Abstract
Water extract from Pinus densiflora (WPD) was investigated for its antioxidant activity and its ability to provide protection from DNA damage. A series of antioxidant assays, including a 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical-scavenging assay, a reducing power assay, a metal-chelating assay, a superoxide radical scavenging assay, and a nitrite scavenging ability, as well as a DNA damage protection assay were performed. Total phenolic content was found to be 211.32 mg Tan/g WPD. The extract scavenged 50% DPPH free radical at a concentration of 21.35 μg/mL. At that same concentration, the reducing power ability of WPD was higher than that of α-tocopherol. The extract chelated 68.9% ferrous ion at the concentration of 4 mg/mL. WPD showed better nitrite scavenging effect at the lower pH. Meanwhile, WPD exhibited a strong capability for DNA damage protection at 1 mg/mL concentration. Taken together, these data suggest water extract from Pinus densiflora could be used as a suitable natural antioxidant.
Keywords: antioxidant, DNA damage, phenolic content, reactive oxygen species
INTRODUCTION
Reactive oxygen species (ROS) are formed in vivo and raise the incidence of more than 30 different diseases by damaging cell structures, DNA, lipids, and proteins (1); therefore, it is necessary to employ exogenous anti-oxidants to balance the ROS in human body (2). The large amount of evidence suggesting that oxidative stress is involved in the pathogenesis of various disorders and diseases, has inspired both scientists and the general public to pay attention to the role of antioxidants in maintaining the health of human body and preventing and treating diseases (3). A medical revolution that has been induced as a result of the discovery of the effect of free radicals in cancer, diabetes, cardiovascular diseases, autoimmune diseases, neurodegenerative disorders, aging, and other diseases, and this revolution is promising a new outlook for healthcare (4).
Antioxidants have been used as food supplements for a very long time, but people still pay close attention to the safety of chemical or artificial antioxidants (5). The antioxidants used currently, such as tocopherol, tertiary-butylhydroquinone (TBHQ), butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) have been reported for the toxicity and carcinogenicity and other adverse effects (6). Therefore, natural antioxidants from plant extracts are gaining increasing interest because of consumer’s concern about the security of the synthetic antioxidants that are used as food supplements. Natural antioxidants such as tocopherols, flavonoids, and rosemary (Rosmarinus officinalis L.) extracts avoid the disadvantages of synthetic antioxidants. The synthetic anti-oxidants such as BHA, BHT, and propyl gallate (PG) may cause toxicity problems (7). Plants are the main source of natural antioxidants, such as vitamin E (α-tocopherol), vitamin C (ascorbate) and phenolic compounds. The antioxidant effects of some plants are mainly due to the phenolic component (8), and phenolic compounds found in food also have been demonstrated to have potential antioxidant activity (9). Some extracts from fruits, vegetables, cereals, and their by-products exhibit valid antioxidant activity in a model system and researchers have also tested natural antioxidants in food systems (10).
Pinus (P.) densiflora belongs to the Pinaceae family and is mainly distributed in Northeastern China (Heilongjiang, Jilin, Liaoning, Shandong), the extreme Southeast of Russia (Southern Primorsky Krai), Korea and Japan. The forest area is more than 65% of total area in South Korea and P. densiflora accounts for about 87% of coniferous forests as the most abundant conifer (11). P. densiflora has been used as an herbal medicine to treat stroke, atherosclerosis, hypertension, and diabetes mellitus in the Orient (12). Pine bark accounts for about 10~15% of the total tree weight and could be an attention-getting and significant biomass resource (13). Pine bark extract is reported to be rich in bioflavonoids, procyanidins and phenolic acids and has been shown to possess the ability to decrease the level of blood glucose and decrease the undesirable complications of the diabetes (14). Our main work focuses on the antioxidant activity of water extract from P. densiflora (WPD), as determined by a series of antioxidant assays.
MATERIALS AND METHODS
Preparation of extract
The P. densiflora bark was harvested from the P. densiflora tree grown in Chuncheon, Korea. P. densiflora bark was dried and immersed in ten times the weight of distilled water at room temperature for 12 hr. The extract was filtered and dried by evaporation of water. Dry extract was stored in a refrigerator before analysis. The WPD was dissolved in distilled water with the concentration of 10 mg/mL and kept at −20°C for stock.
