Abstract
The purpose of this study was to investigate the anti-allergy activities of persimmon leaf extract (PLE) on a phthalic anhydride (PA)-induced allergic mouse model. A human leukemic mast cell line (HMC-1) was used to examine the inhibitory activity of PLE on the histamine release by human leukemic mast cells. PLE inhibited histamine release from HMC-1 cells in response to cross-linkage of high-affinity IgE receptor-α (FcεRIα). Additionally, a PA-induced allergic mouse model was used to investigate the effects of PLE in vivo. Mice were orally administrated with or without PLE of single dose (250 mg/kg/day) for 31 days. Oral intake of PLE significantly inhibited passive cutaneous reactions. Oral administration of PLE to PA-induced allergic mice also led to a striking suppression of the development of contact dermatitis, ear swelling and lymph node weight. In addition, PA-specific IL-4 production of draining lymph node cells was markedly diminished by PLE oral administration, but not IFN-γ. Furthermore, PLE treatment suppressed PA-induced thymus and activation-regulated chemokine (CCL17) and cutaneous T cell-attracting chemokine (CCL27) expressions in ear tissues. Based on these results, we suggest that PLE may have therapeutic potential as an effective material for management of irritant contact dermatitis or related inflammatory diseases.
Keywords: persimmon leaf extract, phthalic anhydride, contact dermatitis, IgE, chemokines
INTRODUCTION
Phthalic anhydride (PA) is a low-molecular-weight compound and an important industrial chemical, especially for the large scale production of plasticizers for plastics. Exposure to PA causes irritation and can lead to immune-mediated allergic diseases such as contact urticaria, rhinitis, conjunctivitis, and atopic dermatitis upon repeat exposure (1). These disorders affect 10∼15% of Western populations, and their prevalence has doubled in the last 10∼15 years (2). This increase has included allergic skin disorders such as atopic or contact dermatitis accompanied by irritant dermatitis in the site at which contact with an allergen has occurred (3). Such allergic responses are characterized by infiltration by specific cells, including inflammatory cells such as T lymphocytes, neutrophils, eosinophils, macrophages and mast cells (4–6). Activation of inflammatory cells by this infiltration is mediated by various cytokines and chemokines at the site of injury.
For example, interleukin (IL)-4, IL-5, and IL-13 produced by Type 2 helper (Th2) cells are responsible for increased serum IgE levels and the presence of blood eosinophilia (7). In particular, enhanced IL-4 is a hallmark of acute allergic skin disease (8) and inhibits ceramide synthesis, and exogenous applications of IL-4 impede recovery of the permeability barrier after acute perturbations (9). Additionally, chemokines such as keratinocyte-derived chemokine were recently found to be the most potent molecules involved in the regulation of selective recruitment of inflammatory cells. These mainly include thymus and activation-regulated chemokine (CCL17), which acts specifically on the chemokine receptor CCR4, and cutaneous T cell-attracting chemokine (CCL27), which acts specifically on the chemokine receptor CCR10 (10,11). The serum concentrations of these Th2 chemokines correlate with the severity of allergic skin diseases (12). Therefore, agents that regulate these inflammatory molecules could provide beneficial anti-inflammatory effects.
Traditionally, persimmon (Diospyros kaki) leaf tea is believed to be a health-promoting beverage, similar to green tea in Korea and Japan (13). Recently, it was reported that flavonoids from persimmon leaf inhibited the adhesion between lymphocytes and neurons (14); however, there is no report on persimmon leaf’s protective effect against PA-induced skin inflammation. Therefore, the purpose of this study was to investigate the protective effect of PLE on PA-induced inflammation in HMC-1 cells and mouse models.
MATERIALS AND METHODS
Plant material
Persimmon (D. kaki) leaf materials used in the present study were collected on 20 Jun 2010 from Bugui-myen and Jeongcheon-myen, Jinan-gun (Jeonbuk, Korea). The plant was identified and authenticated by Prof. Hong Jun Kim at the College of Oriental Medicine, Woosuk University. A voucher specimen (PL-10-06) has been deposited in the author's laboratory (Prof. Seon Il Jang).
Preparation of the extract
The dried persimmon leaf (250 g) was steeped in boiling distilled water (3 L) for 5 minutes and filtered through a nylon mesh (pore size, 40 μm). After centrifugation at 3,500×g for 30 minutes at 4°C, the super-natant of the persimmon leaf extract (PLE) was freeze-dried and kept at −20°C. The contaminated endotoxin level in persimmon leaf was less than 1 ng/mL.
