Abstract
6-Shogaol, a dehydrated form of 6-gingerol, is a minor component in ginger (Zingiber officinale Roscoe) and has recently been reported to have more potent bioactivity than 6-gingerol. Based on the thermal instability of gingerols (their dehydration to corresponding shogaols at high temperature), we aimed to develop an optimal process to maximize the 6-shogaol content during ginger extraction by modulating temperature and pH. Fresh gingers were dried under various conditions: freeze-, room temperature (RT)- or convection oven-drying at 60 or 80°C, and extracted by 95% ethanol at RT, 60 or 80°C. The content of 6-shogaol was augmented by increasing both drying and extraction temperatures. The highest production of 6-shogaol was achieved at 80°C extraction after drying at the same temperature and the content of 6-shogaol was about 7-fold compared to the lowest producing process by freezing and extraction at RT. Adjustment of pH (pH 1, 4, 7 and 10) for the 6-shogaol-richest extract (dried and extracted both at 80°C) also affected the chemical composition of ginger and the yield of 6-shogaol was maximized at the most acidic condition of pH 1. Taken together, the current study shows for the first time that a maximized production of 6-shogaol can be achieved during practical drying and extraction process of ginger by increasing both drying and extracting temperatures. Adjustment of pH to extraction solvent with strong acid also helps increase the production of 6-shogaol. Our data could be usefully employed in the fields of food processing as well as nutraceutical industry.
Keywords: ginger, Zingiber officinale, 6-shogaol, 6-gingerol, 6-shogaol-rich extract, optimization, nutraceuticals
INTRODUCTION
The rhizome of ginger (Zingiber officinale Roscoe) originated in Southeast Asia and widely spread to all around the world (1), being used not only as a food ingredient but also as a traditional medicinal herb to treat many diseases such as gastrointestinal, stomachic, rheumatic disorders and muscular discomfort for thousands of years (2). Several recent studies have reported anti-oxidant and anti-inflammatory effects of ginger (3–5). In addition, the non-volatile pungent compounds of oleoresin from ginger including gingerol, paradol, shogaol, and zingerone, reportedly to have chemotherapeutic effects (6).
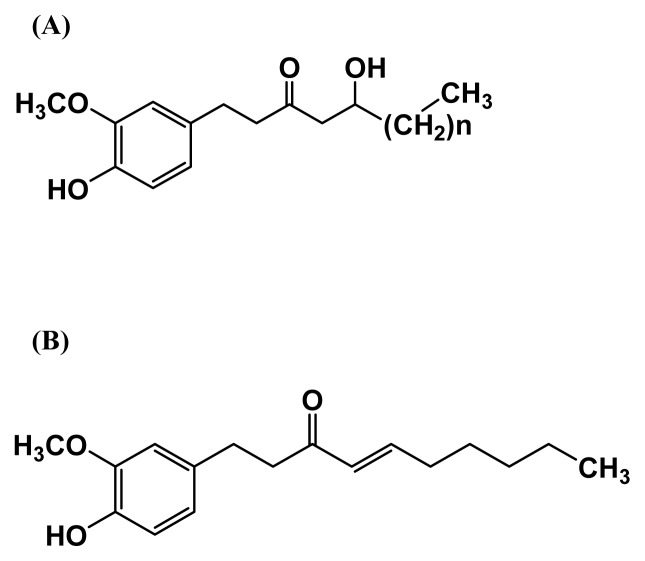
Among the representative bioactive compounds in ginger, gingerols are known as homologous phenolic ketones and exist as 6, 8, and 10-gingerols with different lengths of their unbranched alkyl chains (Fig. 1A) (7). Gingerols have prominent cancer preventive effects against gastric and colon cancer in vitro(8,9) and skin cancer in vivo(10). Of the gingerols, 6-gingerol has been found to possess various pharmacological effects including anti-inflammatory, analgesic, antipyretic, chemopreventive, angiogenesis, and antioxidant application (11–13).
Fig. 1.
Structures of the major bioactive compounds in ginger. (A) n= 6, 8 and 10; 6, 8, and 10-gingerol, respectively; (B) 6-shogaol.
