Abstract
This study was designed to investigate whether the fatty acid composition could make a significant contribution to the oxidation stability of vegetable oils marketed in Korea. Ten kinds, 97 items of vegetable oils that were produced in either an industrialized or a traditional way were collected and analyzed for their fatty acid compositions and lipid oxidation products, in the absence or presence of oxidative stress. Peroxidability index (PI) calculations based on the fatty acid composition ranged from 7.10 to 111.87 with the lowest value found in olive oils and the highest in perilla oils. In the absence of induced oxidative stress, malondialdehyde (MDA), the secondary lipid oxidation product, was generated more in the oils with higher PI (r=0.890), while the tendency was not observed when the oils were subjected to an oxidation-accelerating system. In the presence of the oxidative stress, the perilla oils produced in an industrialized manner generated appreciably higher amounts of MDA than those produced in a traditional way, although both types of oils presented similar PIs. The results implicate that the fatty acid compositions could be a predictor for the oxidation stability of the vegetable oils at the early stage of oil oxidation, but not for those at a later stage of oxidation.
Keywords: vegetable oil, oxidation stability, fatty acid composition, peroxidability index (PI), malondialdehyde (MDA)
INTRODUCTION
Vegetable oils are used for various food applications, such as cooking oils, fats for frying, and as ingredients in emulsions-based products or bakery products (1). However, they are somewhat limited in their usage because of their susceptibility to oxidation (2). Polyunsaturated fatty acids (PUFA), which are specifically abundant in the vegetable oils, are major substrates for oil oxidation, and their oxidative degradation products deteriorate the chemical, sensory, and nutritional properties of the oils (1–4). Thus, the oxidation state of vegetable oils has been recognized as one of the most important parameters for the quality assessment of the oils (3). In particular, the levels of hydroperoxides and malondialdehyde (MDA), corresponding to the primary and secondary lipid oxidation products, respectively, have been used as indicators to reflect oxidation state of the oils.
During the oil’s lipid oxidation, there is a web of complex oxidation processes involved in the generation of oxidative degradation products from their precursor fatty acids. That is, external factors, such as light, elevated temperatures, and oxygen (1,2), and intrinsic factors, such as antioxidants, prooxidants and water in the oils, might simultaneously operate in a complementary or opposite way to affect the fatty acids (1,4). Thus, it has been difficult to single out a strong predictor that could assume future oil behavior before the oil oxidation actually proceeds.
Various types of vegetable oils from different sources and with different fatty acid composition have been marketed in Korea. Korean daily intake of vegetable oils has increased 2.5-fold from 3.1 to 7.7 g/day for the last three decades (5). Considering the fact that the Korean vegetable oils are rich in PUFAs that are easily oxidized (6,7), it would be useful to have a strong predictor for the oxidation stability of the vegetable oils in term of food safety as well as oil quality.
In this study, we investigated whether the fatty acid composition alone could be used as a strong predictor for the oxidation susceptibility of the vegetable oils. To achieve this goal, various vegetable oils that were produced in either an industrialized or a traditional way were collected and analyzed for their fatty acid compositions and oxidation products under two specific conditions, which were: (i) in the absence of induced oxidative stress, which was to reflect the early stage of oil auto-oxidation, and (ii) in the presence of induced oxidative stress using an oxidation-accelerating system, which was to reflect the condition where oxidation was facilitated, such as occurs in the late stages of oxidation.
MATERIALS AND METHODS
Chemical materials
All the reagents were of the highest grade commercially available. HPLC-grade methanol, isooctane and benzene were purchased from Fisher Scientific (Chicago, IL, USA). Tridecanoic acid, acetyl chloride, cumene hydroperoxide, xylenol orange, and malondialdehyde precursor 1,1,3,3-tetraethoxypropane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fatty acid methyl ester mixture was purchased from Supelco (Bellefonte, PA, USA). Butylated hydroxy toluene (BHT) and ascorbic acid were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Trichloroacetic acid (TCA) and Karl Fisher reagent (Hydranal-Composite 5) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Riedel-de Haën® (Seelze, Germany), respectively. p-Anisidine (4-methoxyaniline) and 2-thiobarbituric acid (TBA) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Diethylether and ferrous sulfate (FeSO4) were obtained from Showa Chemical Industry Co., Ltd. (Tokyo, Japan). Distilled and deionized water was used for the preparation of all solutions.
