Abstract
Interruption or prolonged reduction and subsequent restoration of blood flow into the kidney triggers the generation of a burst of reactive oxygen species (ROS), leading to injury in the tubular epithelial cells. In this study, we determined whether methanol extract of goat’s-beard (Aruncus dioicus) (extract) could prevent this ischemia/re-perfusion injury. When in vitro radical scavenging activity of the extract was measured using a DPPH radical quenching assay, the extract displayed slightly lower activity than ascorbic acid. One hour after administration of the extract (400 mg/kg) by intraperitoneal injection in rats, renal ischemia/reperfusion injury was generated by clamping the left renal artery for forty minutes, followed by 24 hr restoration of blood circulation. Prior to clamping the left renal artery, the right renal artery was removed. Compared with the vehicle-treated group, pre-treatment with the extract significantly reduced the tubular epithelial cell injury by 37% in the outer medulla region, and consequently reduced serum creatinine concentration by 39%. Reduction in the cell injury was mediated by attenuation of Bax/Bcl-2 ratio, inhibition of caspase-3 activation from procaspase-3, and subsequent reduction in the number of apoptotic cells. Thus, goat’s-beard (Aruncus dioicus) might be developed as a prophylactic agent to prevent acute kidney injury.
Keywords: goat’s-beard, methanol extract, kidney, ischemia/reperfusion, reactive oxygen species, apoptosis
INTRODUCTION
Ischemic acute renal failure, a subset of acute renal failure (ARF) (also referred to more recently as acute kidney injury), is attributed to the reduction in blood flow to the kidney, leading to an increase of creatinine in the blood (1,2). In severe cases of the event, such as sepsis or cardiac surgery, the interruption or prolonged reduction of blood flow to the kidney can result in injury to the outer medulla region, a result of the combination of countercurrent oxygen exchange and selective reduction in blood supply (3), subsequently causing damage to the tubular cells due to depletion of ATP (1,4). Although early restoration of blood flow to the ischemic kidney is one way to prevent the ischemic ARF, it further augments production of the reactive oxygen species (ROS), predisposing more injury to the cells already weakened by ischemia (5,6). As a result of ischemia followed by reperfusion (ischemia/reperfusion), the tubular cells die through apoptosis as well as necrosis (7). Thus, one way to prevent ischemic ARF caused by I/R injury is to attenuate tubular cell death by eliminating ROS and, consequently, inhibiting apoptosis (6).
Goat’s-beard (Aruncus dioicus var. kamtschaticus) grows naturally in alpine regions of Korea (7) and has edible leaves that contain high antioxidant activities (8,9). In our previous study, we reported that water extract of goat’s beard and its ethyl acetate fraction reduced brain damage in a rat model of ischemia/reperfusion when the agents were administered by intraperitoneal injection prior to occlusion (10).
In the present study, we determined whether goat’s beard was also effective in reducing ischemic acute renal failure because the underlying mechanisms inducing ischemia/reperfusion injury in kidney and brain are similar (11). To explore this possibility, we investigated the effect of ablating ROS and consequently attenuating apoptosis.
MATERIALS AND METHODS
Extraction
Methanol extract of the whole plant of goat’s beard was prepared as previously described (12). Briefly, whole plants of goat’s beard collected in Uiseong area, a county of Gyeongsangbuk-do, South Korea, were washed and dried. Then 100 g of the plant was put into an ultrasonicator (8210R-DTH, Branson Ultrasonic Corp., Danbury, CT, USA) and extracted in 1 L methanol twice for 1 hr each at room temperature, and the extract was filtered with filter papers (Whatman No. 3, Whatman Inc., Piscataway, NJ, USA). The filtrate was vacuum- dried with a rotary evaporator [NP-1, Tokyo Rikakikai Co. (EYELA), Tokyo, Japan] with a 10 g yield, which will be referred to as the extract.