Shogaols, the dehydrated form of the gingerols, are homologous phenolic alkanones (Fig. 1B) (14). Of the shogaols, 6-shogaol has many biological effects such as antibacterial, antifouling and anti-oxidative properties in vitro and in vivo(5,8,11). Recent studies have demonstrated that 6-shogaol is more potent than 6-gingerol in anti-inflammatory, antioxidant and chemopreventive effects (5,11,15).
Various extraction methods have been performed to obtain bioactive compounds from ginger, such as reflux (16), shaking at room and warm temperatures (17), sonication (18), and high-pressure Soxhlet extraction (19) etc. However, until now, studies on ginger have focused on the gingerols due to their abundant amount rather than the shogaols. Yonei et al. (20) showed that 6-gingerol content in ginger was largest, amounting to about 15 wt %, while 6-shogaol accounted for less than 2 wt %. Shogaols are more pungent than gingerols in dried ginger but are scarcely present in fresh ginger (21). The effects of drying and extraction conditions on content of 6-gingerol and 6-shogaol have conversely not yet been studied. Also, a recent study has been reported that 6-gingerol can isomerize to 6-shogaol in a model system such as an acidic condition with high temperature (22). Accordingly, we tried to apply this model system to a practical extraction environment to maximize the production of the potent 6-shogaol from the abundant but less active 6-gingerol in ginger. This paper presents the effect of drying conditions including freeze-drying and extraction conditions by modulating temperature and pH on the extract yield of 6-shogaol and 6-gingerol.
MATERIALS AND METHODS
Materials
Fresh gingers (Zingiber officinale Roscoe) were acquired from a local farm in Busan, Korea on March 2008. The standards (6, 8, 10-gingerols and 6-shogaol) for high performance liquid chromatography (HPLC) analysis were obtained from Chromadex Inc. (Irvine, CA, USA). All other reagents used in this study were of the highest grade available.
Extraction procedures
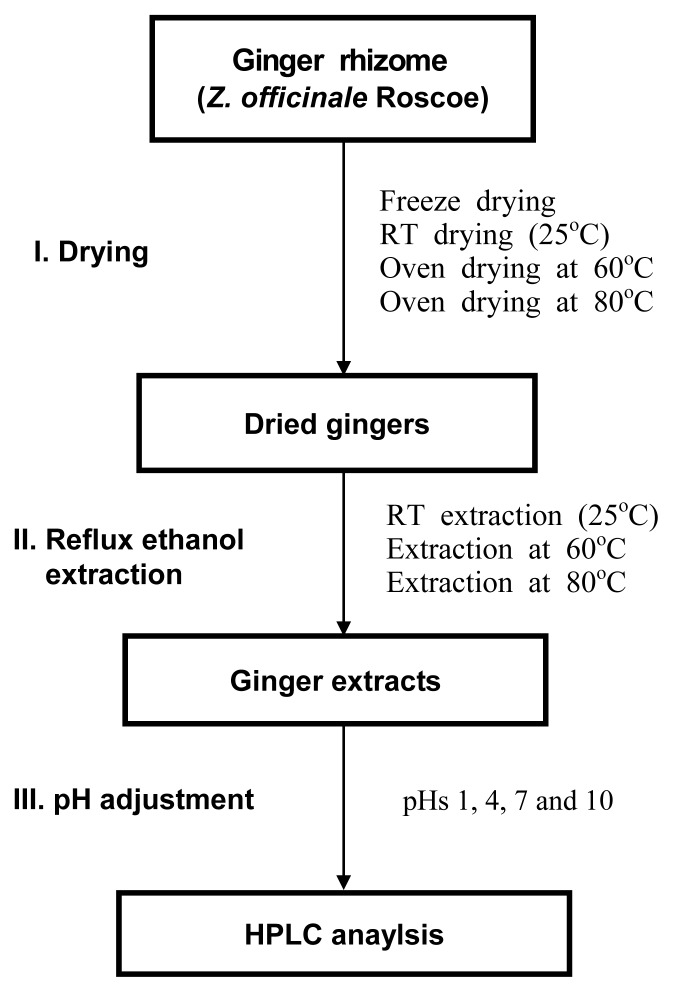
Overall process for drying and extracting ginger is illustrated in Fig. 2.