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), BHA, α-tocopherol, Folin-Ciocalteu reagent, ethylenediamine tetra-acetic acid (EDTA), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), tannic acid, quercetin, trichloroacetic acid (TCA), aluminium chloride hexahydrate (AlCl3), phenazonium methosulphate (PMS), β-nicotinamide adenine dinucleotide reduced disodium salt (NADH), and nitro blue tetrazolium tablet (NBT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Determination of total phenolic content
The concentration of WPD was determined at 1 mg/mL and 0.1 mL of solution or tannic acid (20, 40, 80, 160, and 320 μg/mL) was mixed with 0.5 mL of Folin-Ciocalteu reagent (10%). After shaking, 0.4 mL of 7.5% sodium carbonate solution was added and the mixture was kept at room temperature for 30 min. The absorbance was measured at 750 nm using an absorbance microplate reader (ELX800, Bio-Tek, Vermont, IL, USA). Tannic acid was used as a standard to present the total phenolic content of WPD. The results were expressed as mg Tan/g WPD.
DPPH free radical scavenging activity
DPPH was dissolved in methyl alcohol and the solution was prepared at concentration of 0.1 mM. A volume of 0.5 mL WPD at five different concentrations (3.125, 6.25, 12.5, 25, and 50 μg/mL) and 0.5 mL of DPPH solution were mixed in a 1.5 mL test tube. After shaking adequately, then allowed to react at room temperature in the dark for 30 min, absorbance was measured at 515 nm. BHA and α-tocopherol were used as positive controls. For calculating the capability to scavenge the DPPH free radical, the following formula was used:
Where Ab is absorbance of extract without DPPH solution (0.5 mL of extract plus 0.5 mL of methanol); Ac is absorbance of DPPH solution without extract (0.5 mL of DPPH solution plus 0.5 mL of methanol); Ai is absorbance of extract with DPPH solution (0.5 mL of extract plus 0.5 mL of DPPH solution).
Reducing power assay
The reducing power of WPD was evaluated by the method of Hu et al. (15). Five different concentrations (0.1, 0.2, 0.4, 0.8 and 1 mg/mL) of extract were mixed with 2.5 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 mL of 0.1% potassium ferricyanide. The mixture was incubated at 50°C for 30 min, then 2.5 mL of 10% TCA solution was added. Next, the mixture was centrifuged at 3,000 rpm for 10 min at room temperature. After that, 2.5 mL of the upper layer solution was removed and put into a new test tube. 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride were added and then the mixture was mixed evenly. The absorbance was measured at 700 nm against a reagent blank. α-Tocopherol was used as a positive control.
Metal chelating activity
Various concentrations (0.5, 1, 2, and 4 mg/mL) of WPD and 2 mM FeCl2 (in methanol surroundings) were prepared. 0.2 mL of WPD and 0.02 mL of FeCl2 were mixed and 0.04 mL of 5 mM ferrozine was added. The mixture was vigorously shaken then left at room temperature for 10 min. The absorbance was then measured at 562 nm. EDTA was used as a positive control. The following formula was used to calculate the metal chelating activity:
Where Ai is the absorbance of WPD or EDTA against the blank and Ac is the absorbance of the control.
Superoxide radical scavenging assay
The superoxide radical scavenging activity of WPD was assessed using the model PMS-NADH described by Singh and Rajini (16). 0.1 mL of the extract solution was mixed with 0.1 mL of 150 μM NBT and 0.1 mL of 468 μM NADH in 1 mL of 0.1 M phosphate buffer (pH 7.4). Then 0.02 mL of 60 μM PMS was added. The reaction was carried out at room temperature for 8 min. The absorbance was measured at 560 nm and gallic acid was used as a positive control. The superoxide radical scavenging ability of WPD was evaluated by calculating the inhibition of superoxide radical generation according to the following formula:
Where Ai is the absorbance of samples against the blank and Ac is the absorbance of the control.