Assessment of radical scavenging activity of extract with DPPH
Various amounts of the extract dissolved in 25 μL of dimethyl sulfoxide (DMSO) were mixed with 2.5 mL reaction solution containing α,α-diphenyl-β-picrylhydrazyl (DPPH) [0.1 mM DPPH, 40% ethanol and 0.04 M acetate buffer (pH 5.5)]. Thirty minutes after incubation of the samples at 37°C, the absorbance was measured at 517 nm with a UV-visible spectrophotometer (U2800, Hitachi, Tokyo, Japan). The experiments were repeated three times, and the absorbance was averaged. Final concentrations of the extract in the samples were 0 (negative control), 10, 50, 100, and 200 μg/mL. Ascorbic acid with the concentrations corresponding to the samples was used as a positive control. Radical scavenging activity of the extract and ascorbic acid represented by electron donating activity (EDA) was calculated with the following equation
in which extract, ascorbic acid, and blank absorbance represent absorbance of solutions containing the extract, ascorbic acid, and nothing (negative control), respectively (13,14).
Animals
Eight-week-old male Sprague-Dawley (SD) rats were purchased from Samtaco Inc. (Osan, Korea). Experiments were carried out according to the guidelines for the animal care and use of laboratory animal protocols approved by the Institutional Animal Care and Research Advisory Committee of Catholic University of Daegu. Animals were housed with food and water available ad libitum under diurnal lighting conditions and in a temperature-controlled environment until the day of the experiment.
Rat renal ischemia/reperfusion model
Renal ischemia/reperfusion injury was generated by clamping of the left renal artery in male SD rats (250~300 g) after surgical removal of the right kidney, as previously described (13). Briefly, rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg), and the body temperature was maintained at 36±0.5°C throughout the experiments, with a heating pad. After midline incision, the right kidney was surgically removed. Then the remaining left kidney underwent renal artery occlusion by clamping for 40 min (ischemia), followed by recirculation of blood for another 24 hr (reperfusion), after which the rats were sacrificed for further examinations.
Extract administration
Rats were randomly assigned to one of three groups: (1) extract-treated group (n=10), (2) vehicle-treated group (n=10), or (3) sham group (n=6). In the extract-treated group, rats received the extract (400 mg/kg) dissolved in 0.3% carboxymethyl cellulose (CMC) by intraperitoneal injection 1 hr prior to occlusion. In the vehicle-treated group, rats received 0.3% CMC only by intra-peritoneal injection 1 hr prior to occlusion. In the sham group, experimental procedures were the same as in the vehicle-treated group, except that there was no occlusion by clamping.
Serum creatinine levels
At the end of reperfusion period (24 hr), blood was collected from renal vein under anesthesia with ketamine and xylazine. The serum creatinine concentration was measured by the Jaffė reaction on a Hitachi 747 analyzer (Roche Diagnostics, Mannheim, Germany).
Histological analysis
The remaining left kidney was harvested, bisected along its long axis, fixed in 10% neutral formalin solution for 24~28 hr, embedded in paraffin, and sectioned on slides at 5 μm thickness. The sections were deparaffinized as described previously (15), and stained with hematoxylin and eosin. Twenty representative photomicrographs were randomly taken in the outer medulla region of one representative section at 200× magnification under light microscope. Percentage of damaged area in the section was assessed by computer-aided image analysis (Image J, NIH, Bethesda, MD, USA), from which the degree of damage was graded by a 5-point scale (0~4) adopted by Kelly et al. (16), with a slight modification in assigning the range of scores: 0, none; 1, <5%; 2, 5~30%; 3, 30~50%; 4, >50%. Scores of the 20 sections were averaged to represent a score for each rat. For the histological analysis, tissues of some rats were excluded as they did not meet the quality required for the analysis. Thus, the number of rats assessed for histological examination was as follows: (1) extract- treated group (n=7), (2) vehicle-treated group (n=7), or (3) sham group (n=5).