Sample preparation
A total 10 kinds, 97 items of different vegetable oils commercially available on the Korean markets were selected for the determination of the oxidative stability of edible oils (Table 1). The oil samples were purchased from food sections in supermarkets or grocery markets in December 2009. The perilla oil and sesame oil samples include some purchased from traditional local markets (n=12 for sesame oil and n=18 for perilla oil) as well as some purchased from food sections in department stores or grocery markets (n=14 for sesame oil and n=7 for perilla oil). Compared with soybean oils and corn oils, which are mostly manufactured in an industrialized manner, perilla and sesame oils are frequently pressed and distributed through local shops. Thus, they were selected to determine whether the oxidative stability depended on their manufacturing process. The oil samples were kept at room temperature (around 19°C) without opening until analysis to mimic a typical circumstance where oils were stored prior to being consumed.
Table 1.
Information on the vegetable oils used in this study1)
| Vegetable oils (Abbr., n) | Raw materials | Raw material origin | Bottle materials |
|---|---|---|---|
| Soybean oil (SO, 7) | Soybean 100% | Imported | Polyethylene terephthalate or polyethylene |
| Corn oil (CO, 4) | Germ of corn (maize) 100% | Imported | Polyethylene terephthalate or polyethylene |
| Olive oil (OvO, 16) | Olive 100% | Imported | Polyethylene terephthalate or glass (amber or transparent) bottle |
| Canola oil (CaO, 4) | Canolar (rapeseed) seed 100% | Imported | Polyethylene terephthalate |
| Grapeseed oil (GsO, 11) | Grapeseed 100% | Imported | Polyethylene terephthalate or glass (amber or transparent) bottle |
| Red pepper flavored oil (RpO, 1) | Soybean oil (93%), red pepper powder, red pepper seed | Imported | Glass transparent bottle |
| Hazelnut oil (HO, 1) | Hazelnut 99.9% | Imported | Glass transparent bottle |
| Functional oil (FO, 2) | Esterified canola oil with medium chain fatty acids (MCFA) | Imported | Polyethylene terephthalate |
| Sesame oil (SeO, 26) | |||
| Industrialized (14) | Sesame seed 100% | Domestic (n=2)/Imported (n=12) | Glass (amber) bottle |
| Traditional (12) | Sesame seed 100% | Domestic (n=3)/Imported (n=9) | Glass (amber) bottle |
| Perilla oil (PO, 25) | |||
| Industrialized (7) | Perilla seed 100% | Domestic (n=1)/Imported (n=6) | Glass (amber) bottle |
| Traditional (18) | Perilla seed 100% | Domestic (n=9)/Imported (n=9) | Glass (amber) bottle |
Among the various contents written on the ‘product label’ of the vegetable oils, the information that might be related to the lipid oxidation of the oils was extracted and listed. The vegetable oils produced in an industrialized manner were purchased after checking their ‘best by’ dates, thus there were no oil samples whose ‘best by’ dates passed by.
Fatty acid analysis
Fatty acids in the oil samples were converted to their corresponding fatty acid methyl esters (FAME) according to the method of Lepage and Roy (8). In detail, 2 mL of methanol/benzene 4:1 (v/v) solution was added to 0.1 g of the oil sample. One-hundred μg of tridecanoic acid (C13:0) was included in the solution as an internal standard. While stirring, 200 μL of acetyl chloride was slowly added. Glass sample tubes were tightly closed with Teflon-lined caps and subjected to methanolysis at 100°C for 1 hr. Tubes were weighed before and after heating to check the leakages of any volatile contents. After the tubes had been cooled in water, 5 mL of 6% aqueous K2CO3 was slowly added to stop the reaction and neutralize the mixture. The reaction mixture was centrifuged (5810R, Eppendorf, Hamburg, Germany) at 1,811 × g, 10°C for 10 min and an aliquot of benzene upper phase was subjected to GC. All of these reactions were performed in triplicate for each sample.