Reagents
Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from GIBCOBRL (Invitrogen Co., Grand Island, NY, USA), Phthalic anhydride, penicillin-streptomycin, hematoxylin & eosin (H&E), Congo red or toluidine blue were purchased from Sigma (St. Louis, MO, USA). CCL2 and CCL27 enzyme-linked immunosorbent assay (ELISA) kits for human and mouse were obtained from R&D Systems (Minneapolis, MN, USA). Anti-mouse CCL17 and anti-mouse CCL27 mAb conjugated with phycoerythrin (PE) were obtained from BD Biosciences (San Jose, CA, USA). Anti-FcεRIα mAb was purchased from Kyokuto Pharmaceutical Industry Co., Ltd. (Tokyo, Japan). Other chemicals for reagent grade were purchased from Sigma (St. Louis, MO, USA).
Cells
Human leukemic mast cells, HMC-1, were kindly provided by Dr J. H. Butterfield (Mayo Clinic, Rochester, MN, USA). Cells were cultured with DMEM containing 10% heat-inactivated FBS, penicillin G (100 IU/mL), and streptomycin (100 μg/mL) and incubated at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The medium was changed every 2 days. In this experiment, cells were cultured to 70∼80% confluence in a round plate (10 cm in diameter) and expanded by subculturing into four plates.
Histamine release assay
HMC-1 cells were suspended in a histamine release buffer (30 mmol/L Tris-HCl (pH 7.6), 120 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.03% BSA). The first incubation of 100 μL of HMC-1 cells (105 cells/mL) was performed with 100 μL of persimmon leaf extract (250 μg/mL) in an ice bath for 30 minutes. After centrifugation at 800×g for 5 minutes at 4°C, 200 μL of histamine release buffer was added to the cells, which were then incubated with 15 μg/mL Anti-FcεRIα mAb at 37°C for 30 minutes. After centrifugation at 800×g for 5 minutes at 4°C, the histamine contents of the supernatant and cell lysate were measured by means of ELISA. Positive and negative controls were used to indicate histamine content obtained with or without anti-FcεRIα mAb.
Animals
Seven-week-old Balb/c mice were purchased from Central Lab. Inc. (Seoul, Korea), the Korean branch of Charles River Japan (Kanagawa, Japan). Mice were maintained in an environmentally controlled rearing system and used for experiments at 8 weeks of age. All experiments in this study were performed in accordance with Jeonju University Institutional Animal Care and Use Committee guidelines.
Experimental design
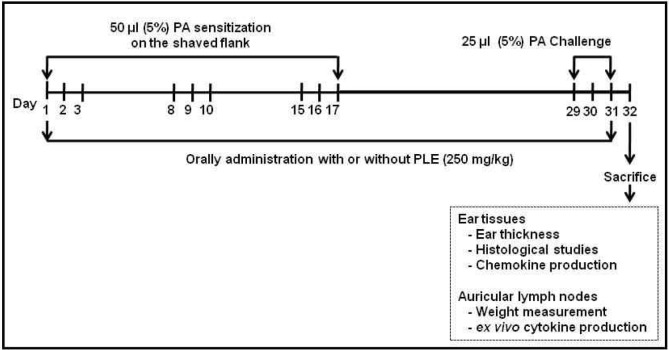
The mice were divided into four groups. Control and positive control groups (PA alone) were orally administrated with 200 μL saline, daily for 31 weeks. Also, active control group (PA plus EGCG) was administrated with 50 mg/kg epigallocatechin-3-gallate (EGCG) and the last group (PA plus EGCG) was orally administrated with 250 mg/kg PLE (the determinated optimum dose by our previous study) (15) daily for 31 weeks. Each experimental group consisted of five mice. Fig. 1 depicts the experimental protocol used. On days 1∼3, 8∼10 and 15∼17, a 50 μL aliquot of PA solution or solvent alone was applied to the dorsum of both ears of each mouse for dermal sensitization. On the day after challenge (day 32), all mice were anesthetized with pentobarbital sodium (75 mg/kg) and sacrificed. Blood samples were taken from the portal vein, and serum samples were assayed for IgE levels. Each animal’s ear thickness was measured and then ears for each group were removed to investigate histological changes and chemokine expression. Also, each animal’s auricular lymph nodes (LN) were removed to measure weight of LN and cytokine production.
Fig. 1.
Schematic diagram of experimental protocol. Mice were sensitized on shaved flank skin with and without 50 μL of 10% phthalic anhydride (PA) on days 1∼3, 8∼10 and 15∼17. On day 29, 30, and 31, mice were challenged with or without 25 μL of 10% PA on the dorsum of both ears. Control group received only saline for 31 days. Experimental groups received the single dose of persimmon leaf extract (PLE) (250 mg/kg body weight) or EGCG (50 mg/kg body weight) one time per day for 31 days.