TUNEL staining
To measure DNA nicks, terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed, using ApopTag®in situ apoptosis detection kit (Chemicon, Temecula, CA, USA) according to the manufacturer’s protocol. Briefly, after successive pretreatment with Proteinase K and hydrogen peroxide, the sections were incubated with terminal deoxynucleotidyl transferase (TdT) and dNTP covalently linked with digoxigenin for 30 min at 37°C, then with anti-digoxigenin peroxidase conjugate, finally incubated with 3,3′-diaminobenzidine tetrahydrochloride (DAB), a substrate for staining. For negative controls, TUNEL staining was performed in the absence of TdT. For the counter-staining, the sections were stained with methyl green. To determine the percentage of apoptotic cells, micrographs of TUNEL-positive nuclei and methyl green-stained nuclei were captured using an Olympus microscope (Tokyo, Japan), and the cell number was counted using the Image J software (Image J version 1.43r, NIH, from 20 random fields at 400× magnification.
Assessment of Bcl-2, Bax, and caspase-3
To assess the presence of Bcl-2, Bax, and cleaved (activated) caspase-3, immunohistochemical techniques were utilized, as described previously (15). Briefly, rabbit polyclonal anti-Bcl-2, anti-Bax (1:50 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or anti- (cleaved caspase-3) primary antibody (1:50 dilution, Cell Signaling, Beverly, MA, USA) was added to the 0.1% BSA solution, and the sections were incubated overnight at 4°C in a humidified chamber. A Vectastain Elite ABC kit (Vector Laboratories, Burlington, ON, USA) was used for the staining, which was carried out according to the manufacturer’s instructions. Color was developed by incubating the sections for 5 min in PBS containing 1 mg/mL 3,3′-diaminobenzidine tetrahydrochloride (DAB) and 0.3% H2O2. The sections were evaluated at 200× magnification. The color intensity of DAB staining was measured using Image J software (NIH). For the quantitative comparison in the assessment of activated caspase- 3, the value for the sham group was arbitrarily set 1.0.
Statistical analysis
Data is given as means±SEM. Comparisons between groups were performed using standard statistical methods using SPSS software (SPSS Inc., Chicago, IL, USA). The data was analyzed with one-way ANOVA, Kruskal–Wallis one-way ANOVA on ranks, or unpaired t-test. Statistical significance was determined at the p<0.05 level.
RESULTS
Radical scavenging activity of the extract
To determine whether the extract has antioxidant activity, radical scavenging activity of the extract was measured with a DPPH assay. Absorbance at 517 nm decreased as concentrations of the extract and ascorbic acid, a strong antioxidant used as control, increased (data not shown). Thus, the electron donating ability (EDA) (%) of the extract, which represents its radical scavenging activity, increased with the addition of more extract (Fig. 1). IC50s, concentrations representing the half maximal EDA, of the extract and ascorbic acid were 28.2 and 12.0 μg/mL, respectively.
Fig. 1.
Assessment of DPPH radical scavenging activity of methanol extract. Absorbance at 517 nm was measured after various concentrations of the extract and ascorbic acid were reacted with DPPH, and then electron donating ability (EDA) of the methanol extract of goat’s beard and ascorbic acid was calculated using the following equation: EDA (%)= (1 – extract or ascorbic acid absorbance/blank absorbance) ×100 in which extract, ascorbic acid, and blank absorbance represents absorbance of solutions containing the extract, ascorbic acid, and nothing (negative control), respectively.
Improvement of renal function by the extract
Serum creatinine levels were selected as a biomarker to determine whether the extract preserved the renal function from ischemia/reperfusin injury. In a rat model of ischemia/reperfusion, rats underwent removal of the right kidney and subsequent occlusion of the remaining left kidney by clamping for 40 min to induce ischemia, followed by 24 hr reperfusion. The extract (400 mg/kg) was administered by intraperitoneal injection 1 hr prior to occlusion of the remaining left kidney. Serum creatinine levels in the extract-treated group (n=10) were significantly reduced (39%) compared with those in the vehicle-treated group (n=10) (1.4±0.2 versus 2.3±1.2 mg/dL, p<0.05), whereas those in the sham group (n=6) remained low (0.3±0.1 mg/dL) (Fig. 2).