Analysis of FAME was performed by a GC (YL 6100GC, Yonglin, Anyang, Korea) on a fused silica column (J&W Durawax 30.0 m × 320 μm × 0.25 mm i.d., J&W Scientific, Folsom, CA, USA). The carrier gas was nitrogen at a flow rate of 1.0 mL/min and detector mode was flame ionization detector (FID). Ten μL of the sample was injected with a split ratio of 5:1 and was run in constant flow mode. Chromatographic conditions were as follows: injector and detector temperatures, 200°C; initial oven temperature 90°C for 5 min, rising to 180°C at 10°C/min with a hold time of 3 min, to 195°C at 3 °C/min, to 199°C at 1°C/min, to 202°C at 4°C/min with a hold time of 5 min, and then to 240°C at 1°C/min with a final hold time of 20 min. Each peak was identified by comparison with the retention times of 33 known fatty acids standards. Fatty acids were quantified from the standard curves of individual fatty acids because saturated fatty acids were reported to show higher GC-FID response than unsaturated fatty acids (8). Based on the fatty acid compositions of the oils, intrinsic peroxidability index (PI) of each type of oil was calculated as follows (9); PI=(% monoenoic FA × 0.025) + (% dienoic FA × 1) + (% trienoic FA × 2) + (% tetraenoic FA × 4) + (% pentaenoic FA×6) + (% hexaenoic FA × 8).
Moisture determination
Moisture in the vegetable oils was determined according to Karl Fischer titration that could quantify the trace amounts of the water in a sample (10). The method is based on the following reaction: one mole of I2 in Karl Fisher reagent that consists of methanol, pyridine, SO2, and a known concentration of I2 was consumed for each mole of water. The titration was performed using Karl Fisher potentiometric titrator (702 SM Titrino, Metrohm, Flawil, Switzerland).
Lipid hydroperoxide determination
Lipid hydroperoxides formed in the oils were spectrophotometrically measured according to ferrous oxidation/xylenol orange method that based on the ability of lipid peroxides to oxidize ferrous ions at low pH (11). 0.1 g of oils were weighed into a glass tube and added with BHT (0.01 g per sample) to prevent further oxidation. Then, they were added with 9.9 mL of chloroform/methanol 7:3 (v/v) solution and vortexed for 5 sec. The sample solutions were sequentially added with 50 μL of 10 mM xylenol orange and 50 μL of iron (II) chloride and vortexed thoroughly. A tube that contained all of the reaction solutions except the oil sample was used as a blank. After allowing the reaction mixtures to stand exactly 5 min at room temperature, their absorbances were determined at 560 nm using UV-visible spectrophotometer (UV-1650, Shimazu, Kyoto, Japan). Lipid hydroperoxide in the oils was quantified by referencing to a calibration line constructed with cumene hydroperoxide as an external standard.
MDA determination
Malondialdehyde (MDA), as secondary oxidation product, was measured according to TBA method (12). The assay is based on the reaction between 2 molecules of TBA with 1 molecule of MDA during heating at acid pH to produce a red pigment with a maximum absorbance at 532 nm. 0.1 g of the oil was accurately weighed into a 25-mL volumetric flask, and BHT (0.01 g per sample) was immediately added to prevent further oxidation. Then, 5 mL of 1-butanol was added and vortexed thoroughly for dissolution. Five mL of 0.2% TBA in 1-butanol was added to the sample solution. After vortexing, the mixture was incubated at 95°C in a water bath for 2 hr, and then it was quickly cooled under cold running tap water to stop the reaction. The resulting solution was allowed to stand at room temperature for 10 min to stabilize the chromogenic MDA-TBA complex, and then its absorbance was measured using UV-visible spectrophotometer at 532 nm. Standard curve for the quantification of MDA was constructed by plotting the content of MDA against the absorbance of its complex, using 1,1,3,3-tetraethoxypropane as an MDA precursor. The MDA content in the oil was determined by referencing to the calibration curve.
p-Anisidine value determination
The amount of non-volatile aldehydes (principally 2-alkenals) in the oils was measured using p-anisidine test, the AOCS official method for determining levels of the aldehydes (13). A 0.5 g of the oil sample was accurately weighed in a 25-mL volumetric flask and mixed with 10 mL isooctane for dissolution. The solution was filled up to 25 mL with additional isooctane, and its absorbance (Ab) at 350 nm was measured with the iso-octane as a reagent blank. 5 mL of the solution was then pipetted into a test tube and 5 mL of isooctane into another test tube. 1 mL of the p-anisidine solution (0.25 g p-anisidine/100 mL glacial acetic acid) was added to both test tubes and the solutions were mixed. After 10 minutes, the absorbance (As) of the sample solution was read with the tube containing isooctane and p-anisidine as a blank. The p-anisidine value (p-AV) was calculated using the following equation: p-AV=25(As − Ab)/m, where As is the absorbance of the oil solution after reaction with the p-anisidine reagent, Ab is the absorbance of the oil solution, and m is the mass (g) of the test portion. The test was conducted in triplicates for all samples.
