Abstract
Gelidium (G.) amansii is a red alga widely distributed in the shallow waters around East Asian countries. We investigated the effect of G. amansii on lipid accumulation and ROS (Reactive Oxygen Species) production in 3T3-L1 cells. G. amansii extracts dose-dependently inhibited lipid formation and ROS generation in cultured cells. Our results showed that anti-adipogenic effect of G. amansii was due to the reduction in mRNA expressions of PPARγ peroxisome proliferator-activated receptor-γ and aP2 (adipocyte protein 2). G. amansii extracts significantly decreased mRNA levels of a ROS-generator, NOX4 (nicotinamide adenine dinucleotide phosphate hydrogen oxidase 4), and increased the protein levels of antioxidant enzymes including SOD1/2 (superoxide dis-mutases), Gpx (glutathione peroxidase), and GR (glutathione reductase), which can lead to the reduction of ROS in the cell. In addition, the G. amansii extract enhanced mRNA levels of adiponectin, one of the adipokines secreted from adipocytes, and GLUT4, glucose uptake protein. Taken together, our study shows that G. amansii extract inhibited lipid accumulation and ROS production by controlling adipogenic signals and ROS regulating genes.
Keywords: Gelidium amansii, 3T3-L1 cells, lipid accumulation, ROS production, adiponectin
INTRODUCTION
Obesity results from malfunctions of the metabolic system responsible for energy homeostasis in the body. Long-term imbalance of energy leads to obesity, as well as secondary complications like diabetes and cardiovascular diseases (1). Adipocyte differentiation is a key process in the development of obesity, as well as in normal development and function of adipose tissue. Adipose tissue has an important role not only in energy storage, but also in endocrine metabolism (2). During adipogenesis, the cells generate a variety of biologically active molecules, including reactive oxygen species and fatty acids (3). Recent studies have shown that ROS status is closely related to the pathogenesis and development of metabolic diseases such as obesity and diabetes (4,5). ROS generation is generally performed by NOX4 (NADPH (nicotinamide adenine dinucleotide phosphate hydrogen oxidase 4) during metabolism in cytoplasm (6). On the other hand, ROS scavenging enzymes such as SOD (superoxide dismutase), GPx (glutathione peroxidase), and GR (glutathione reductase) modulate excessive ROS level in the cell.
ROS status in the cell modulates cellular physiology including growth and proliferation by affecting signaling molecules (3).
PPARγ (peroxisome proliferator-activated receptor-γ) is one of the most important adipogenic transcription factors required in adipogenesis (7). PPARγ controls adipocyte differentiation and lipogenic expression by interacting the other transcription factors like C/EBPα (CCAAT/enhancer binding protein-α (8). PPARγ also regulates many genes that stimulate lipid uptake and glucose metabolism in adipocytes (9). These genes include aP2 (adipocyte protein 2), fatty acid transporter, GLUT4 (glucose transporter 4), lipoprotein lipase, and acetyl-CoA oxidase (9).
Adiponectin is one of adipocyte-derived adipokines that affect cell physiology (10). Its level is reduced in patients with obesity and diabetes (11).
Gelidium (G.) amansii is an edible red alga which has been in common use for a long time. It is also used as an agar as well as a part of a salad in East Asia. Several studies have shown various biological activities of G. amansii including antitumor, cytotoxicity, immunomodulation, and antioxidant effects (1–4,12–14).
However, the ROS-reducing and anti-adipogenic effect of G. amansii has not been investigated. In this study, we show that G. amansii extract inhibits lipid accumulation by suppressing adipogenic factors and reduces ROS production by controlling antioxidant enzymes and oxidant enzyme during adipogenesis in 3T3-L1 cells.
MATERIALS AND METHODS
Materials
80% ethanol extract of G. amansii was purchased from Jehihidi (Jeju, Korea). Dulbecco’s modified Eagle’s medium (DMEM), bovine calf serum (BCS), fetal bovine serum (FBS), penicillin-streptomycin (P/S), phosphate buffered saline (PBS), and trypsin-EDTA were purchased from Gibco (Gaithersburg, MD, USA). Dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), insulin, Oil red O, nitroblue tetrazolium (NBT), and N-acetyl cystein (NAC) were purchased from Sigma (St. Louis, MO, USA). Unless noted, all chemicals also were purchased from Sigma Chemical Co.