Fig. 2.
Measurement of serum creatinine levels in a rat model of ischemia/reperfusion injury. Serum creatinine levels in the sham (n=6), vehicle-treated (n=10) and extract-treated (n=10) groups were measured after renal ischemia/reperfusion injury. I/R injury was generated by 40 min clamping of the left renal artery in Sprauge-Dawley rats after surgical removal of the right kidney, followed by 24 hr restoration of blood circulation. In the extract-treated group, rats received the extract (400 mg/kg) dissolved in 0.3% carboxymethyl cellulose (CMC) by intraperitoneal injection 1 hr prior to occlusion. In the vehicle-treated group, rats received 0.3% CMC only by intra-peritoneal injection 1 hr prior to occlusion. In the sham group, experimental procedures were the same as in the vehicle-treated group, except that there was no occlusion by clamping. The numbers of rats used in sham, vehicle-treated, and extract- treated group were 6, 10, and 10, respectively. Each column represents the means±SE. *p<0.05 vs. control group.
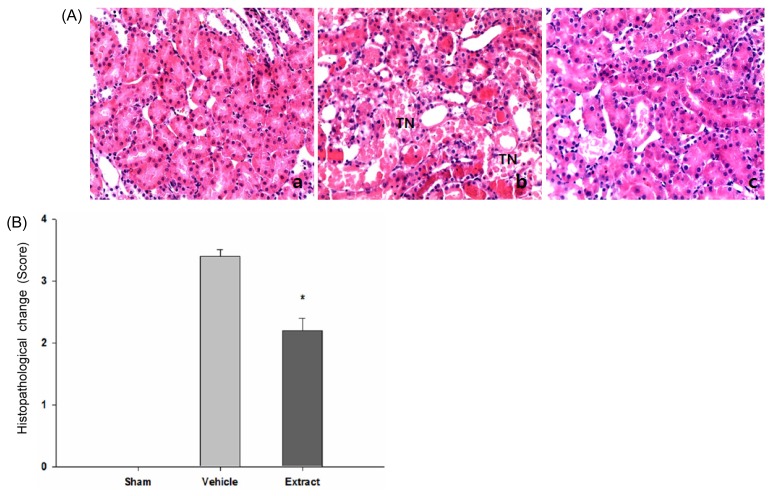
Attenuation of tubular damage by the extract
To determine whether the extract protected tubular cells in outer medulla regions against ischemia/reperfusion injury, sections stained with H&E were analyzed (Fig. 3). Representative micrographs revealed that the tubules in the vehicle-treated group underwent damage, as represented by distinctive morphological changes of the tubules, loss of nuclei and concomitant death of tubular epithelial cells, and desquamation of viable and necrotic cells, compared with those in the sham group (Fig. 3A). The extent and severity of damage of the tubules in the extract-treated group were markedly reduced, compared with those in the vehicle-treated group. To quantify the extent of damage, a grading system with 5-point scale (0~4) was adopted as described in Materials and Method section (Fig. 3B). Consistent with the qualitative findings, tubular damage in the extract-treated group (n=7) were significantly attenuated compared with that in the vehicle-treated group (n=7) (average score 2.2±0.2 versus 3.5±0.3, p<0.05), where “score 0” represents no damage as was confirmed in the sham group (n=5).
Fig. 3.