Measurement of nitrite scavenging ability
200 μL of 1 mM nitrite sodium was added to 200 μL of extract solution, then 1.6 mL of 0.1 M HCl buffer (pH 1.2 adjusted with NaOH) or 0.2 M citrate buffer at pH 4.2 or 6.0 was mixed. The mixture was incubated at 37°C for 1 hr and then 200 μL of solution was taken out and mixed with 400 μL of 2% acetic acid. Then, 80 μL of Griess reagent [the mixed solution of 1% sulfanilamide (in 5% phosphoric acid) and 0.1% aqueous solution of naphthyl-ethylenediamine dihydrochloride] was added. The mixture was held at room temperature for 15 min after shaking. The absorbance was measured at 515 nm. The calculation was performed according to the method in superoxide radical scavenging assay.
DNA damage protection assay
Genomic DNA was isolated from RAW 264.7 cells. 0.5 μg of DNA was mixed with 3 μL of 50 mM phosphate buffer (pH 7.4) and 3 μL of 1 mM FeSO4. Then, 10 μL of WPD was added. After adding 4 μL of 0.1 mM H2O2 the mixture was incubated at 37°C for 30 min. After that, 5 μL of gallic acid with high concentration was added immediately to end the reaction. Finally, the DNA was analyzed with 1% agarose gel electrophoresis. A solution of hydrogen peroxide and a ferrous iron catalyst is called Fenton’s reagent. Fenton’s reagent is usually used to oxidize organic contaminants or waste waters. It was used to destroy the DNA in this assay.
RESULTS AND DISCUSSION
Determination of total phenolic content
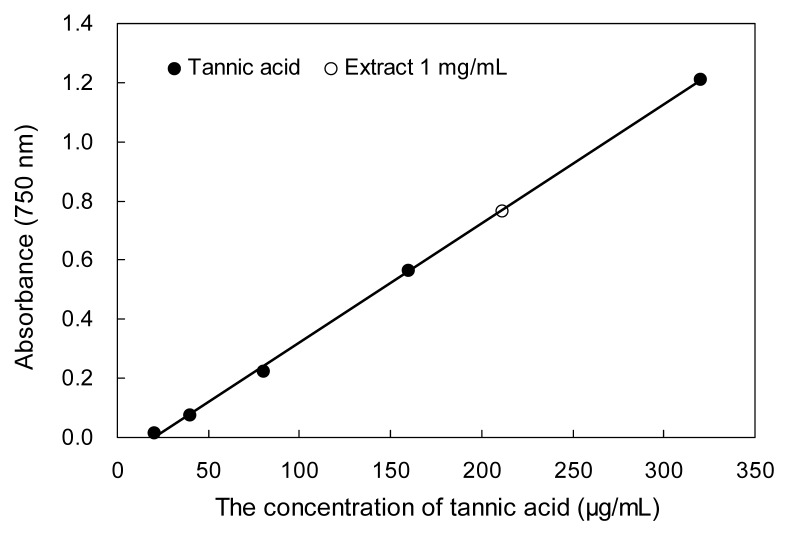
Plant phenolic compounds with reducing and anti-oxidant properties can inhibit the formation of super-oxide anion radicals, which has been reported by Bursal et al. (17); therefore, the total phenolic content can be used as a useful indicator of antioxidant potential. In this study, the yield of P. densiflora bark extract was 3.6% and the total phenolic content of this sample was 211.32 mg Tan/g WPD (Fig. 1). Compared to similar research, the total phenolic content of WPD reported in this paper is high. For example, Li et al. (18) reported that the total phenolic content of Crataegus pinnatifida Bunge methanol extract was 101.56 Tan/g. 80% methanol extract of Pinus cembra L. bark has higher content of total phenolic compounds (299.3 mg gallic acid equivalens/g extract), however, the content of total phenolic compound of Pinus cembra L. needle extract was low (78.22 mg gallic acid equivalents/g extract), as presented by Apetrei et al. (19).
Fig. 1.
Total phenolic content of water extract from P. densiflora. Tannic acid was used as a standard for measuring the total phenolic content. Each value is expressed as the mean±SD (n=3).
DPPH free radical scavenging activity
The DPPH free radical scavenging activity of WPD is presented in Table 1. WPD showed an IC50 (the concentration at which WPD scavenges 50% of the DPPH free radical) at a concentration of 21.35 μg/mL, which is significantly higher than BHA and α-tocopherol. The significantly lower IC50 value of BHA or α-tocopherol indicates that the free radical scavenging effects of BHA and α-tocopherol are better than that of WPD. However, the DPPH free radical scavenging activity of WPD was found to be strong compared to the water and methanol extracts of P. densiflora needles, which showed an IC50 value of 25.1 μg/mL and 32.5 μg/mL (20), respectively.