Susceptibility to lipid oxidation
Lipid oxidation susceptibility of the oils was determined by measuring MDA that was induced by the addition of iron and ascorbic acid into the oil samples (14). 0.5 g of oil was weighed into a 50 mL-tube and mixed thoroughly with 21 mL of 1.15% KCl. 2.5 mL of the oil solution was added with 12.5 mL of 80 mM Tris-ma-late buffer (pH 7.4), 5 mL of 5 mM FeSO4·7H2O and 5 mL of 2 mM ascorbic acid, sequentially. The reaction mixture was vortexed for 30 sec, and then incubated at 37°C in a water bath. At specific times (0, 20, 40, 60, 90, 150, 210, 270, and 330 min), a 2 mL aliquot was taken from each of the incubated reaction mixtures, and mixed with 4 mL TCA-TBA-HCl reagent (15% TCA, 0.37% TBA in 0.25 N HCl). After vortexing thoroughly, it was heated at 100°C for 15 min. Then, the reaction mixture was cooled under cold tap water and centrifuged at 1,811 × g for 15 min (5810R, Eppendorf). The absorbance reflecting the amounts of MDA-TBA complex was measured at 532 nm, as described above.
Statistical analysis
All experiments were conducted in triplicate. Averages and standard deviations were calculated from these triplicate measurements. Analysis of variance (ANOVA) and Duncan’s multiple range tests were performed using the SAS program version 9.1 for windows (Cary, NC, USA) to express the difference among the oil groups. Pearson’s correlation between the assessed indexes was done with the SPSS 12.0 for Windows statistical package (Chicago, IL, USA).
RESULTS AND DISCUSSION
Fatty acid composition of the vegetable oils
Fatty acids as the precursors of lipid oxidation products were determined for the 10 kinds 97 items of vegetable oils commercially available on the Korean market (Table 2). Fatty acid composition of the vegetable oils varied appreciably with the type of vegetable oils, but not with oil processing (traditional vs. industrial) within the same type of vegetable oils (e.g., sesame oils and perilla oils). The vegetable oils covered a wide range of polyunsaturated fatty acid (PUFA) to saturated fatty acid (SFA) ratios (P/S; 0.24 ~ 3.80) and peroxidability index (PI; 7.10 ~ 111.87) calculated based on the fatty acids composition, with both values being the lowest in olive oils and highest in perilla oils. Thus, the selected oils appeared proper for determining whether the fatty acid composition was a dominating factor of the vegetable oils’ oxidation over various intrinsic antioxidants/pro-oxidants. It should be noted that the perilla oils presented 3-fold higher PI value compared to the sesame oils, although both oils have been used for the same cooking purpose (i.e. seasoning) in Korea. Therefore, for the remainder of this study, we investigated whether the difference in PI could make a significant contribution to the oxidation susceptibility of the vegetable oils which Koreans have been consuming. For this purpose, the oils were evaluated for their oxidation states (i) as they were (i.e., without induced oxidative stress) and (ii) under an oxidation-accelerating system (i.e., with induced oxidative stress) as well.
Table 2.