Histopathological evaluation of tubular injury in the outer medulla region in a rat model of ischemia/reperfusion injury. (A) Representative photomicrographs of renal tissue sections stained with hematoxylin & eosin (H&E) at magnification 200× were presented: (a) sham (n=5), (b) vehicle-treated (n=7), and (c) extract-treated group (n=7), respectively. (B) Semi-quantitative assessment of the histological alterations due to tubular damage was presented with 5-point scale system: 0, none (no change); 1, <5% (mild); 2, 5~30% (moderate); 3, 30~50% (severe); 4, >50% (very severe). Each column represents the means±SE. *p<0.05 vs. control group.
Involvement of apoptosis in the attenuation of tubule damage by extract
Levels of DNA nicks and cleaved caspase-3 measured by TUNEL staining and immunohistochemistry, respectively, were selected as indices to assess the extent of apoptosis that occurred in the tubular epithelial cells during ischemia/reperfusion (Fig. 4). Representative micrographs stained with TUNEL revealed apoptotic cells in the tubules of the sham, vehicle-treated and extract-treated group (Fig. 4A). The number of apoptotic cells in the vehicle-treated group increased markedly, compared with that in the sham group, whereas the number of apoptotic cells in the extract-treated group was attenuated, compared with that in the vehicle treated-group. To quantify these findings, the ratio of apoptotic cells to total cells, [Apoptotic cell (% total cell)], was assessed (Fig. 4B). The ratio was significantly reduced (39%) in the extract-treated group (n=7) compared with those in the vehicle-treated group (n=7), with a value of 22.1±1.4% versus 36.0±8.4%, p<0.05), whereas those in the sham group (n=5) remained low (0.7±0.1%). As another index for assessing apoptosis, representative micrographs stained with an antibody that specifically recognizes cleaved caspase-3 (activated caspase-3) also revealed apoptotic cells in the tubules of the sham, vehicle-treated and extract-treated group (Fig. 5). Consistent with the findings observed with TUNEL staining, color intensity in the vehicle-treated group that reflects the number of apoptotic cells increased markedly, compared with that in the sham rats, whereas the color intensity in the extract-treated group was attenuated, compared with that in the vehicle-treated group (Fig. 5A). To quantify these findings, color intensity in each group was compared (Fig. 5B). The color intensity was significantly reduced in the extract-treated group (n=7) compared with that in the vehicle-treated group (n=7) (5.8±0.3% versus 10.2±0.2%, p<0.05), when the color intensity in the sham group (n=5) was arbitrarily set at 1.0±0.3.
Fig. 4.
Assessment of apoptotic cells by TUNEL staining in a rat model of ischemia/reperfusion injury. (A) Representative photomicrographs of renal tissue sections stained with TUNEL at two different magnifications were presented: (a) & (d) sham (n=5) at 200× and 400×, respectively, (b) & (e) vehicle-treated (n=7) at 200× and 400×, respectively, and (c) & (f) extract- treated group (n=7), at 200× and 400×, respectively. (B) Quantitative assessment of TUNEL-positive cells was presented with TUNEL-positive cells/total cells ratio. Each column represents the means±SE. *p<0.05 vs. control group.
Fig. 5.
Assessment of caspase-3 activation by immunohistochemistry in a rat model of ischemia/reperfusion injury. (A) Representative photomicrographs of renal tissue sections stained with immunohistochemistry at magnification 400× were presented: (a) sham (n=5), (b) vehicle-treated (n=7), and (c) extract-treated group (n=7), respectively. (B) Quantitative assessment of activated caspase-3 produced from cleavage of procaspase-3 was presented, with the value for the sham group arbitrarily set 1.0. Each column represents the means±SE. *p< 0.05 vs. control group.