Cell culture
3T3-L1 preadipocyes obtained from American Type Culture Collection (ATCC, CL-173) were cultured, maintained, and differentiated as described by Lee et al. (15). Briefly, cells were plated and grown in DMEM with 3.7 g/L sodium bicarbonate, 1% P/S and 10% BCS. Adipocyte differentiation was induced by treatment of 2 day post-confluent cells with 10% FBS and a hormonal mixture (MDI), consisting of 0.5 mM IBMX, 1.0 μM DEX and 1.67 μM insulin. Two days after the initiation of differentiation, the culture medium was replaced with DMEM supplemented with only 1.67 μM insulin and 10% FBS. This medium was then replenished every other day. For the treatments, 2 day post-confluent cells were differentiated with MDI in the presence of 1, 10, 20 and 40 μg/mL concentrations of G. amansii extract. Positive control sample was also treated 10mM NAC, which reduces the ROS by regulating glutathione with GPx and GR.
Determination of lipid accumulation by Oil red O staining
The extent of differentiation reflected by the amount of lipid accumulation was determined at day eight by Oil red O staining. Briefly, cells were fixed in 10% formaldehyde in PBS for 1 hr, washed with distilled water, and completely dried. Cells were stained with 0.5% Oil red O solution in 60:40 (v/v) isopropanol : H2O for 30 min at room temperature and washed four times with water, and dried. Differentiation was also monitored under microscope and quantified by elution with isopropanol and an optical density (OD) measurement at 490 nm.
ROS production in 3T3-L1 preadipocyte
3T3-L1 preadipocytes were grown to confluence and induced to differentiate into adipocyte, as described above. ROS production was detected by NBT assay. NBT is reduced by ROS to a dark-blue, insoluble form of NBT called formazan (4). At day 8 after induction, the cells were incubated for 90 min in PBS containing 0.2% NBT. Formazan was dissolved in 50% acetic acid, and the absorbance was determined at 570 nm.
RNA extraction and semi-quantitative RT-PCR
Total RNA was extracted from 3T3-L1 adipocyte cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol. RNA samples with OD260/OD280 ratios higher than 2.0 were employed for semi-quantitative RT-PCR. One microgram of total RNA was employed for the production of cDNA, using a reverse transcription-polymerase chain reaction (RT-PCR) system. The oligonucleotide primer sequence was as follows: PPARγ, sense (5′-CCAGAGTCTGCT-GATCTGCG-3′) and antisense (5′-GCCACCTCTTTG-CTCTGATC-3′), aP2, sense (5′-GACCTGGAAACTC-GTCTCCA-3′) and antisense (5′-CATGACA CATTCC-ACCACCA-3′), NOX4, sense (5′-GAAGCCCATTTG-AGGAGTCA-3′) and antisense (5′-GGGTCCACAGC-AGAAAACTC-3′), GLUT4, sense (5′-ACTGCTGAAG-ACAAGCATCC-3′) and antisense (5′-AAATGTGAGT-AGGGGCATGA-3′), adiponectin, sense (5′-CTGCAA-CATTCCGGGACTCT-3′) and antisense (5′-CCTGGT CCACATTCTTTTCC-3′) GAPDH, sense (5′-AACTTT-GGCATTGTGGAAGG-3′) and antisense (5′-ACACAT-TGGGGGTAGGAACA-3′). The PCR products were then run on 1.5% (v/v) agarose gels, stained with ethidium bromide, and photographed. The expression levels were quantified via scanning with a gel documentation and analysis system (Image J Program, NIH, Bethesda, MD, USA).
Western blot analysis
Protein extracts (30 μg) were separated via SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were then blocked and immunoblotted with primary antibodies specific for Cu/Zn-SOD, Mn-SOD, GPx, GR, GLUT4, adiponectin and GAPDH. Secondary antibodies conjugated with horseradish peroxidase (1:1000) were applied for 1 hr. The bands were visualized via enhanced chemiluminescence (ECL), and proteins were detected with LAS image software (Fuji, New York, NY, USA).
Statistical analysis
All experiments were repeated three times. The results were statistically analyzed via ANOVA and Duncan’s multiple range tests. A p-value of <0.05 was considered statistically significant (SAS Institute, Cary, NC, USA).