Measurement of ear thickness and skin severity score
The ear thickness and skin severity scores of the mice were measured to determine the degree of contact irritant dermatitis induced by PA treatment. Briefly, the ear thickness was also measured using a dial caliper (Mitutoyo, Japan) 24 hr after final PA treatment. In addition, changes in the skin on the dorsum of the ear and the back skin of mice were observed 24 hr after final PA treatment using the method described in a previous study (16). In this method, the degree of four characteristics, erythema/hemorrhage, edema, excoriation/erosion and dryness, was scored from 0 points (none) to 3 points (severe) depending on the severity of the dermatitis. Finally, the total skin score was calculated from the sum of the scores of the four characters.
Preparation of lymph node cell
Auricular LN cells were derived from the auricular region. Freshly isolated lymph node cells were made into single-cell suspensions, and contaminated erythrocytes were lysed by hypotonic shock with sterile distilled water. Cells (1×106/well) were stimulated with soluble anti-CD3 (1 μg/mL) plus anti-CD28 (1 μg/mL) antibodies (BD Pharmingen, San Jose, CA, USA) in RPMI1640 medium (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% heat-inactivated FBS (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μ g/mL amphotericin B (Sigma-Aldrich, St. Louis, MO, USA), and 50 μM 2-mercaptoethanol. After 48 hours, cytokines were measured from supernatants using IL-4 and IFN-γ ELISA kit.
ELISA
IgE levels in the sera and cytokine levels in the culture supernatants were determined by sandwich ELISA using antibody pairs against murine IgE, IL-4 or IFN-γ from R&D Systems, following the manufacturer's instructions. Mouse chemokines were analyzed in the lysate of ear tissues. The tissues were lysed in 100 μL of protein extraction buffer (PRO-PREP, Intron, Seoul, Korea) with a sonicator (Ultra-Turrax-25, Ika-Werke, Staufen, Germany). Lysates were collected from the sonicator mixture using centrifugation and stored at −70°C until further analysis. Chemokine levels in the lysate were determined in appropriate dilutions by ELISA using antibody pairs against CCL17 and CCL27 from R&D Systems, following the manufacturer's instructions.
Histological analysis
The ear tissues were fixed with 4% paraformaldehyde, embedded in paraffin and then 5 μm thin sections were made on saline coated micro slides (Muto-glass, Tokyo, Japan). The de-paraffinized skin sections were stained with H&E and examined by light microscopy (Olympus, Tokyo, Japan) to assess the histological changes. The sections for immunohistochemistry were de-paraffinized with xylene, rinsed with distilled water and then rinsed with PBS. The nonspecific antigen–antibody reactions were inhibited by one hour treatment with 10% normal goat serum. The sections were reacted with diluted solutions of each of the primary antibodies such as purified rat monoclonal antibodies against mouse CCL17 or CCL27 for overnight at 4°C. The sections were rinsed with distilled water and PBS and then were covered with a coverslip. They were examined by fluorescence microscopy (Olympus) to assess the molecule expressions.
Statistical analysis
Differences in data among the groups were analyzed by one-way ANOVA, and all values were expressed as mean±SE. The differences between groups were considered to be significant at p<0.05.
RESULTS
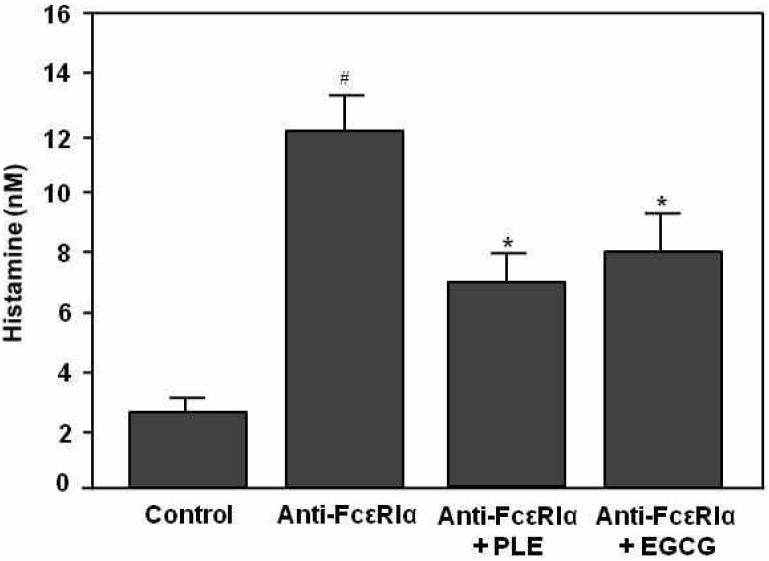
Effect of PLE on histamine release from HMC-1 cells in response to FcεRI cross-linkage. First, to investigate whether PLE could inhibit histamine release, we used human leukemic mast cells, HMC-1. As shown in Fig. 2, histamine release was significantly increased in the anti-FcεRIα mAb alone group (p<0.001), compared to the control group. However, histamine level of PLE or EGCG treated group was significantly lower than that for anti-FcεRIα mAb alone group (p<0.05). The inhibitory effect of PLE on histamine release was slightly higher than that for the EGCG group.
