [Google Scholar]
- 23.Tepe B, Sokmen M, Akpulat HA, Sokmen A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006;95:200–204. [Google Scholar]
- 24.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 25.Hwang IG, Kim HY, Joung EM, Woo KS, Jeong JH, Yu KW, Lee J, Jeong HS. Changes in ginsenosides and antioxidant activity of Korean ginseng (Panax ginseng C.A. Meyer) with heating temperature and pressure. Food Sci Biotechnol. 2010;19:941–949. [Google Scholar]
- 26.Hwang IG, Kim HY, Lee J, Kim HR, Cho MC, Ko IB, Yoo SM. Quality characteristics of Cheongyang pepper (Capsicum annuum L.) according to cultivation region. J Korean Soc Food Sci Nutr. 2011;40:1340–1346. [Google Scholar]
- 27.NRLSI. Food Composition Table. 7th ed. National Rural Living Science Institute; Suwon, Korea: 2006. p. 104. [Google Scholar]
- 28.Alajaji SA, El-Adawy TA. Nutritional composition of chickpea (Cicer arietinum L.) as ffected by microwave cooking and other traditional cooking methods. J Food Compos Anal. 2006;19:806–812. [Google Scholar]
- 29.Somsub W, Kongkachuichai R, Sungpuag P, Charoensiri R. Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. J Food Compos Anal. 2008;21:187– 197. [Google Scholar]
- 30.Lešková E, Kubíková J, Kováciková E, Košická M, Porubská J, Holcíková Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J Food Compos Anal. 2006;19:252–276. [Google Scholar]
- 31.Masrizal MA, Giraud DW, Driskell JA. Retention of vitamin C, iron and β-carotene in vegetables using different cooking methods. J Food Quality. 1997;20:403–408. [Google Scholar]
- 32.Bernhardt S, Schlich E. Impact of different cooking methods on food quality: Retention of lipophilic vitamins in fresh and frozen vegetables. J Food Eng. 2006;77:327–333. [Google Scholar]
- 33.Kao FJ, Chiu YS, Tsou MJ, Chiang WD. Effects of Chinese domestic cooking methods on the carotenoid composition of vegetables in Taiwan. LWT-Food Sci Tech-nol. 2012;46:485–492. [Google Scholar]
- 34.Hart DJ, Scott J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed on the UK. Food Chem. 1995;54:101–111. [Google Scholar]
- 35.Mazzea T, N’Dri D, Chiavaro E, Visconti A, Fogliano V, Pellegrini N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011;128:627– 633. [Google Scholar]
- 36.Ismail A, Marjan ZM, Foong CW. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004;87:581–586. [Google Scholar]
- 37.Faller ALK, Fialho E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res Int. 2009;42:210–215. [Google Scholar]
- 38.Hwang IG, Kim HY, Woo KS, Lee J, Jeong HS. Biological activities of Maillard reaction products (MRPs) in a sugar-amino acid model system. Food Chem. 2011;126:221–227. [Google Scholar]
- 39.Roy MK, Takenaka M, Isobe S, Tsushida T. Antiox-idant potential, anti-proliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: Effects of thermal treatment. Food Chem. 2007;103:106–114. [Google Scholar]