RESULTS AND DISCUSSION
Effect of G. amansii extract on cell viability of 3T3-L1 adipocytes
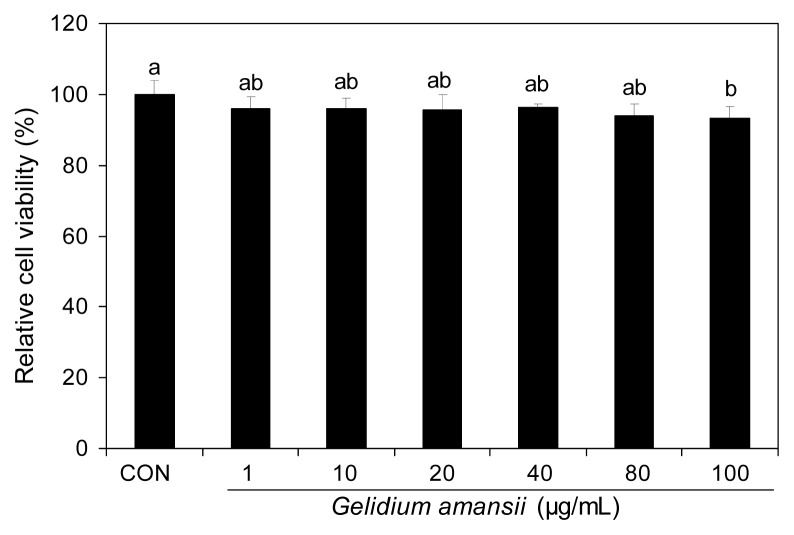
The G. amansii extract didn’t show any toxicity to the 3T3-L1 cells within the experimental range of concentrations (Fig. 1). We performed the subsequent experiments with concentrations less than 50 μg/mL of G. amansii extract.
Fig. 1.
Effect of Gelidium amansii on cell viability of 3T3-L1 adipocytes. 3T3-L1 adipocyte were cultured for a day and treated Gelidium amansii (1, 10, 20, 40, 80 and 100 μg/mL). XTT reagent treat after 24 hour and measure at 450 nm after incubate 4 hours. These data were measured as the standard deviation of six replicates. Means in the same column not sharing a common letter (a,b) are significantly different (p<0.05) based on Duncan’s multiple range test.
Effect of G. amansii extract on intracellular lipid accumulation and ROS production during differentiation
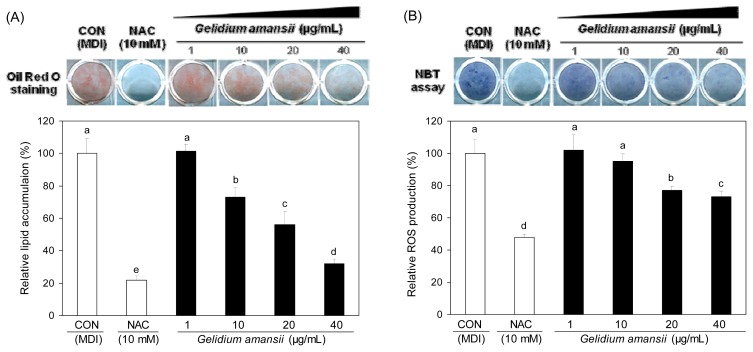
The effect of G. amansii extract on lipid accumulation was evaluated by Oil red O staining. The level of stained lipids was significantly decreased in a dose-dependent manner in the groups treated with the G. amansii extract (Fig. 2A). The 40 μg/mL concentration showed a 75% decrease in oil drop staining when compared to that of the control (Fig. 2). Our data shows that G. amansii extract inhibits lipid accumulation during differentiation in adipocyte. The NBT assay was performed to analyze the effect of G. amansii extract on ROS generation in the cells. G. amansii extract dose-dependently decreased formazan formation compared to that of the control (Fig. 2B), suggesting a significant effect of the extract on ROS production.
Fig. 2.
Effect of Gelidium amansii has inhibiting lipid accumulation (A) and ROS production (B) in 3T3-L1 cells during adipogenesis. 3T3-L1 adipocyte were cultured during differentiation (for 8 days) treatments with NAC (10 mM), Gelidium amansii (1, 10, 20 and 40 μg/mL). These result express lipid accumulation and ROS production during the differentiation of the 3T3-L1 adipocyte. Oil red O staining to measure lipid accumulation was determined by using Oil red O reagent and absorbance at 490 nm. NBT assay to measure ROS production was determined by using nitrotetrazolium blue chloride (TLC) and absorbance at 570 nm. These data were measured as the standard deviation of three replicates. Means in the same column not sharing a common letter (a–e, a–d) are significantly different (p<0.05) based on Duncan’s multiple range test.