Fig. 2.
Inhibitory effect of PLE treatment on histamine release from HMC-1 cells stimulated with anti-FcεRIα mAb. Cells (104) were incubated with 250 μg/mL PLE or 50 μg/mL EGCG on ice for 30 minutes and then incubated with 15 μg/mL anti-FcεRIα mAb at 37°C for 30 minutes. Histamine content of the supernatant was measured by means of ELISA. Control and anti-FcεRIα mAb alone groups indicate histamine content with or without anti-FcεRIα mAb. Data represent the mean ±SE of 3 determinations. #p<0.001 compared with the control group. *p<0.05 compared with the anti-FcεRIα alone group.
Effect of PLE on PA-induced ear thickness and skin severity score
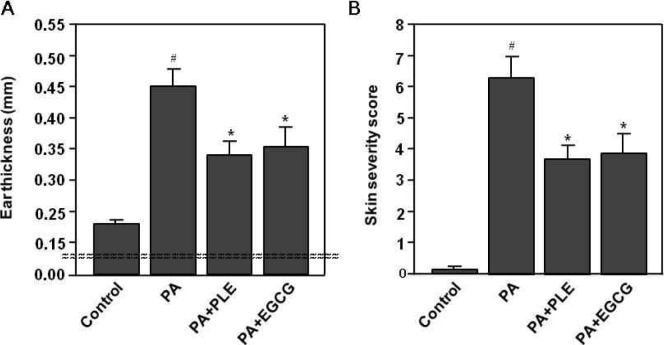
Next, to investigate the skin irritation response in Balb/c mice, ear thickness and skin severity score were measured in mice following topical application of PA solution. The ear thickness was measured in the ears of mice using a thickness gauge. As shown in Fig. 3A, the ear thickness was significantly increased in the PA alone group (p<0.001), while it was not changed in the control group. In contrast, the ear thickness of PLE or EGCG treated group was significantly lower than those for PA alone group (p<0.05). The skin severity score was used to determine the degree of the erythema/hemorrhage, edema, excoriation/erosion and dryness on the flank and ear skin of mice. As shown in Fig. 3B, the skin severity scores increased significantly in PA alone group (p<0.001). However, the skin severity scores of PLE or EGCG treated group were significantly lower than those for PA alone group (p<0.05). The inhibitory effects of PLE on the changes in ear thickness and skin severity score were slightly higher than that for the EGCG group.
Fig. 3.
Effect of PLE treatment on PA-induced changes in the ear thickness and the skin severity score. Mice were sensitized on shaved flank skin with and without 50 μL of 10% phthalic anhydride (PA) on days 1∼3, 8∼10 and 15∼17. On day 29, 30, and 31, mice were challenged with or without 25 μL of 10% PA on the dorsum of both ears. Control group received only saline for 31 days. Experimental groups received the single dose of persimmon leaf extract (PLE) (250 mg/kg body weight) or EGCG (50 mg/kg body weight) one time per day for 31 days. (A) Ear thickness was measured using a dial caliper on day 32. (B) The total clinical severity score was defined as the sum of scores graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for each of five aspects: itch, erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness on day 32. Data represent the mean±SE of 3 determinations. #p< 0.001 compared with the control group. *p<0.05 compared with the PA alone group.
Effect of PLE on PA-induced serum IgE production and lymph node weight
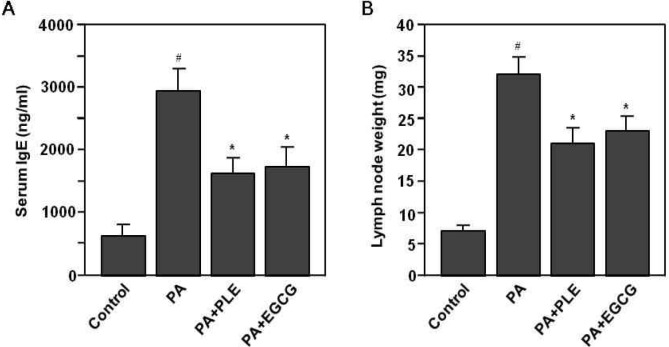
We also investigated whether PLE might be effective for allergic response in vivo by measuring the serum IgE and auricular lymph node in PA treated mice. As shown in Fig. 4A, repeated topical application of the PA solution induced a significant increase in serum IgE levels in mice, compared to those of the control group (p<0.001). However, the serum IgE level of PLE or EGCG treated group was significantly lower than that for PA alone group (p<0.05). In addition, PA treatment was found to induce an increase in the weight of auricular lymph node in mice, whereas the weight of auricular lymph node of PLE or EGCG treated group was significantly lower than that for PA alone group (p<0.05) (Fig. 4B).