Fig. 2.
Overall process for drying & extraction of ginger.
Drying conditions
After washing each ginger sample to remove debris, samples were sliced using a mechanical slicer and spread onto drying trays under various conditions including either freeze-drying (Biotron, Gyeonggi, Korea), room temperature (RT) drying or convection oven drying (VISION, Gyeonggi, Korea) at 60°C and 80°C. The dried samples were ground with a mortar and pestle prior to extraction. The moisture contents of dried ginger were about 6.95±1.55 wt % (n=10).
Extraction conditions
Each ginger powder (10 g) was extracted with 100 mL of 95% ethanol (EtOH) either at RT or refluxing at 60 and 80°C for 24 hr. The extracts were filtered by Whatman No. 1 filter paper and the filtrates were centrifuged (Supra22K, Hanil, Incheon, Korea) at 5,500 rpm for 10 min at 4°C. The supernatant was subsequently filtered through a 0.2 μm nylon membrane filter (Corning, Baden Bath, Germany). Two mL of each sample was transferred into Eppendorf tubes and dried by speed vacuum from Biotron. For HPLC analysis, the remained volumes were concentrated by evaporator from EYELA (Tokyo, Japan) under 40°C.
pH adjustment
EtOH was adjusted to pH 1, 4, 7, or 10 with 1 N NaOH or 1 N HCl before the above extraction process. A hundred mL of EtOH extraction at 80°C after ginger dried at 80°C was added into a mixture of water and dichloromethane (DCM) (each 100 mL) and partitioned. The DCM layer was collected from the mixture and solvent was removed by vacuum evaporation.
HPLC analysis
The ginger extracts were analyzed by a HPLC system (Agilent, Foster, CA, USA) consisting of a quaternary pump, photodiode array detector, and autosampler. Chromatographic analysis was performed on a 250×4.6 mm, 5 μm Alltima HP C18 reversed phase column (Grace, Deerfield, IL, USA) with acetonitrile- water (v/v) as the mobile phase. The HPLC operating parameters were: injection volume, 5 μL; column flow rate, 1.0 mL/min; chromatographic run time, 48 min; UV spectra recording, 230 nm. The mobile phase consisted of water (A) and acetonitrile (B). The gradient elution was as follows; 0 min 45% B, 8 min 50% B, 17 min 65% B, 32 min 100% B, 38 min 100% B, 43 min 45% B, 48 min 45% B.
Calibration of 6-gingerol and 6-shogaol for quantitative analysis
The quantification was carried out using the external standard method. The contents of 6-gingerol and 6-shogaol from gingers were determined using a calibration graphic established with dilutions of each standard at concentrations ranging from 0.1 mM to 1 mM injected into the HPLC system (correlation coefficient ≥0.996). Each concentration was measured in triplicate. The corresponding peak areas were plotted against the concentration of the peak amount injected and identification of peaks was achieved by comparison of both the retention time and UV absorption spectrum with standards.
Statistical analysis
Data were expressed as the means±SD. The statistical analyses were performed using the SPSS 10.0 program (Systat Software Inc., Chicago, IL, USA). Values were compared to control using analysis of variance (ANOVA) followed by Duncan’s post hoc test. P values <0.05 were considered significant.