Thus, we observed an inhibitory effect of the G. amansii extract on lipid accumulation and ROS production.
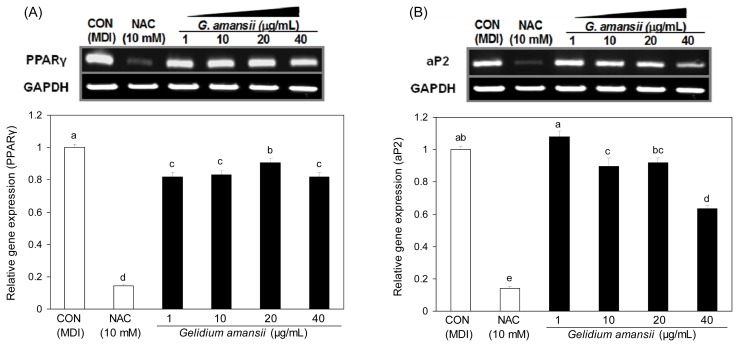
Effect of G. amansii extract on PPARγ and aP2 mRNA expressions during differentiation
To understand the molecular events of the inhibitory effect of G. amansii extract on lipid accumulation, we determined mRNA levels of PPARγ, a major adipogenic transcription factor, and ap2, a fatty acid binding protein. G. amansii extract markedly inhibited PPARγ and ap2 mRNA expression (Fig. 3A and B). Down-regulation of ap2 may be due to the reduction of PPARγ mRNA expression because ap2 is a major target gene of PPARγ. This result suggests that G. amansii extract inhibits adipocyte differentiation by decreasing the expression of PPARγ and aP2. However, PPARγ is not only factor necessary for adipogenesis. There are other downstream and upstream factors, including C/EBP (CCAAT/enhancer binding protein). C/EBPβ and C/EBPδ are expressed during the early phase of adipogenesis and induce expression of PPARγ and C/EBPα, which are present at the next stage of adipogensis (8). C/EBPα is required for normal adipogenesis by interacting with PPARγ (8).
Fig. 3.
Effect of Gelidium amansii on the mRNA expression of PPARγ and aP2. 3T3-L1 adipocyte were cultured during differentiation (for 8 days) treatments with NAC (10 mM), Gelidium amansii (1, 10, 20 and 40 μg/mL). Total cellular RNA was extracted on day 8 and performed RT-PCR. Gene expression of PPARγ and aP2 were quantified and normalized with GAPDH. These data were measured as the standard deviation of three replicates. Means in the same column not sharing a common letter (a–d, a–e) are significantly different (p<0.05) based on Duncan’s multiple range test.
In addition, lipid synthesis is associated with many other genes in metabolism. For example, DGAT (diacyl-glycerolacyltransferase) and lipin1 (phosphatidic acid phosphatase) are involved in lipid accumulation by catalyzing the TAG synthesis pathway (16). Thus, an analysis of these genes should be conducted to better understand the effect of the G. amansii extract on lipid accumulation.