Fig. 4.
Effects of PLE treatment on PA-induced changes in the serum level of IgE and the weight of auricular lymph node (LN). Mice were sensitized on shaved flank skin with and without 50 μL of 10% PA on days 1∼3, 8∼10 and 15∼17. On day 29, 30, and 31, mice were challenged with or without 25 μL of 10% PA on the dorsum of both ears. Control group received only saline for 31 days. Experimental groups received the single dose of PLE (250 mg/kg body weight) or EGCG (50 mg/kg body weight) one time per day for 31 days. (A) Ear thickness was measured using a dial caliper on day 32. (A) Sera were obtained from each mouse group on day 32. Serum level of IgE was measured using the specific ELISA kit for IgE molecule. (B) Weight of auricular LN was measured using a electric balance on day 32. Data represent the mean ±SE of 3 determinations. #p<0.001 compared with the control group. *p<0.05 compared with the PA alone group.
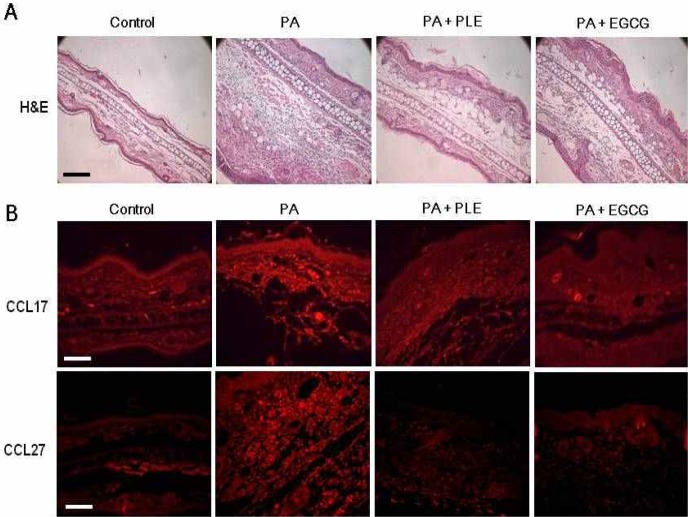
Effect of PLE on PA-induced histological changes and chemokine expressions
To evaluate the inhibitory effect of PLE on PA-induced skin lesions, we performed H&E staining on specimens of the ears of mice. As shown in Fig. 5A, sections of the ears in the PA alone group revealed a hyperplastic epidermis and edematous dermis, with a large number of infiltrated mononuclear cells together with some eosinophils. In contrast, sections of the ears of PLE or EGCG-treated groups showed markedly diminished epidermal hyperplasia and cellular infiltration into the dermis. We also conducted immunohistochemistry for CCL17 and CCL27 chemokines to assess whether oral administration of PLE has an effect on CCL17 and CCL27 expression in PA-induced skin lesions. As shown in Fig. 4B, CCL17 and CCL27 expressions were increased in the skin lesions in PA alone group. In contrast, they were markedly decreased with oral administration of the PLE or EGCG. The inhibitory effect of PLE on histological changes and chemokine expression were slightly higher than that of the EGCG group. These results demonstrate that PLE suppressed PA-induced histological changes and chemokine expression in mice treated with PA.
Fig. 5.
Effect of PLE treatment on PA-induced skin lesions in mice. Mice were sensitized on shaved flank skin with and without 50 μL of 10% PA on days 1∼3, 8∼10 and 15∼17. On day 29, 30, and 31, mice were challenged with or without 25 μL of 10% PA on the dorsum of both ears. Control group received only saline for 31 days. Experimental groups received the single dose of PLE (250 mg/kg body weight) or EGCG (50 mg/kg body weight) one time per day for 31 days. (A) Sections were stained with hematoxylin and eosin, and then observed at an original magnification of ×200, scale bar= 120 μm. (B) CCL17 and CCL27 chemokine expressions were determined by immunochemical staining with anti-mouse CCL17 or CCL27 antibody. The sections were observed at an original magnification of ×200, scale bar=60 μm.