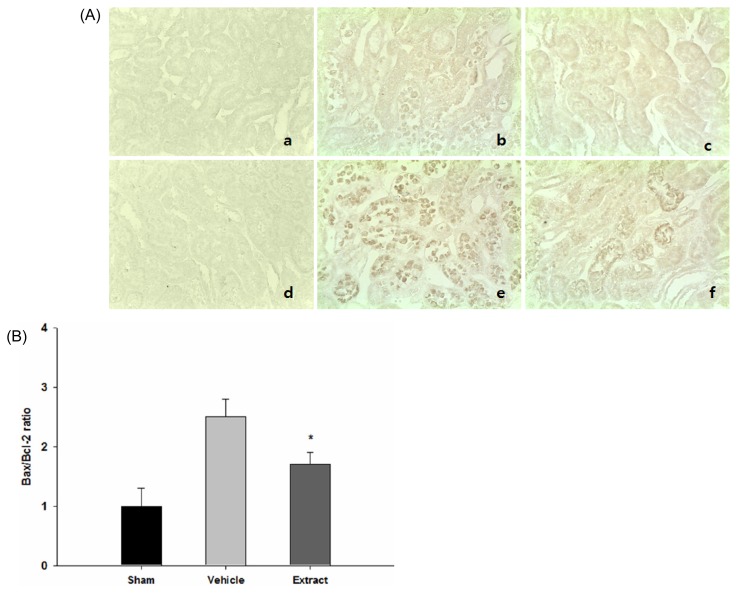
Association of Bcl-2 and Bax in mediating the antiapoptotic effect of extract
Bcl-2 and Bax act as antiapoptotic and proapoptotic proteins, respectively. Their levels of expression were evaluated by immunohistochemistry, with the Bax/Bcl-2 ratio as the key determinant (17) (Fig. 6). Representative micrographs revealed that color intensity of Bcl-2 in the vehicle-treated group (n=7) was markedly increased, while the color intensity of Bax was markedly decreased, compared with the sham group (n=5). The color intensity of Bcl-2 and Bax in the extract-treated group (n=7) was elevated and attenuated, respectively, compared with that in the vehicle-treated group (Fig. 6A). To quantify these findings, Bax/Bcl-2 ratios were assessed (Fig. 6B). The ratio was significantly reduced (32%) in the extract- treated group (n=7) compared with those in the vehicle- treated group (n=7) (1.7±0.2 versus 2.5±0.3, p<0.05), whereas those in the sham group (n=5) remained relatively low (1.0±0.3).
Fig. 6.
Assessment of Bcl-2 and Bax expression by immunohistochemistry in a rat model of ischemia/reperfusion injury. (A) Representative photomicrographs of renal tissue sections stained with TUNEL at magnification 400× were presented: (a), (b), and (c); Bcl-2 for sham (n=5), vehicle-treated (n=7), and extract-treated group (n=7), respectively, (d), (e), and (f); Bax for sham (n=5), vehicle-treated (n=7), and extract-treated group (n=7), respectively. (B) Quantitative assessment of Bax/Bcl-2 ratio was presented. Each column represents the means±SE. *p<0.05 vs. control group.
DISCUSSION
In the present study, we demonstrated that the methanol extract of goat’s-beard (Aruncus dioicus) protected the rat kidney against ischemia/reperfusion injury by attenuating apoptosis when administered by intraperitoneal injection prior to ischemia.
Obstruction of the renal artery by clamping in a rat leads the kidney to an anoxic state, and consequent depletion of ATP triggers cascades of events in the cells, which eventually result in the cell death by apoptosis or necrosis, depending on the severity and duration of ATP depletion (7,18,19). Furthermore, restoration of blood flow exacerbates the situation by generating more reactive oxygen species (ROS), which mediates further damage in cellular structures, such as membranes and proteins (20). Consequently, ROS augments cell death initiated by ischemia. The cells and cell debris detached from the tubules block tubules located further downstream and wastes, such as creatinine in the tubular fluid, leaks back into the peritubular capillaries (1,4). These events contribute to the reduction of the effective glomerulus filtration rate (GFR), resulting in the elevation of serum creatinine level in the blood, a classical bio-marker to assess renal function (21).