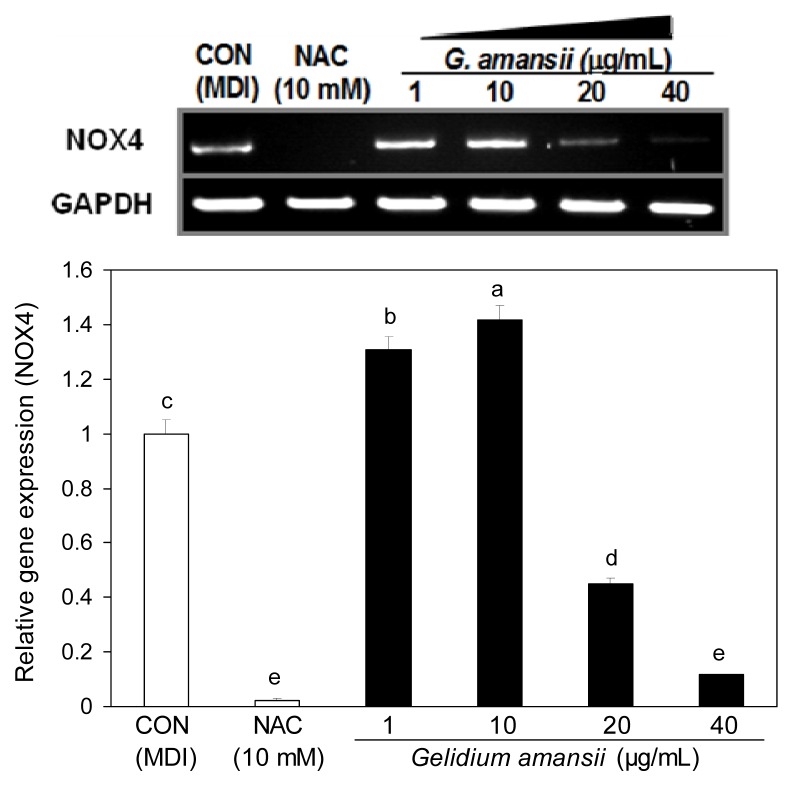
Effect of G. amansii extract on ROS regulating mRNA and proteins during differentiation
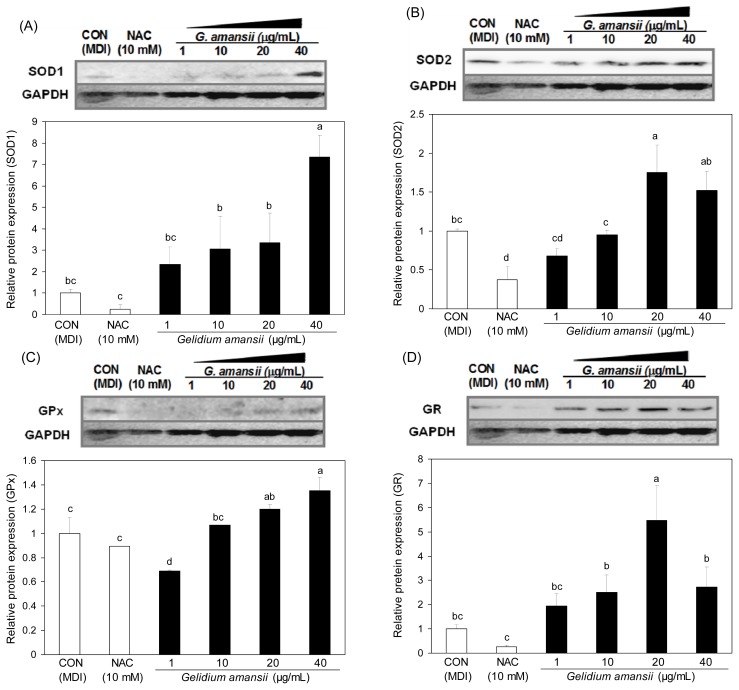
ROS generation is selectively enhanced in adipocytes to play an important role in lipid accumulation (17). Oxidative stress in adipocytes by ROS is one of the major factors associated with metabolic diseases and cell damage (18). NOX4 is a transmembrane enzyme that produces ROS in the cytosol by using NADPH. We examined the inhibitory effect of the G. amansii extract on ROS production by determining NOX4 mRNA levels. NOX4 mRNA expression in G. amansii extract 40 μg/mL treatment group was greatly reduced (~90%) compared with control group. This data indicates that G. amansii extract inhibits ROS production by downregulating the NOX4 gene during adipogenesis. Moreover, we evaluated the effect of the extract on the expression of ROS scavenging enzymes such as SOD1, SOD2, Gpx, and GR, which are in charge of ROS elimination from the cytoplasm and mitochondria in the cell. SOD1 and SOD2 are major enzymes that remove ROS by converting O− (superoxide) to hydrogen peroxide in the cytoplasm. Gpx and GR also play an important role in the ROS decrease by participating in the chain reaction to generate NADPH. At concentrations of 20 or 40 μg/mL, the extract greatly increased the levels of these ROS scavenging proteins, compared with the effects of the control group (Fig. 5). This result shows that up-regulation of ROS scavenging enzymes leads to the inhibition of ROS production. Taken together, these data indicate that G. amansii extract suppresses ROS production, and protects the cells against oxidative stress by activating ROS-scavenging enzymes and inhibiting an ROS-producing gene.
Fig. 5.