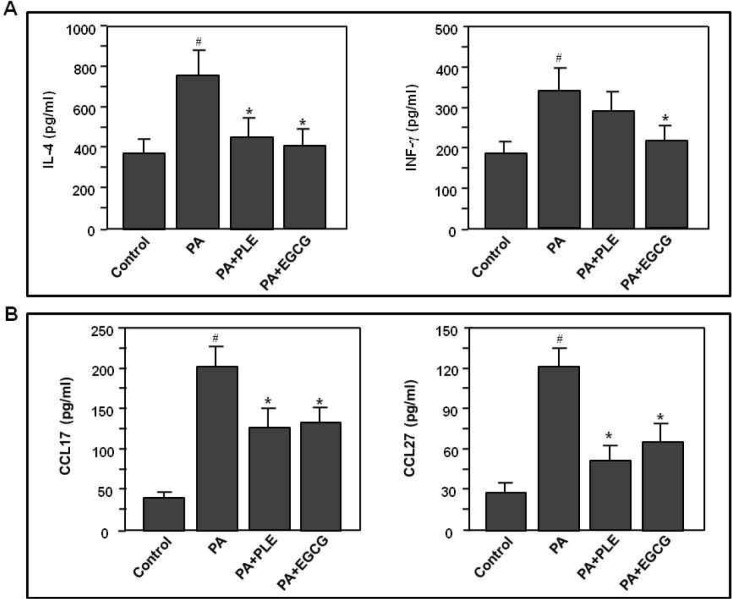
Effect of PLE on PA-induced cytokine and chemokine productions
Finally, to examine whether PLE affects cytokines of auricular LN cells or chemokines of ear tissues, we measured cytokine levels of supernatants stimulated with anti-CD3 plus CD28 antibodies for 48 hr in PA-sensitized cells and also measured chemokine levels of ear tissue lysates of PA-sensitized mice. As shown in Fig. 6A, the levels of cytokines (IL-4 and IFN-γ) in the draining LN cells were significantly increased in PA-treated mice (p<0.001), compared to those of the control group. In contrast, IL-4 production of lymph node cells was markedly diminished by PLE or EGCG treatment (p<0.05), compared to those of PA alone group. However, IFN-γ level of the PLE treated group, but not the EGCG group, was not changed. The levels of CCL17 and CCL27 were significantly increased in PA-treated mice (p<0.001), compared to those of the control group. In parallel with the protein expression of CCL17 and CCL27 in the ear tissue (Fig. 5B), PLE or EGCG treatment markedly suppressed PA-induced CCL17 and CCL27 productions in the lysate of ear tissues (Fig. 6B). These results demonstrate that PLE suppressed PA-induced Th2 cytokine production and Th2 chemokine expression in mice sensitized with PA chemical antigen.
Fig. 6.
Effects of PLE treatment on cytokine production of auricular LN cells and chemokine production of ear tissue. Mice were sensitized on shaved flank skin with and without 50 μL of 10% PA on days 1∼3, 8∼10 and 15∼17. On day 29, 30, and 31, mice were challenged with or without 25 μL of 10% PA on the dorsum of both ears. Control group received only saline for 31 days, experimental groups received the single dose of PLE (250 mg/kg body weight) or EGCG (50 mg/kg body weight) one time per day for 31 days. (A) At the last day of experiments, auricular LN cells were isolated and stimulated with anti-CD3 plus anti-CD28 antibodies for 2 days and then cytokine productions of supernatants were examined by ELISA. (B) At the last day of experiments, ear tissues were removed and lysed with tissue lysis buffer. CCL17 and CCL27 levels of the tissue lysate were measured using the each specific ELISA kit for CCL17 or CC27. Data represent the mean±SE of 3 determinations. #p<0.001 compared with the control group. *p<0.05 compared with the PA alone group.
DISCUSSION
Irritant contact dermatitis accounts for 80% of all contact dermatitis reactions. It occurs as a result of xenobiotic chemicals penetrating the skin and chemically reacting with self proteins, which results in a hapten-specific immune response (17). Exposure to PA causes irritation and can lead to allergic skin disorders such as atopic or contact dermatitis accompanied by irritant dermatitis in the site at which contact with an allergen has occurred (3).
First, to investigate whether PLE could inhibit histamine release from human leukemic mast cells, we stimulated HMC-1 cells with anti-FcεRIα antibody. The cross-linkage of FcεRIα by the anti-FcεRIα antibody in mast cells induces histamine release, as previously reported (18). The histamine level of the PLE treated group was significantly lower than that for anti-FcεRIα antibody alone group. For the inhibitory effect of PLE on histamine release, our result was similar to a previous report (19).