Classified fatty acid compositions, fatty acids-related nutritional index, and peroxidability index (PI) of various vegetable oils1)
| Vegetable oils | SFA | MUFA | PUFA | n-3 PUFA | n-6 PUFA | P/S | n-6/n-3 | PI |
|---|---|---|---|---|---|---|---|---|
| SO | 34.64 | 15.84 | 49.52 | 0.42 | 49.10 | 1.43 | 117.84 | 50.33 |
| CO | 23.89 | 31.34 | 44.76 | 0.37 | 44.39 | 1.87 | 119.81 | 45.92 |
| OvO | 21.08 | 73.97 | 4.95 | 0.30 | 4.66 | 0.24 | 15.62 | 7.10 |
| CaO | 18.07 | 61.48 | 20.45 | 0.52 | 19.93 | 1.13 | 38.28 | 22.51 |
| GsO | 20.89 | 22.91 | 56.21 | 0.17 | 56.04 | 2.69 | 326.01 | 56.95 |
| RpO | 23.15 | 28.15 | 48.70 | 0.43 | 48.27 | 2.10 | 113.12 | 49.83 |
| HO | 10.72 | 78.64 | 10.64 | 0.21 | 10.43 | 0.99 | 48.62 | 12.82 |
| FO | 43.41 | 45.76 | 10.84 | 0.37 | 10.47 | 0.25 | 28.12 | 12.35 |
| SeO, industrial | 26.00 | 39.36 | 34.64 | 0.45 | 34.18 | 1.33 | 80.90 | 36.08 |
| SeO, traditional | 27.21 | 37.73 | 35.06 | 0.43 | 34.63 | 1.30 | 87.52 | 36.44 |
| PO, industrial | 16.15 | 22.51 | 61.35 | 49.96 | 11.39 | 3.80 | 0.23 | 111.87 |
| PO, traditional | 16.61 | 21.91 | 61.48 | 49.59 | 11.89 | 3.70 | 0.24 | 111.62 |
Values for SFA, MUFA, PUFA, n-3 PUFA, and n-6 PUFA are average percentages (%) of total fatty acids. The relative standard deviation of each value was less than 10%. P/S and n-6/n-3 are ratios of PUFA to SFA and n-6 to n-3, respectively. PI of the vegetable oils was calculated using the equation described in MATERIALS AND METHODS.
In the absence of induced oxidative stress
Moisture contents of the various vegetable oils, as a factor influencing lipid oxidation, satisfied the standard specification (≤0.1%) for fresh vegetable oils by ranging 0.03 to 0.08%, except the perilla oils that were produced in a traditional way (0.11%, relative standard deviation=163%). Generally, as the water content is increased in a food system, the rate of lipid oxidation tends to be increased proportionally, which is attributed to the increase in the mobility of reactants such as transition metals and oxygen (15).
Oxidation states of the 10 kinds 97 items of vegetable oils were assessed by measuring hydroperoxides as primary oxidation products, and MDA and non-volatile 2-alkenals as secondary oxidation products. The initial hydroperoxide and MDA contents were differed by up to two orders of magnitude, depending on the type of vegetable oils, with each ranging from 6.4 to 700.3 μg/g and 36.5 to 1,087.2 μg/g, respectively (Table 3). However, the p-anisidine values, which reflect the levels of α,β-unsaturated aldehydes, were narrowly distributed and so were not significantly different (Table 3). There was little quantitative correlation between the hydroperoxide levels and PI of the oils, presumably due to the decomposition following the accumulation of the initially formed hydroperoxides (15). On the contrary, the MDA contents detected in the oils were proportionally correlated to the PI of the oils (r=0.890), e.g., the olive oils and hazelnut oil that presented the lowest PI values produced the lowest amounts of MDA (each 50.4 and 36.5 μg/g), while the perilla oils that presented the highest PI generated the highest amounts of MDA without regard to the oil processing method (1,080.3 μg/g for industrial and 1,087.2 μg/g for traditional). The resistance of the olive oils and hazelnut oil to oxidation might have been partially due to their compositions being low in polyunsaturated fatty acids compared to other types of vegetable oils (Table 2). It has been well established that the ease of formation of fatty acid radicals increases with increasing unsaturation, e.g., oleic acid (C18:1) has been estimated to be 10 ~ 40 times less susceptible to oxidation than linoleic acid (C18:2) (15). On the other hand, the MDA levels observed in the perilla oils were up to 20-fold higher than those of the sesame oils (Table 3). The difference could be explained by the fact that the perilla oils had a higher proportion of linolenic acid (C18:3, n-3), which was far more readily oxidized than linoleic acid (C18:2, n-6), the major fatty acid of the sesame oils. In most cases, linolenic acid has been known to oxidize twice as fast as linoleic acid because of the decrease in bond dissociation energy caused by the addition of a methylene-interrupted carbon (15). The above results confirmed that the initial composition of fatty acids was one of the factors that could determine the levels of the aldehyde (1). Among the fatty acids, n-3 PUFAs have been of particular interest since epidemiological and clinical researches have revealed possible effects of the n-3 PUFA on brain development and curative and/or preventive effects on cardiovascular disease (16,17). However, the current results imply that the n-3 PUFA-containing foods (here, perilla oils) might adversely affect human health because of their susceptibility to lipid oxidation.