Effect of Gelidium amansii on the protein expression of SOD1, SOD2, GPx and GR. 3T3-L1 adipocyte were cultured during differentiation (for 8 days) treatments with NAC (10 mM), Gelidium amansii (1, 10, 20 and 40 μg/mL). Data are representative of three independent experiments, and GAPDH and target protein signals were from one representative blot. Individual antibodies were applied after stripping the membrane continuously. Less GAPDH amounts in control or NAC group were seems to be due to the technical error in loading. Means in the same column not sharing a common letter (a–c, a–d) are significantly different (p<0.05) based on Duncan’s multiple range test.
However, the inhibitory effect of G. amansii extract on the ROS-regulating enzymes (Fig. 4) was not completely reflected in the result from NBT assay, which showed 25% ROS inhibition (Fig. 2), suggesting that the ROS regulation is much more complicated and that other genes may be associated in the counter-inhibition of ROS generation.
Fig. 4.
Effect of Gelidium amansii on the mRNA expression of NOX4. 3T3-L1 adipocyte were cultured during differentiation (for 8 days) treatments with NAC (10 mM), Gelidium amansii (1, 10, 20 and 40 μg/mL). Total cellular RNA was extracted on day 8 and performed RT-PCR. Gene expression of NOX4 was quantified and normalized with GAPDH. These data were measured as the standard deviation of three replicates. Means in the same column not sharing a common letter (a–e) are significantly different (p<0.05) based on Duncan’s multiple range test.
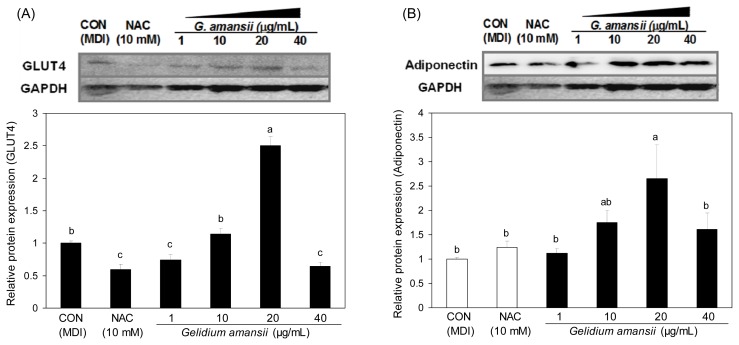
Effect of G. amansii extract on protein expressions of GLUT4 and adiponectin during differentiation
We further determined adiponectin and GLUT4 protein levels in 3T3-L1 cells in the presence of the G. amansii extract (Fig. 6(A)). GLUT4, a glucose uptake protein, is stimulated by insulin, and translocates to the cell membrane to uptake the glucose. The downgraded function of GLUT4 results in insulin resistance, which is closely associated to obesity (19). Adiponectin is reported to be downregulated in insulin resistance and obesity, and modulates glucose regulation and insulin sensitivity (11) (Fig. 6(B)). Adiponectin has shown to be at lower levels in patients with diabetes and obesity (19); however, Fu et al. showed that adiponectin promotes adipocyte differentiation (20), and Kusminski et al. reported that an increase in adiponectin level is accompanied by the treatment of PPARγ agonist (21). The mechanistic action of adiponectin is still being debated. Our data shows that GLUT4 and adiponectin protein expression in the G. amansii extract treatment group was significantly increased compared with control group. Its increasing behavior showed a dose-dependent manner up to a concentration of 20 μg/mL. However, the expression of both proteins decreased in samples receiving 40 μg/mL of extract. In particular, our adiponectin result was the opposite of some studies (20,21). Further study will be performed for precise understanding on the physiological effect and action of adiponectin.
Fig. 6.
Effect of Gelidium amansii on the protein expression of GLUT4 and adiponectin. 3T3-L1 adipocyte were cultured during differentiation (for 8 days) treatments with NAC (10 mM), Gelidium amansii (1, 10, 20 μg/mL). Total cellular protein was extracted on day 8 and performed western blotting. Protein expression of GLUT4 and Adiponectin were quantified and normalized with GAPDH. Data are representative of three independent experiments, and GAPDH and target protein signals were from one representative blot. Individual antibodies were applied after stripping the membrane continuously. Less GAPDH amounts in control or NAC group were seems to be due to the technical error in loading. Means in the same column not sharing a common letter (a–c, a–b) are significantly different (p<0.05) based on Duncan’s multiple range test.