Next, we examined whether oral PLE administration ameliorates the development of irritant contact dermatitis in PA-sensitized mice and whether PLE alters IgE level of serum, cytokine production of the auricular LN cells, and chemokine expression of ear tissue in PA-sensitized mice. Allergic response to chemical allergens is generally acknowledged to be dependent on IgE-mediated mechanisms, and the hyper-production of IgE is one of the characteristic features of allergic response (20,21). The administration of PLE showed an inhibitory effect on serum IgE level and auricular LN weight induced by PA (Fig. 3). PLE also significantly reduced the clinical signs provoked by PA, such as erythema, edema, scaling and excoriation. A suppressive effect of PLE was similar to that of EGCG, which was used as an active control. It is also well known that the weight of the local lymph node increases in response to topical application of agents that have allergenic or sensitizing potential (22).
Balance between the Th1 and Th2 responses is regarded as important for efficient immune function, and imbalances lead to various immune diseases, such as autoimmune and allergic diseases (10). Th2-dominant phenotype is one of the aspects of human irritant contact dermatitis (23). Irritant contact dermatitis is typically characterized by the infiltration of inflammatory cells (6). It has been reported that several cytokines and chemokines could regulate the irritant contact dermatitis (24). IL-4 secreted from Th2 cells is an important factor for immunoglobulin class switching to IgE in B cells, but IFN-γ strongly suppresses IgE production in B cells via suppression of CD40L mRNA expression in Th2 cells (25). We found that anti-CD3 plus anti-CD28 antibody-induced IL-4 production of draining auricular LN cells was markedly diminished by oral administration of PLE, but not IFN-γ (Fig. 6A). These changes in the cytokine profile might contribute to IgE suppression. Since many studies demonstrated that CCL17 and CCL22 play a critical role in infiltration CCR4+ lymphocytes into the skin area, the down-regulation of CCL17 and CCL22 production in keratinocytes may be an effective approach for treatment of inflammatory skin diseases (12,26). In addition, CCL27 is a skin-specific CC chemokine which is produced constitutively by keratinocytes and is highly upregulated in inflammatory skin conditions such as atopic dermatitis and allergic contact dermatitis (27). Therefore, CCL17 and CCL27 may contribute to the pathogenesis of a Th2-dominant disease, such as atopic dermatitis and allergic contact dermatitis. We found that PLE significantly inhibited the serum CCL17 and CCL27 levels in PA-treated mice (Fig. 5B). A suppressive effect of PLE was similar to that of EGCG used as an active control. These findings indicate that these cytokines and chemokines play a role in determining the degree of skin irritation.
On the other hand, current treatments for inflammation include aspirin, nonsteroidal anti-inflammatory drugs and dexamethasone. These compounds tend to have adverse side effects, which may be ameliorated by the use of effective botanical alternatives (4). D. kaki is widely distributed in East Asia, and its leaf is a traditional plant medicine for the treatment of hypertension, angina and internal hemorrhage. PLE is used to promote maternal health that in Korea and Japan (13) and Persimmon leaf has been shown to contain bioactive compounds, including polyphenols, flavonoids, hydrocarbons, triterpene and steroids (28,29).
In conclusion, we have provided evidence that PLE has an inhibitory effect on the development of irritant contact dermatitis symptoms, especially ear thickness and skin symptoms, with alterations of Th2 cytokine and chemokines, as well as IgE level in PA-treated mice. These results suggest that PLE may be an effective material for management of irritant contact dermatitis.
Acknowledgments
This work was supported by Jeollabukdo & Wanjugun in 2011.