RESULTS AND DISCUSSION
Analysis of compounds from ginger extraction
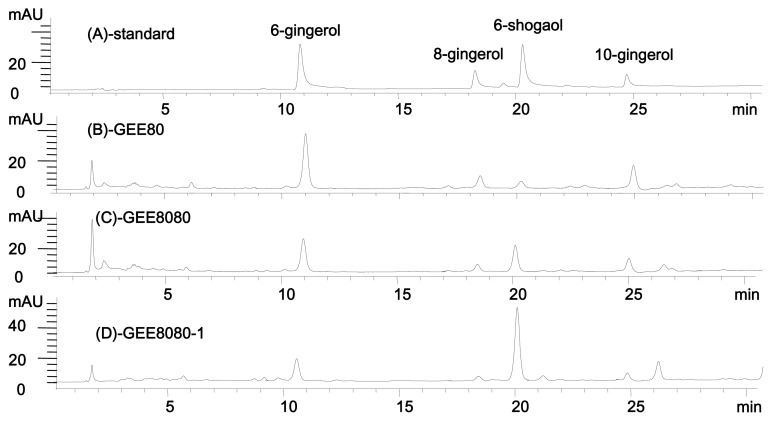
Many qualitative and/or quantitative methods have been reported for analysis of fresh (16–18,23) and dried ginger (24). A variety of solvents such as methanol (16, 17), ethanol (18,25), DCM (23), acetone (25), and pressurized CO2(19,20) have been used for ginger extraction. Extraction methods to obtain bioactive compounds from ginger also include reflux (16), sonication (18,26), and high-pressure Soxhlet extraction (19,26) etc. Based on the literature, the most effective method for extracting gingerol compounds might be the reflux method (26). In the present study, therefore, we employed reflux method for the extraction of ginger. After the extraction followed by concentration process, we analyzed the extracts by HPLC with wavelength scanning. The HPLC analysis of each ginger extract was carried out with standard compounds of 6, 8, and 10-gingerols and 6-shogaol. HPLC (16) and Gas Chromatography (GC) (27) have been typically used to analyze gingerols and shogaols in ginger. In the present study, we chose HPLC analysis since the GC method might cause a conversion of 6-gin-gerol to 6-shogaol through the high temperatures (28). HPLC chromatograms of standard compounds and some representative ginger extracts (GEE80, GEE8080 or GEE8080-1) are listed in Fig. 3.
Fig. 3.
HPLC chromatograms of standard ginger compounds and representative ginger extracts. (A) standards components of ginger, (B) GEE80, ginger ethanol extract after drying at 80°C, (C) GEE8080, ginger ethanol extract dried and extracted at 80°C, (D) GEE8080-1, ginger ethanol extract dried and extracted at 80°C (pH 1).
Effects of drying and extraction conditions on 6-gin-gerol and 6-shogaol contents
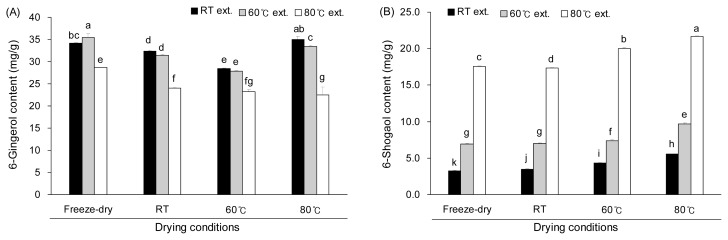
6-Gingerol content was analyzed after applying various drying and extraction conditions and the results are shown in Fig. 4A. When ginger was extracted at RT, 6-gingerol content tended to decrease as the drying temperature increased up to 60°C while drying at 80°C (GEE80) resulted in an increase in 6-gingerol yield. 6- Gingerol seems to degrade as the drying temperature increases to 60°C. The reason for the highest 6-gingerol content drying at 80°C is not clear at this moment. The extraction temperature also affected the 6-gingerol content. In all drying conditions, the highest extraction temperature of 80°C resulted in the lowest 6-gingerol content, probably due to the degradation of 6-gingerol at high temperature.
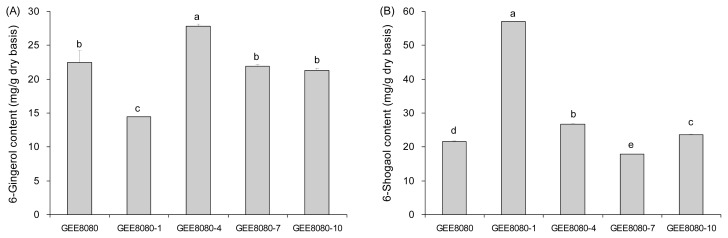
Fig. 4.
Contents of 6-gingerol (A) and 6-shogaol (B) after drying and extraction. Values are represented as mean±SD (n=5). Means with the different superscripts (a-k) are significantly different at p<0.05 by Duncan’s multiple range test.