Considering these cascades of events occurring in renal ischemia/reperfusion, the findings of the present study clearly demonstrate that the extract attenuated damage and subsequent death of the tubular epithelial cells in the outer medulla region, as revealed with H&E staining. It was also reported that the outer medulla region is a very susceptible region in a similar rat or mouse model of ischemia/reperfusion, and the extent of damage in the region could be graded with score systems (16,22).
We have demonstrated with DPPH, acting as a radical donor in this in vitro system (23), that, at a concentration twice that of the strong antioxidant ascorbic acid, the extract has a similar radical scavenging activity. Furthermore, ascorbic acid has been shown by others to reduce acute kidney injury in a rat model of ischemia/reperfusion (24). In addition, radical scavenging activity of some natural products measured in vitro correlated well with antioxidant activity in vivo(25,26). Thus, attenuation of the damage by the extract is likely to be initiated by removal of ROS mediated by radical scavenging activity of the extract.
We have also shown that suppressing the generation of ROS by the extract contributed to the attenuation of apoptosis mediated by a blockade of the cascade of apoptotic pathways; i.e., the reduction of Bax/Bcl-2 ratio and the inhibition of caspase-3 activation. Both of these activities were revealed by immunohistochemistry, while the resulting attenuation in the generation of DNA nicks was observed through TUNEL staining. Similar means of assessing apoptosis caused by caspase-3 activation and the resulting generation of DNA nicks were previously used in studies involving rats (27,28). Finally, the extract-mediated reduction of the tubular epithelial cell death by apoptosis, along with necrosis, likely contributed to the improvement of renal function, as assessed with the reduction in serum creatinine level. In agreement with the findings of the present study, earlier studies showed that, under ischemia/reperfusion conditions, overexpression of Bcl-2 protein in tubular cells in vitro and in vivo by viral vector-mediated gene transfer triggered inhibition of caspase-3 expression, attenuation of apoptosis as confirmed by TUNEL staining, and inhibition of elevated blood serum creatinine levels (29).
In summary, prophylactic administration of goat’s- beard (Aruncus dioicus) could become a means to prevent ischemic acute kidney injury by inhibition of apoptosis in tubular epithelial cells through removal of ROS produced during ischemia/reperfusion. Thus, the extract might be developed as a prophylactic agent to prevent acute kidney injury in clinical settings where acute kidney injury is expected to occur, such as following cardiac surgery. In fact, several renoprotective agents have been clinically tried, from which some agents, such as fenoldopam and atrial natriuretic peptide, showed evidence of renoprotection against injury after cardiac surgery (30,31).
ACKNOWLEDGMENTS
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu (2009) (K. S. Ahn), and a grant from Jangwon Co. (J. W. Lee).
ABBREVIATIONS
- ROS
reactive oxygen species
- ARF
acute renal failure
- DMSO
dimethyl sulfoxide
- DPPH
α,α-diphenyl-β-picrylhydrazyl
- EDA
electron donating activity
- SD
Sprague Dawley
- CMC
carboxymethyl cellulose
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick- end labeling
- TdT
deoxynucleotidyl transferase
- DAB
3,3′-diaminobenzidine tetrahydrochloride
- GFR
glomerulus filtration rate
REFERENCES
- 1.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 2.Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria. J Intensive Care Med. 2007;22:187–193. doi: 10.1177/0885066607299510. [DOI] [PubMed] [Google Scholar]
- 3.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JW. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 5.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:655–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunym-Schmiedeberg’s Arch Pharmacol. 2007;376:1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 7.Pandanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 8.Shin JW, Lee SI, Woo MH, Kim SD. Effect of ethanol extracts of goat’s beard on streptozotocin induced diabetic symptoms and oxidative stress in rats. J East Asian Soc Dietary Life. 2008;18:939–948. [Google Scholar]