Table 3.
Moisture and lipid oxidation products in various vegetable oils1)
| Vegetable oils | Moisture (%) | Hydroperoxides (μg/g) | MDA (μg/g)2) | p-Anisidine value3) |
|---|---|---|---|---|
| SO | 0.08 ± 0.01a | 341.0 ± 9.3e | 180.7 ± 13.8bc | 4.8 ± 5.5ab |
| CO | 0.04 ± 0.01b | 280.8 ± 6.8ef | 85.5 ± 3.3bcd | 10.0 ± 7.0a |
| OvO | 0.08 ± 0.01a | 618.7 ± 32.5bc | 50.4 ± 2.2cd | 5.8 ± 3.6ab |
| CaO | 0.07 ± 0.01a | 569.9 ± 5.9cd | 187.5 ± 14.3b | 6.8 ± 1.7ab |
| GsO | 0.07 ± 0.01a | 660.9 ± 47.5ab | 62.8 ± 9.7bcd | 5.0 ± 5.1ab |
| RpO | 0.05 ± 0.02b | 598.0 ± 26.3bc | 172.8 ± 13.9bc | 0.4 ± 0.4b |
| HO | 0.03 ± 0.01b | 700.3 ± 17.2a | 36.5 ± 2.4d | 3.2 ± 4.8ab |
| FO | 0.08 ± 0.00a | 249.9 ± 8.9f | 124.0 ± 5.8bcd | 3.6 ± 0.7ab |
| SeO, industrial | 0.05 ± 0.03b | 44.5 ± 49.7h | 56.4 ± 5.2bcd | 1.4 ± 0.3b |
| SeO, traditional | 0.04 ± 0.02b | 6.4 ± 11.7i | 83.0 ± 27.1bc | 1.1 ± 5.0b |
| PO, industrial | 0.05 ± 0.02b | 157.8 ± 55.9g | 1080.3 ± 291.5a | 1.2 ± 4.1b |
| PO, traditional | 0.11 ± 0.18a | 66.6 ± 23.6h | 1087.2 ± 169.2a | 3.8 ± 1.0ab |
|
| ||||
| Significance | p<0.001 | p<0.001 | p<0.001 | NS |
Values with different superscripts in the same column are significantly different. NS means not significant.
The method for MDA determination employed the incubation step of reaction mixtures (sample + chemicals) for 2 hr in a 95°C water bath, thus MDA could be artificially formed from its precursor fatty acids during the analysis.
The value reflects the amount of non-volatile aldehydes, principally 2-alkenals.
The observation that the MDA contents formed in the vegetable oils without oxidative stress were somewhat directly proportional to their PI (r=0.890), suggested that the fatty acid composition (or PI derived from them) of the oils appeared to be a strong indicative factor that could predict the oxidative state and quality of the oils at the early stage of oil oxidation (i.e., right after opening); however, whether the fatty acid composition could predict later oil behavior (i.e., the late stage of oil oxidation where oxidation rate increases exponentially) was uncertain. For this reason, the resistance of oils was further assessed in a condition where oxidation was facilitated. Then, it was determined if the fatty acid composition (or PI) could still serve as a strong predictor for the oxidation stability of Korean vegetable oils that were at later stage of oxidation or exposed to a severe oxidation condition.