Effect of drying and extraction temperature on 6-shogaol content seems more apparent than on the 6-gingerol. As shown in Fig. 4B, 6-shogaol content increased with both drying and extraction temperatures. The lowest 6- shogaol content was achieved when the freeze-dried ginger was extracted at RT while the highest 6-shogaol content was obtained when the ginger was dried and extracted at 80°C. Between the drying and extraction conditions, the extraction condition was more critical for 6-shogaol production.
6-Shogaol content of the ginger extract at RT (GEERT) in the present study ranged from 3 to 5 mg/g and our result is similar to the results from previously reported studies (about 2~2.5 mg/g in EtOH extraction) (26,29). However, drying and extraction at 80°C (GEE 8080) resulted in 5- to 7-fold increase in the 6-shogaol content (about 22 mg/g) compared to the 6-shogaol content of drying and extraction at RT. These results imply that the drying and extraction temperature of ginger directly affects the yield of 6-shogaol.
Effect of pH extraction conditions on 6-gingerol and 6-shogaol contents
To examine the effect of pH on 6-shogaol content during extraction, the extraction solvent was adjusted to pH 1, 4, or 10 with HCl or NaOH. For the pH adjustment experiment, we used only GEE8080 because it displayed the highest 6-shogaol content (Fig. 4).
Among the pH-adjusted GEE8080s, GEE8080-4 showed the highest 6-gingerol content (about 28 mg/g) due to its stability at pH 4 than at pH 1 and 7 (22) and the lowest 6-gingerol content (about 14 mg/g) was observed at GEE8080-1 (Fig. 5). Conversely, GEE8080-1 exhibited the highest 6-shogaol content (about 57 mg/g), which was more than a 2-fold increase than GEE8080 (about 22 mg/g). The lowest 6-shogaol content was obtained with GEE8080-7 (about 18 mg/g). 6-Gingerol can convert to 6-shogaol with strong acid or base due to dehydration of 6-gingerol either by protonation with strong acid or deprotonation by a strong base, while 6-gingerol is more degradable in acid than in alkaline solution (22).
Fig. 5.
Contents of 6-gingerol (A) and 6-shogaol (B) after pH adjustment of GEE8080. GEE8080, ginger ethanol extract dried and extracted at 80°C; GEE8080-1, 4, 7 and 10, GEE8080 adjusted to pH 1, 4, 7 and 10, respectively. Values are represented as mean±SD (n=5). Means with the different superscripts (a-e) are significantly different at p<0.05 by Duncan’s multiple range test.
Overall, the current study shows for the first time that a maximized production of 6-shogaol can be achieved during a practical drying and extraction process of ginger by increasing both drying and extracting temperatures. Adjusting the pH of the extraction solvent with strong acid also help increase the production of 6-shogaol. Our data could be usefully employed in the fields of food processing as well as nutraceutical industry.
ACKNOWLEDGMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF- 2007-331-1-F00062).
ABBREVIATIONS
- GEE
ginger ethanol extract
- GEERT
GEE extracted at RT
- GEE60
GEE extracted at 60°C
- GEE80
GEE extracted at 80°C
- GEE60FD
GEE60 after freeze-drying
- GEERTFD
GEERT after freeze-drying
- GEE8080
GEE80 after drying at 80°C
- GEE80-1
GEE80 adjusted by pH 1; GEE8080-1, 4, 7 and 10, GEE8080 adjusted by pH 1, 4, 7 and 10, respectively
REFERENCES
- 1.Ravindran PN, Sasikumar B, Johnson KG, Ratnambal MJ, Babu KN, Zachariah JT, Nair RR. Genetic resources of ginger (Zingiber officinale Rosc.) and its conservation in India. Plant Genet Resour Newsl. 1994;98:1–4. [Google Scholar]
- 2.Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact. 2001;18:159–190. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 3.Thomson M, Al-Qattan KK, Al-Sawana SM, Alnaqeeba MA, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and an-tithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. 2002;67:475–478. doi: 10.1054/plef.2002.0441. [DOI] [PubMed] [Google Scholar]