Table 1.
DPPH free radical scavenging activity of water extracts from P. densiflora
| Sample | DPPH free radical scavenging activity (IC50: μg/mL) |
|---|---|
| WPD | 21.35±0.71c |
| BHA | 8.52±0.69a |
| α-tocopherol | 9.56±0.56b |
BHA and α-tocopherol were used as positive control. Each value is expressed as the mean±SD (n=3). Different letters of upper index in the same column are significantly different by Duncan’s multiple range test (p<0.05).
Reducing power assay
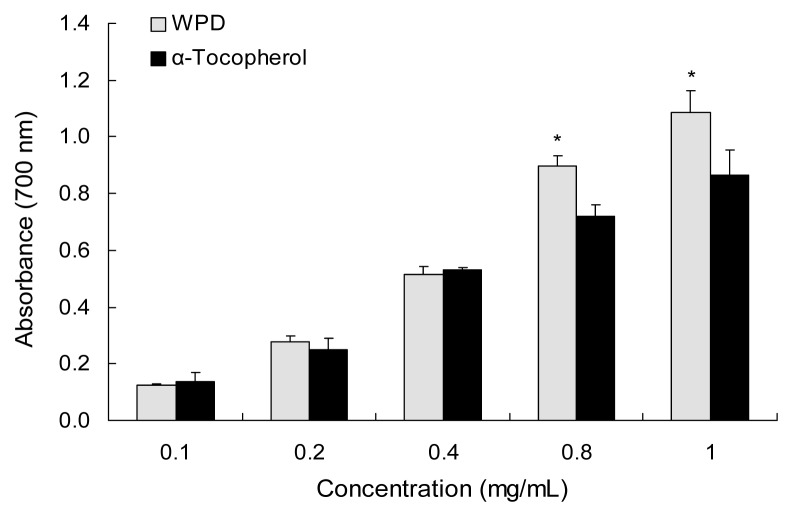
Results of the reducing power assays are presented in Fig. 2. The higher absorbance of the reaction mixture reflects a stronger reduction capability. The extract showed a dose-dependent reducing power. At 1 mg/mL, the reducing power of WPD was the strongest. With higher concentration (0.8 and 1 mg/mL) the reducing power of WPD was higher than that of α-tocopherol. Ustun et al. (21) reported the reducing power ability of needle acetone extracts from four Turkish Pinus species which include Pinus brutia, Pinus nigra, Pinus halepensi, and Pinus sylvestris. The reducing power (absorbance at 700 nm) of these extracts at a concentration of 1 mg/mL was 0.889, 0.893, 0.941, and 1.015, respectively, while the reducing power of WPD was 1.087 at the same concentration.
Fig. 2.
Reducing power ability of water extract from P. densiflora. Each value is expressed as the mean±SD (n=3). *Significantly different from α-tocopherol (p<0.05).
Metal chelating activity
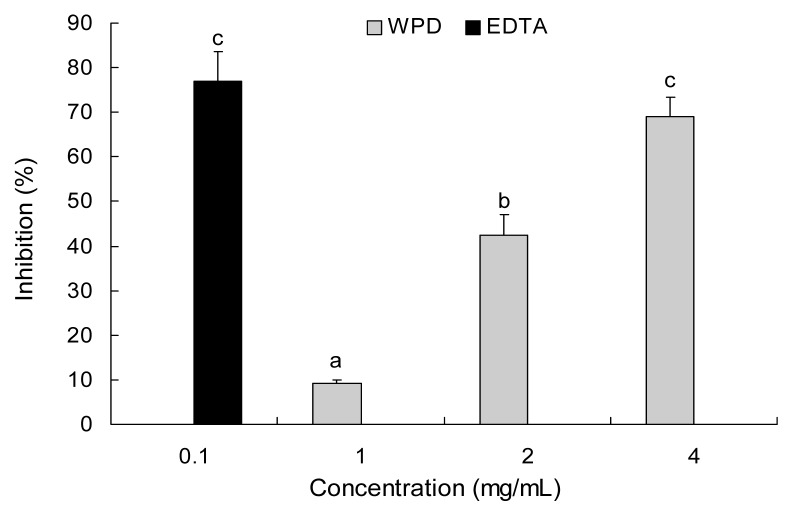
The extract was able to chelate ferrous ion, as shown in Fig. 3, in a concentration-dependent fashion. WPD did not show an obvious metal-chelating effect when the concentration was low. WPD exhibited 68.9% chelating ability of ferrous ion at concentration of 4 mg/mL. On the other hand, EDTA exhibited 70.0% inhibition at 0.1 mg/mL concentration. Similar research was carried out by Lantto et al. (22) who showed that Siberian pine seeds chelated 50% of the iron (II) at a concentration of 20.1 mg/mL. Chelating agents could be used as an effective secondary antioxidants due to reducing their redox potential and steadying the oxidized form of the metal ion (23). The results in this study showed that WPD had an available activity for metal chelating.