In the presence of induced oxidative stress
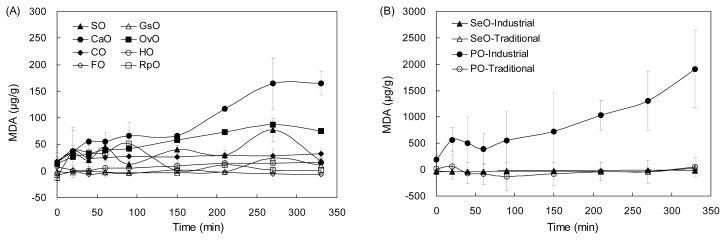
To predict the oxidation stability of the various vegetable oils over long periods of time, the oils were subjected to the oxidation-accelerating system where radicals could be generated, and then the secondary oxidation product MDA was determined as a function of incubation time (Fig. 1). The oxidation susceptibility varied with the types of vegetable oils as well as with the oil processing (traditional vs. industrial) within the same type of vegetable oils (e.g., perilla oils). As the incubation time increased, there was a small amount of MDA generated in the corn oils, grape seed oils, red pepper flavored oil, hazelnut oil, functional oils, and sesame oils. On the contrary, MDA contents tended to increase through the whole incubation time in the olive oils, canola oils, and perilla oils. As for the olive oils and sesame oils, the PI value of the sesame oils (~36) was 5-fold higher than that of olive oils (~7). Thus one might have expected that the olive oils would be less prone to oxidation, compared with the sesame oils. However, when the both oils were subjected to an oxidation-accelerating system, the olive oils generated MDA increasingly with the incubation time while there was no noticeable increase observed for the sesame oils (Fig. 1). In spite of the favorable fatty acid composition of the olive oils, their weak resistance to oxidation might be due to the presence of endogenous chlorophylls and carotenoids (1). The chlorophylls and carotenoids could add radicals because of their conjugated double bond structures, which might accelerate the lipid oxidation chain reaction in the olive oils and thus finally generated the MDA increasingly. On the other hand, the sesame oils showed superior oxidative stability despite their rather unsaturated fatty acid composition, which could be attributed to the abundance of anti-oxidants in the oils, such as sesamin, sesamolin, sesamol, γ-tocopherol, and Maillard reaction products with high antioxidative potential (1,18). As observed here, the fatty acid compositions of the oils could not explain why sesame oils showed better oxidation stability than the olive oils under the oxidation-accelerating system. Similar phenomenon was also observed for the perilla oils. According to the fatty acids composition, the PI values of the perilla oils were not appreciably different depending on the oil processing, with the linolenic acid being predominant and its level being narrowly-distributed (relative standard deviation=2%). In addition, there was no significant difference observed in the amounts of MDA when the both types of perilla oils were tested for their oxidation state as they were (Table 3). However, under the oxidation-accelerating system, the perilla oils produced industrially generated considerable amounts of MDA with a great variation among the individual oil samples (i.e., large standard deviation at each incubation time), while MDA levels remained constant for the perilla oils produced traditionally (Fig. 1B). All the above results implicated that the oxidative stability of the vegetable oils under the conditions where radicals were constantly generated (such as frying at high temperature or long-term storage at room temperature) might depend more on factors other than the fatty acids composition, e.g., endogenous components, including prooxidants and antioxidants. There are a couple of possible explanations for the different oxidation susceptibility of the perilla oils depending on the processing type. Firstly, the oils produced in an industrial manner might have gone through additional processing steps following roasting and pressing, which might have removed or destroyed compounds that could protect the oils from oxidation (4,19), consequently much lower amounts of antioxidants might have been present in the oils. It was reported that filtered oils were less stable than cloudy oils containing suspended and dispersed materials because these suspended materials could play a stabilizing role by acting as antioxidants (4). Apparently, the perilla oils produced in a traditional way contained more amounts of suspended materials than their counterpart oils. Secondly, the parameters conditions (roasting conditions such as temperature and duration) at the individual processing step might have varied from company to company, which could have eventually led to the large variation of MDA values at each incubation time. Lastly, initial state of source seeds subjected to processing might have varied from company to company (e.g., whole seeds vs. seeds flours) or their origins might have varied (domestic seeds vs. imported ones) (Table 1). Oils made from whole seeds had higher oxidative stability than those from their flour-counterparts, and antioxidants contents of the oils varied with the origins of seeds (20).
Fig. 1.
MDA contents that were generated from the various vegetable oils with induced oxidative stress. The MDA was monitored as a function of incubation time under an oxidation-accelerating system. Refer to Table 1 for the abbreviations.
Taken together, the results indicate the fatty acid composition (or PI derived from them) could be used as a predictor for initial oxidation state or quality of the vegetable oils. However, under the condition where oil oxidation could be facilitated (e.g., frying at high temperature or storing for a long time after opening), the fatty acid composition might not make an appreciable contribution to determining the oxidative stability of the vegetable oils.
Additionally, it should be noted that, in this study, the oxidation stability of the perilla oils depended on the processing method. The current findings suggest that the n-3 PUFA in the perilla oils could be consumed without loss of functionality under well-defined processing conditions. Therefore, future studies exploring major endogenous components that make a significant difference to the oxidation stability of the perilla oils depending on the processing type are warranted.