REFERENCES
- 1.Ban M, Hettich D. Effect of Th2 cytokine antagonist treatments on chemical-induced allergic response in mice. J Appl Toxicol. 2005;25:239–247. doi: 10.1002/jat.1062. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 3.Incorvaia C, Frati F, Verna N, D'Alò S, Motolese A, Pucci S. Allergy and the skin. Clin Exp Immunol. 2008;153(Suppl 1):27–29. doi: 10.1111/j.1365-2249.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J Food Sci. 2010;75:H87–92. doi: 10.1111/j.1750-3841.2010.01541.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- 6.Dubois GR, Bruijnzeel PL. IL-4-induced migration of eosinophils in allergic inflammation. Ann N Y Acad Sci. 1994;725:268–273. doi: 10.1111/j.1749-6632.1994.tb39809.x. [DOI] [PubMed] [Google Scholar]
- 7.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 8.Boguniewicz M. Update on atopic dermatitis: insights into pathogenesis and new treatment paradigms. Allergy Asthma Proc. 2004;25:279–282. [PubMed] [Google Scholar]
- 9.Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008;128:1329–1331. doi: 10.1038/sj.jid.5701138. [DOI] [PubMed] [Google Scholar]
- 10.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastiani S, Denelon G, Gerber B, Uguccioni M. CCL22 induced responses are powerfully enhanced by synergy inducing chemokines via CCR4: evidence for the involvement of b strand of chemokine. Eur J Immunol. 2005;35:746–756. doi: 10.1002/eji.200525800. [DOI] [PubMed] [Google Scholar]
- 12.Nakazato J, Kishida M, Kuroiwa R, Fujiwara J, Shimoda M, Shinomiya N. Serum levels of Th2 chemokines, CCL17, CCL22, and CCL27, were the important markers of severity in infantile atopic dermatitis. Pediatr Allergy Immunol. 2008;19:605–613. doi: 10.1111/j.1399-3038.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Kang S, Choue R, Kim H, Leem K, Chung S, Kim C, Chung J. Free radical scavenging effect of Diospyros kaki, Laminaria japonica and Undaria pinnatifida. Fitoterapia. 2002;73:710–712. doi: 10.1016/s0367-326x(02)00236-8. [DOI] [PubMed] [Google Scholar]
- 14.Bei WJ, Xu AL, Li CY, Cabot PJ, Hermanussen S. Flavonoids from Diospyros kaki inhibit the adhesion between lymphocyte and dorsal root ganglion. Zhong Yao Cai. 2009;32:740–744. [PubMed] [Google Scholar]
- 15.Cho JK, Jeon IH, Park JM, Kim HS, Jang SI. Inhibitory effect of persimmon leaf extract on development of atopic dermatitis-like skin lesions. J Korean Soc Appl Biol Chem. 2011;54:653–657. [Google Scholar]
- 16.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:21–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 18.Queralt M, Brazís P, Merlos M, de Mora F, Puigdemont A. In vitro inhibitory effect of rupatadine on histamine and TNF-alpha release from dispersed canine skin mast cells and the human mast cell line HMC-1. Inflamm Res. 2000;49:355–360. doi: 10.1007/PL00000216. [DOI] [PubMed] [Google Scholar]
- 19.Kotani M, Matsumoto M, Fujita A, Higa S, Wang W, Suemura M, Kishimoto T, Tanaka T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin Immun. 2000;106:159–166. doi: 10.1067/mai.2000.107194. [DOI] [PubMed] [Google Scholar]
- 20.Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of allergic dermatitis in NC/ Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004;27:1376–1381. doi: 10.1248/bpb.27.1376. [DOI] [PubMed] [Google Scholar]
- 21.Dearman RJ, Skinner RA, Humphreys NE, Kimber I. Methods for the identification of chemical respiratory allergens in rodents: comparisons of cytokine profiling with induced changes in serum IgE. J Appl Toxicol. 2003;23:199–207. doi: 10.1002/jat.907. [DOI] [PubMed] [Google Scholar]
- 22.Stahlmann R, Wegner M, Riecke K, Kruse M, Platzek T. Sensitising potential of four textile dyes and some of their metabolites in a modified local lymph node assay. Toxicology. 2006;219:113–123. doi: 10.1016/j.tox.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Goleva E, Dunlap A, Leung DY. Differential control of Th1 versus Th2 cell responses by the combination of low-dose steroids with beta2-adrenergic agonists. J Allergy Clin Immun. 2004;114:183–191. doi: 10.1016/j.jaci.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohtani T, Nakagawa S, Kurosawa M, Mizuashi M, Ozawa M, Aiba S. Cellular basis of the role of diesel exhaust particles in inducing Th2-dominant response. J Immunol. 2005;174:2412–2419. doi: 10.4049/jimmunol.174.4.2412. [DOI] [PubMed] [Google Scholar]
- 26.Vestergaard C, Yoneyama H, Murai M, Nakamura K, Tamaki K, Terashima Y, Imai T, Yoshie O, Irimura T, Mizutani H, Matsushima K. Overproduction of Th2-specific chemokines in NC/Nga mic exhibiting atopic dermatitis-like lesions. J Clin Invest. 1999;104:1097–1105. doi: 10.1172/JCI7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bünemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami K, Aketa S, Nakanami M, Iizuka S, Hirayama M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their alpha-amylase inhibitory activity. Biosci Biotechnol Biochem. 2010;74:1380–1385. doi: 10.1271/bbb.100056. [DOI] [PubMed] [Google Scholar]
- 29.Chen G, Wei SH, Huang J, Sun J. A novel C-glycosylflavone from the leaves of Diospyros kaki. J Asian Nat Prod Res. 2009;11:503–507. doi: 10.1080/10286020902911512. [DOI] [PubMed] [Google Scholar]