Fig. 3.
Metal chelating activity of water extract from P. densiflora. EDTA was used as a positive control. Each value is expressed as the mean±SD (n=3). Values are significantly different by Duncan’s multiple range test (p<0.05).
Superoxide radical scavenging assay
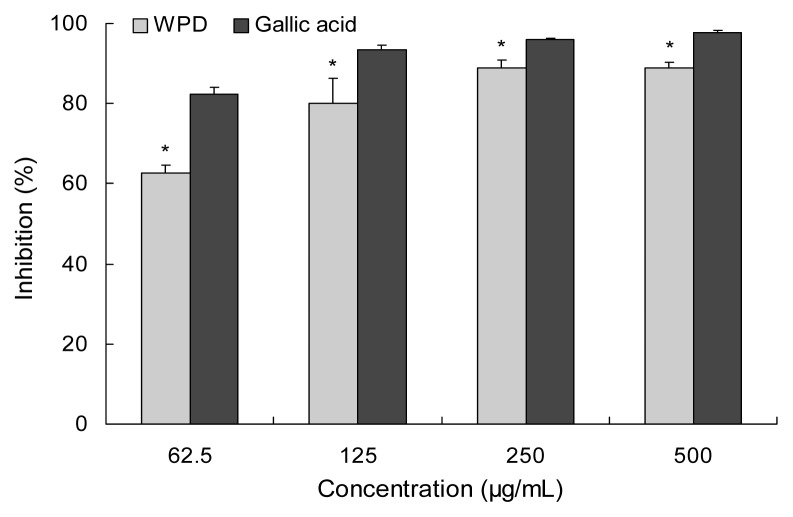
Data in Fig. 4 shows the superoxide radical scavenging capacity of WPD compared with gallic acid. The inhibition of WPD was enhanced with increased concentration. However, the inhibitory effect of WPD was nearly the same at 250 and 500 μg/mL, which means that WPD has achieved a maximum effect on scavenging superoxide radical at concentration of 250 μg/mL. In addition, at that concentration, the superoxide radical scavenging activity of extract was lower than that of gallic acid. Joo et al. (24) have reported the superoxide anion radical scavenging activity of 70% ethyl alcohol extract form Pinus densiflora root to be 50% of the superoxide radical at a concentration of 149.7 μg/mL. WPD exhibits 62.8% of superoxide radical scavenging activity at a concentration of 62.5 μg/mL.
Fig. 4.
Superoxide radical scavenging activity of water extract from P. densiflora. Gallic acid was used as a positive control. Each value is expressed as the mean±SD (n=3). *Significantly different from gallic acid (p<0.05).