ACKNOWLEDGMENTS
This project was conducted by the generous financial support of the Youlchon Foundation (Nongshim Corporation and its affiliated companies) in Korea.
REFERENCES
- 1.Kamal-Eldin A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur J Lipid Sci Technol. 2006;58:1051–1061. [Google Scholar]
- 2.Merrill LI, Pike OA, Ogden LV, Dunn ML. Oxidative stability of conventional and high-oleic vegetable oils with added antioxidants. J Am Oil Chem Soc. 2008;85:771–776. [Google Scholar]
- 3.Kowalski B, Ratusz K, Kowalska D, Bekas W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and rancimat measurements. Eur J Lipid Sci Technol. 2004;106:165–169. [Google Scholar]
- 4.Velasco J, Dobarganes C. Oxidative stability of virgin olive oil. Eur J Lipid Sci Technol. 2002;104:661–676. [Google Scholar]
- 5.KHIDI. National Food & Nutrition Statistics: based on 2008 Korea National Health and Nutrition Examination Survey. Korea Health Industry Development Institute; Seoul, Korea: 2010. pp. 8–60. [Google Scholar]
- 6.Surh J, Kwon H. Quantification of 4-hydroxyalkenals in oils consumed in Korea. Korean J Food Sci Technol. 2002;34:905–910. [Google Scholar]
- 7.Surh J, Kwon H. Estimation of daily exposure to 4-hydroxy-2-alkenals in Korean foods containing n-3 and n-6 polyunsaturated fatty acids. Food Addit Contam. 2005;22:701–708. doi: 10.1080/02652030500164359. [DOI] [PubMed] [Google Scholar]
- 8.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 9.Cortinas L, Galobart J, Barroeta AC, Baucells MD, Grashorn MA. Change in a-tocopherol contents, lipid oxidation and fatty acid profile in eggs enriched with linolenic acid or very long-chain w3 polyunsaturated fatty acids after different processing methods. J Sci Food Agr. 2003;83:820–829. [Google Scholar]
- 10.Ruiz RP. Karl Fischer titration. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Handbook of Food Analytical Chemistry. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2004. pp. 13–16. [Google Scholar]
- 11.Pegg RB. Measurement of primary lipid oxidation products. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Handbook of Food Analytical Chemistry. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2004. pp. 531–564. [Google Scholar]
- 12.Pegg RB. Spectrophotometric measurement of secondary lipid oxidation products. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Handbook of Food Analytical Chemistry. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2004. pp. 547–564. [Google Scholar]
- 13.Tompkins C, Perkins EG. The evaluation of frying oils with the p-anisidine value. J Am Oil Chem Soc. 1999;76:945–947. [Google Scholar]
- 14.Kornbrust DJ, Mavis RD. Relative susceptibility of microsomes from lung, heart, liver, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids. 1980;15:315–322. doi: 10.1007/BF02533546. [DOI] [PubMed] [Google Scholar]
- 15.McClements DJ, Decker EA. Lipids. In: Damodaran S, Parkin KL, Fennema OR, editors. Food Chemistry. CRC Press; Boca Raton, FL, USA: 2008. pp. 155–216. [Google Scholar]
- 16.Simopoulos AP. Summary of NATO advanced research workshop on dietary w-3 and w-6 fatty acids: Biological effects and nutritional essentiality. J Nutr. 1989;19:521–528. doi: 10.1093/jn/119.4.521. [DOI] [PubMed] [Google Scholar]
- 17.Surai PF, Sparks NHC. Designer eggs: from improvement of egg composition to functional food. Trends Food Sci Technol. 2001;12:7–16. [Google Scholar]
- 18.Shahidi F, Amarowicz R, Abou-Gharbia HA, Shehata AAY. Endogenous antioxidants and stability of sesame oil as affected by processing and storage. J Am Oil Chem Soc. 1997;74:143–148. [Google Scholar]
- 19.Koski A, Psomiadou E, Tsimidou M, Hopia A, Kefalas P, Wahala K, Heinonen M. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur Food Res Technol. 2002;214:294–298. [Google Scholar]
- 20.Kim SH, Kim IH, Kim JO, Lee GD. Comparision of components of sesame oils extracted from sesame flour and whole sesame. Korean J Food Preserv. 2002;9:67–73. [Google Scholar]