- 4.Ajith TA, Nivitha V, Usha S. Zingiber officinale Roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxicol. 2007;45:921–927. doi: 10.1016/j.fct.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2009;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Masuda Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidant properties of gingerol related compounds from ginger. Biofactors. 2004;21:293–296. doi: 10.1002/biof.552210157. [DOI] [PubMed] [Google Scholar]
- 8.Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong CH, Bode AM, Pugliese A, Cho YY, Kim HG, Shim JH, Jeon YJ, Li H, Jiang H, Dong Z. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69:5584–5591. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- 10.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- 11.Suekawa M, Ishige A, Yuasa K, Sudo K, Aburada M, Hosoya E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn. 1984;7:836–848. doi: 10.1248/bpb1978.7.836. [DOI] [PubMed] [Google Scholar]
- 12.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96:207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 14.Wu TS, Wu C, Wu PL, Chern CY, Leu YL, Chan YY. Structure and synthesis of (n)-dehydroshogaols from Zingiber officinale. Phytochemistry. 1998;48:889–891. [Google Scholar]
- 15.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52:527–537. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 16.He XH, Bernart MW, Lian LZ, Lin LZ. High-performance liquid chromatography-electrospray mass spectrometric analysis of pungent constituents of ginger. J Chromatogr A. 1998;796:327–334. [Google Scholar]
- 17.Jiang H, Solyom AM, Timmermann BN, Gang DR. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2957–2964. doi: 10.1002/rcm.2140. [DOI] [PubMed] [Google Scholar]
- 18.Wohlmuth H, Leach DN, Smith MK, Myers SP. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) J Agric Food Chem. 2005;53:5772–5778. doi: 10.1021/jf050435b. [DOI] [PubMed] [Google Scholar]
- 19.Chen CC, Kuo MC, Wu CM, Ho CT. Pungent compounds of ginger (Zingiber officinale Roscoe) extracted by liquid carbon dioxide. J Agric Food Chem. 1986;34:477–480. [Google Scholar]
- 20.Yonei Y, Ohinata H, Yoshida R, Shimizu Y, Yokoyama C. Extraction of ginger flavor with liquid or supercritical carbon dioxide. J Supercrit Fluids. 1995;8:156–161. [Google Scholar]
- 21.Baranowski JD. Storage stability of a processed ginger paste. J Food Sci. 1985;50:932–933. [Google Scholar]
- 22.Bhattarai S, Tran VH, Duke CC. The stability of gingerol and shogaol in aqueous solutions. J Pharm Sci. 2001;90:1658–1664. doi: 10.1002/jps.1116. [DOI] [PubMed] [Google Scholar]
- 23.Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8-, and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacterium avium and Mycobacterium tuberculosis. J Agric Food Chem. 1998;46:2504–2508. [Google Scholar]
- 24.Bailey-Shaw YA, Williams LA, Junor GA, Green CE, Hibbert SL, Salmon CN, Smith AM. Changes in the contents of oleoresin and pungent bioactive principles of Jamaican ginger (Zingiber officinale Roscoe.) during maturation. J Agric Food Chem. 2008;56:5564–5571. doi: 10.1021/jf072782m. [DOI] [PubMed] [Google Scholar]
- 25.Balladin DA, Headley O, Chang-Yen I, McGaw DR. Extraction and evaluation of the main pungent principles of solar dried west Indian ginger (Zingiber Officinale Roscoe) rhizome. Renewable Energy. 1997;12:125–130. [Google Scholar]
- 26.Lee S, Khoo C, Halstead CW, Huynh T, Bensoussan A. Liquid chromatographic determination of 6-, 8-, 10- gingerol, and 6-shogaol in ginger (Zingiber officinale) as the raw herb and dried aqueous extract. J AOAC Int. 2007;90:1219–1226. [PubMed] [Google Scholar]
- 27.Pfeiffer E, Heuschmid FF, Kranz S, Metzler M. Microsomal hydroxylation and glucuronidation of [6]-gingerol. J Agric Food Chem. 2006;54:8769–8774. doi: 10.1021/jf062235l. [DOI] [PubMed] [Google Scholar]
- 28.John P, Bartley PF. Supercritical fluid extraction of australian-grown ginger (Zingiber officinale) J Sci Food Agric. 2006;66:365–371. [Google Scholar]
- 29.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10- gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]