Measurement of nitrite scavenging ability
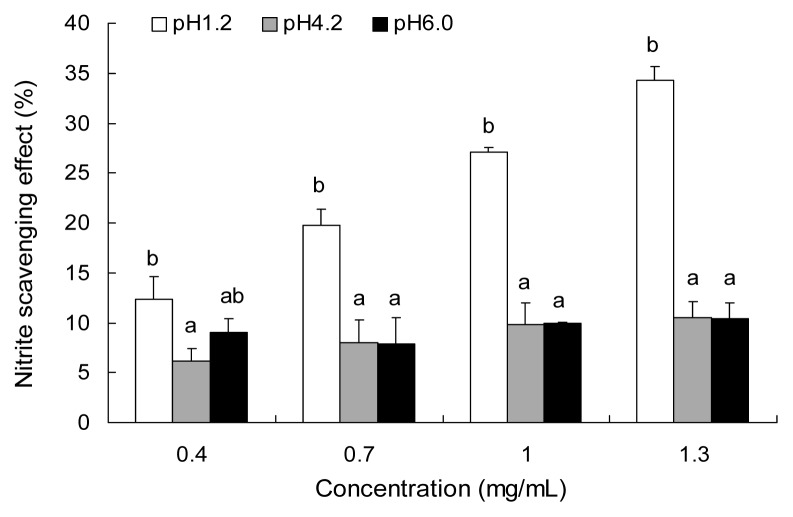
As shown in Fig. 5, the extract scavenged nitrite in a concentration-dependent fashion under a condition of pH 1.2. WPD exhibited 34.28% of NO radical scavenging activity at a concentration of 1.3 mg/mL at pH 1.2, the nitrite scavenging ability was the strongest. The nitrite scavenging ability of WPD at pH 1.2 was stronger than at pH 4.2 or 6.0, which indicated that it was more advantageous for WPD to scavenge nitrite under low pH condition.
Fig. 5.
Nitrite scavenging activity of water extract from P. densiflora at varying pHs. Each value is expressed as the mean±SD (n=3). Different letters in the same concentration are significantly different by Duncan’s multiple range test (p<0.05).
DNA damage protection assay
The stability of the genome and the normal life cycle of the cell are affected by DNA damage, which has been associated with cell cycle regulation, repair pathways, and cell death through a variety of mechanisms (25). Fig. 6 shows the agarose gel electrophoretic pattern of the damage induced by hydroxyl radicals on DNA in the presence and the absence of the various concentrations of pine bark water extracts (200, 500, 1000 μg/mL). The DNA derived from RAW 264.7 cells that was not incubated with Fenton’s reagent showed up as a bright band on agarose gel electrophoresis (lane 1), but a definite DNA band was not found in either lane 2, which contained Fenton’s reagent, or lane 3, which had the same Fenton’s reagent plus 250 μg/mL extract. The lack of DNA in these lanes implies that it has been thoroughly degraded. As the concentration of WPD was increased in lanes 4 and 5 (500 and 1000 μg/mL, respectively), the presence and brightness of the DNA band increased, indicating WPD can confer a protective effect against Fenton’s agent-induced DNA damage.
Fig. 6.
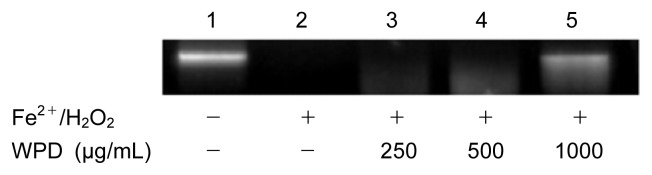
Visualization of the damage induced by hydroxyl radicals on genomic DNA in the presence and absence of water extract from P. densiflora by agarose gel electrophoresis. Lane 1, DNA incubated without Fenton’s reagent; Lane 2, DNA incubated with Fenton’s reagent; Lanes 3~5, DNA incubated with Fenton’s reagent in the presence of 250 μg/mL, 500 μg/mL, and 1000 μg/mL of WPD, respectively.
CONCLUSION
This study demonstrates the antioxidant activity and protective ability of water extract from P. densiflora against DNA damage. In the reducing power assay, the reducing power of WPD even higher than α-tocopherol, which is the most effective antioxidant currently in use. WPD showed some advantages, such as the advantage of abundant resources and the security and low-cost advantages of extraction with water. WPD showed strong antioxidant activities, such as exhibiting stronger DPPH free radical scavenging activity, reducing power, metal-chelating activity, and superoxide radical scavenging ability when compared with other samples including P. densiflora needles, needle acetone extracts from four Turkish Pinus species (Pinus brutia, Pinus nigra, Pinus halepensi, and Pinus sylvestris), Siberian pine seeds, and P. densiflora root in previous publication. These research results suggest P. densiflora could be used as an anti-oxidant source.