- 9.Lee SO, Lee HJ, Yu MH, Im HG, Lee IS. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ullung island. Korean J Food Sci Technol. 2005;37:233–240. [Google Scholar]
- 10.Han HS, Lee JW. Attenuation of brain injury by water extract of goat’s beard (Aruncus dioicus) and its ethyl acetate fraction in a rat model of ischemia-reperfusion. J Food Sci Nutr. 2011;16:217–223. [Google Scholar]
- 11.Tapuria N, Kumar Y, Habib MM, Amara MA, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury-a review. J Surg Res. 2008;150:304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Lee JW. Methanol extract of Cassia mimosoides var. nomame and its ethyl acetate fraction attenuate brain damage by inhibition of apoptosis in a rat model of ischemia-reperfusion. J Food Sci Nutr. 2010;15:255–261. doi: 10.3746/pnf.2012.17.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung HJ, Kim MJ, Lim SH, Park JH, Lee HK, Ahn KS, Lee JW. The effect of extract of Paeonia lactiflora on the improvement of ischemic acute renal failure. Korean J Nephrol. 2009;28:180–189. [Google Scholar]
- 14.Kuriakose GC, Kurup MG. Effects of Aulosura fertilisima against cisplatin-induced nephrotoxicity and oxidative stress in rats. Ren Fail. 2010;32:224–233. doi: 10.3109/08860220903541143. [DOI] [PubMed] [Google Scholar]
- 15.Lim SH, Song KS, Lee JW. Butyrate and propionate, short chain fatty acids, attenuate myocardial damages by inhibition of apoptosis in a rat model of ischemia-reperfusion. J Korean Soc Appl Biol Chem. 2010;53:570–577. [Google Scholar]
- 16.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intracellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1799s. [PubMed] [Google Scholar]
- 18.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 20.Erdogan H, Fadillioglu E, Yagmurca M, Ucar M, Irmak MK. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006;34:41–46. doi: 10.1007/s00240-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Levey AS. Measurement of kidney function. Med Clin N Am. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Kirkby K, Baylis C, Agarwal A, Croker B, Archer L, Adin C. Intravenous bilirubin provides incomplete protection against renal ischemia-reperfusion injury in vivo. Am J Physiol Renal Physiol. 2007;292:F888–F894. doi: 10.1152/ajprenal.00064.2006. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux P. The use of the stable free radical diphe-nylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. [Google Scholar]
- 24.Korkmaz A, Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Ren Fail. 2009;31:36–43. doi: 10.1080/08860220802546271. [DOI] [PubMed] [Google Scholar]
- 25.Leelavinothan P, Kalist S. Beneficial effect of hesperetin on cadmium induced oxidative stress in rats: an in vivo and in vitro study. Eur Rev Med Pharmacol Sci. 2011;15:992–1002. [PubMed] [Google Scholar]
- 26.Xu R, Shang N, Li P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe. 2011;17:226–231. doi: 10.1016/j.anaerobe.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Cadetg P, Ruf R, Mazzuchelli L, Ferrari P, Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kid Int. 2003;63:1564–1573. doi: 10.1046/j.1523-1755.2003.00897.x. [DOI] [PubMed] [Google Scholar]
- 28.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kid Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien CT, Chang TC, Tsai CY, Shyue SK, Lai MK. Adenovirus-mediated Bcl-2 gene transfer inhibits renal ischemia/reperfusion induced tubular oxidative stress and apoptosis. Am J Transplantation. 2005;5:1194–1203. doi: 10.1111/j.1600-6143.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- 30.Coleman MD, Shaefi S, Sladen RN. Preventing acute kidney injury after cardiac surgery. Curr Opin Anesthesiol. 2011;24:70–76. doi: 10.1097/ACO.0b013e3283422ebc. [DOI] [PubMed] [Google Scholar]
- 31.Patel NN, Rogers CA, Angelini GD, Murphy GJ. Pharmacological therapies for the prevention of acute kidney injury following cardiac surgery: a systematic review. Heart Fail Rev. 2011;16:553–567. doi: 10.1007/s10741-011-9235-5. [DOI] [PubMed] [Google Scholar]