Collectively, these data indicate that G. amansii extract provides favorable conditions, such as insulin sensitivity of the cell, by upregulating GLUT4 and adiponectin.
There are very few studies on the biological activity of G. amansii. Yue-Hwa et al. showed the anti-proliferative effect of G. amansii on hepatoma and fibroblast cells (22); however, there is no data on bioactive compounds isolated from G. amansii. A couple of compounds from other seaweeds have been studied, including polysaccharides and polyphenols. A polysaccharide, fucoidan, and a carotenoid, fucoxanthin, from brown algae have been shown to inhibit lipid accumulation in 3T3-L1 (23,24). On the other hand, pigments from red alga such as chlorophyll α, β-carotene and lutein are involved in the anti-mutagenesis effects (25). Among these compounds, pigments except for fucoidan can be obtained by solvent exraction, and fucoxanthin could be potential candidates because of their similar extraction and biological activity.
In conclusion, this study showed an inhibitory effect of a red alga, G. amansii, on the lipid accumulation and ROS production at the molecular level. Our data suggested that ROS suppression through ROS-related gene regulation could lead to the anti-adipogenic effect of a G. amansii extract through inhibition of adipogenic gene expression. This study will be useful for developing various commercial products, including an edible anti-obesity agent from marine resources.
ACKNOWLEDGMENTS
This research was a part of the project titled “Development of Lipid Lowering Food and Drug Biomaterials with Korean Seaweed” funded by the Ministry of Food, Agriculture, Forestry and Fisheries, Korea, to Boo-Yong Lee and “NRF-2011-355-F00035 Nutrigenomic studies of seaweed on fat mudulation by TAG synthetic genes in adipocytes” funded by National Research Foundation of Korea to Hyeon-Son Choi.
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
REFERENCES
- 1.Moller DE, Flier JS. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991;325:938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 2.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 3.Adachi T, Toishi T. Expression of extracellular superoxide dismutase during adipose differentiation in 3T3-L1 cells. Redox Rep. 2009;14:34–40. doi: 10.1179/135100009X392467. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa S, Fujita T. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent HK, Bourguignon CM, Vincent KR, Weltman AL, Bryant M, Taylor AG. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity. 2006;14:2224–2235. doi: 10.1038/oby.2006.261. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MP. How mitochondria produce reactive oxygen species. J Biochem. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attie AD. Adipocyte metabolism and obesity. J Lipid Res. 2009;50:S395–399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Annu Rev Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 9.Pearson SL, Cawthorne MA, Clapharm JC. The thiazolidinedione insulin sensitizer, BRL49653, increase the expression of PPARγ and aP2 in adipose tissue of high fat fed rats. Biochem Biophys Res Commun. 1996;229:752–757. doi: 10.1006/bbrc.1996.1876. [DOI] [PubMed] [Google Scholar]
- 10.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 11.Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Mantzoros CS. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–1054. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, Song J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006;243:228–234. doi: 10.1016/j.canlet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Nagata T. Antioxidative activities in some common seaweeds. Plant Foods Hum Nutr. 1998;52:253–262. doi: 10.1023/a:1008007014659. [DOI] [PubMed] [Google Scholar]
- 14.Fu YW, Hou WY. The immunostimulatory effects of hot-water extract of Gelidium amansii via immersion, injection and dietary administrations on white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007;22:673–685. doi: 10.1016/j.fsi.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Lee OH, Seo MJ, Choi HS, Lee BY. Pycnogenol inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother Res. 2011;26:403–411. doi: 10.1002/ptr.3568. [DOI] [PubMed] [Google Scholar]
- 16.Peterson TR, Sengupta SS, Harris TE. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 19.James DE, Brown R. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1998;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 20.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and athe-roslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 21.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Tu CJ, Wu HT. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol Pharm Bull. 2004;27:180–184. doi: 10.1248/bpb.27.180. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int J Mol Med. 2006;18:147–152. [PubMed] [Google Scholar]
- 24.Pantankar MS, Oehninger S, Barnett T, Williams RL, Clark GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem. 1993;29:21770–21776. [PubMed] [Google Scholar]
- 25.Okai Y, Higashi-Okai K, Yano Y, Otani S. Identification of antimutagenic substances in an extract of edible red alga, Porphyra tenera (Asakusa-nori) Cancer Lett. 1996;100:235–240. doi: 10.1016/0304-3835(95)04101-x. [DOI] [PubMed] [Google Scholar]