REFERENCES
- 1.Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahu A, Bora U. Indian medicinal herbs as sources of antioxidants. Food Res Int. 2008;41:1–15. [Google Scholar]
- 2.Hu W, Shen W, Wang MH. Free radical scavenging activity and protective ability of methanolic extract from Duchesnea indica against protein oxidation and DNA damage. J Food Sci Nutr. 2009;14:277–282. [Google Scholar]
- 3.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 2010;49:503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MNVR. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113:189–270. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Ende WVDE, Peshev D, Gara LD. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends Food Sci Tech. 2011;22:689–697. [Google Scholar]
- 6.Mariod AA, Ibrahim RM, Ismail M, Ismail N. Antioxidant activities of phenolic rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 2010;118:120–127. [Google Scholar]
- 7.Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Kunyanga CN, Imungi JK, Okoth MW, Biesaiski HK, Vadivel V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci Technol. 2012;45:269–276. [Google Scholar]
- 9.Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–417. [Google Scholar]
- 10.Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. [Google Scholar]
- 11.Lim JH, Kim JC, Kim KJ, Son YS, Sunwoo Y, Han JS. Seasonal variations of monoterpene emissions from Pinus densiflora in East Asia. Chemosphere. 2008;73:470–478. doi: 10.1016/j.chemosphere.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Joo CG, Lee KH, Park C, Lee BC. Antioxidative activities and composition analysis of Pinus densiflora root by ultra high pressure extraction. J Ind Eng Chem. 2011;17:712–716. [Google Scholar]
- 13.Mun SP, Ku CS, Kim JP. Adsorption of metal and uranyl ions onto amidoximated Pinus densiflora bark. Wood Sci Technol. 2010;44:283–299. [Google Scholar]
- 14.EI-Zein O, Kreydiyyeh SI. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition. 2011;27:707–712. doi: 10.1016/j.nut.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Heo SI, Wang MH. Antioxidant and anti-inflammatory activity of Kalopanax pictus leaf. J Korean Soc Appl Biol Chem. 2009;52:360–366. [Google Scholar]
- 16.Singh N, Rajini PS. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004;85:611–616. [Google Scholar]
- 17.Bursal E, Gülçin İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa) Food Res Int. 2011;44:1482–1489. [Google Scholar]
- 18.Li C, Son HJ, Huang C, Lee SK, Lohakare J, Wang MH. Comparison of Crataegus pinnatifida Bunge var. typica Schneider and C. pinnatifida Bunge fruits for anti-oxidant, anti-α-glucosidase, and anti-inflammatory activities. Food Sci Biotechnol. 2010;19:769–775. [Google Scholar]
- 19.Apetrei CL, Tuchilus C, Aprotosoaie C, Opera A, Malterud KE, Miron A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules. 2011;16:7773–7788. doi: 10.3390/molecules16097773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung MJ, Chung HY, Choi JH, Choi JS. Antioxidant principles from the needles of red pine, Pinus densiflora. Phytother Res. 2003;17:1064–1068. doi: 10.1002/ptr.1302. [DOI] [PubMed] [Google Scholar]
- 21.Ustun O, Senol FS, Kurkcuoglu M, Orhan IE, Kartal M, Baser KHC. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oil of Turkish Pinus species and pycnogenol. Ind Crop Prod. 2012;38:115–123. [Google Scholar]
- 22.Lantto TA, Dorman HJD, Shikov AN, Pozharitskaya ON, Makarov VG, Tikhonov VP, Hitunen R, Raasmaja A. Chemical composition, antioxidative activity and cell viability effects of a Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009;112:936–943. [Google Scholar]
- 23.Yu L, Zhao M, Wang J, Cui C, Yang B, Jiang Y, Zhao Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Innov Food Sci Emerg. 2008;9:122–128. [Google Scholar]
- 24.Joo CG, Lee KH, Park C, Lee BC. Antioxidative activities and composition analysis of Pinus densiflora root by ultra high pressure extraction. J Ind Eng Chem. 2011;17:712–716. [Google Scholar]
- 25.Liu H, Wang L, Wang MH. Antioxidant and nitric oxide release inhibition activities of methanolic extract from Clerodendrum cyrtophyllum Turcz. Hortic Environ Biotechnol. 2011;52:1–6. [Google Scholar]