Abstract
This study was performed to investigate the effects of pre-treatment and storage temperature and periods on the quality characteristics of ginger paste. The pH of the ginger paste remained constant during room temperature storage but increased with prolonged refrigerated storage periods. During five months of frozen storage, regardless of pre-treatment, the pH of most of the samples decreased slightly and then remained constant. In the color value of ginger paste stored at room temperature, the samples with and without chemical additives changed in color more prominently than fermented or pasteurized samples. Intriguingly, the color value for samples containing chemical additives changed more dramatically when stored under refrigerated conditions. However, the L, a, and b values of samples stored under frozen storage conditions did not change even after ten months. Most of the samples contained glucose and fructose, except for the fermented samples. The free sugar content of samples slowly decreased with increasing storage periods, while the organic acid content generally decreased also, regardless of sample type. Depending on pre-treatment and storage temperature, the gingerol content of the samples was either retained or decreased with prolonged storage time.
Keywords: ginger paste, pre-treatment, storage condition, quality characteristics
INTRODUCTION
Ginger (Zingiber officinals Roscoe) is a rhizome plant that grows in tropical and subtropical countries such as Egypt and Iraq. As a spice of commerce and a perennial herb, ginger has a distinctive spicy flavor and taste properties (1–3). Ginger is used as fresh (green ginger), dried, oleoresin and an essential oil in cooking, and medicinal or cosmetic products (4). The world's total output per year of ginger is about 500,000 tons and approximately 48,000 tons are produced annually in Korea alone. In Korea, after ginger is harvested, most of it is immediately stored in the crypts until the next harvest season. The crypt conditions for ginger storage are not ideal since these storage conditions increase germination, qualitative and quantitative losses of stored ginger. The optimum storage condition for fresh ginger is 13∼15°C and a relative humidity of 90∼95%; however, these conditions are difficult to maintain in the crypts for fresh ginger. In addition, fresh ginger is negatively effected by temperatures below 10°C and higher rates of germination occur when temperatures exceed 18°C (5). Therefore, many issues are associated with ginger storage and distribution including gas production, decay, cold interference etc (6).
To solve these problems, many studies have evaluated the effects of pre-treatment such as cleansing, chilling, freezing, grinding, heating, and drying of fresh ginger. Ginger paste has a spicy taste and flavor similar to fresh ginger, but non-enzymatic browning can occur as well as microbial gas production during ginger paste storage and distribution. In addition, high-temperature sterilization and the addition of antioxidants can influence properties such as unique flavor and color (6,7). Previous studies have found that pre-treatment methods such as freezing, cleansing, creating ginger paste or ginger powder, affected the unique flavor of ginger and its functional properties and the gingerol component (4,8–10). Therefore, many studies have attempted to develop methods to improve storage of ginger paste including a solid-liquid separation inhibitor, a browning reaction inhibitor, and packaging methods (6,11–13).
Meanwhile, fermentation has been widely used for the manufacturing of fruit wines, breads, and cheeses. Lactobaicillus spp. can produce, among many others, lactic acid, acetic acid, and benzoic acid by fermentation. Some anti-microbial substances, such as hydrogen peroxide, prohibit the growth of pathogenic bacteria and increase the retention period of fermentation products (14). In addition, lactic acid fermentation produces a unique flavor in sensory properties and inhibits the growth of pathogenic bacteria in fermented vegetables (15). Furthermore, Lactobacillus spp. was shown to produce a unique taste and change the flavor of ginger (16).
Therefore, this study investigated the effects of pasteurization, lactic acid fermentation and addition of chemical additives on ginger paste and its shelf life according to storage temperature and periods.
MATERIALS AND METHODS
Materials
Ginger harvested in the central region of Korea in October 2010 was purchased at Susan Agricultural Cooperative. Fresh ginger was cleaned and minced with a blender (Dong-A Ozka, Gimhae-si, Gyeongnam, Korea)
Pre-treatment method
Ginger paste samples were divided into four groups: A) ginger paste without pre-treatment (control). B) ginger paste samples pasteurized at 70°C for 20 min to inhibit uncontrolled microbes without degradation of ginger quality. C) Fermented samples were prepared using pasteurized samples inoculated with a 1% microbial strain (activated in MRS broth with 20% ginger juice) and cultured for two to three days at 30°C. The microbial strains (Lactobaicillus brevis, KCCM 35464) were purchased from the Korea Center for Microbial Retention (KCCM). D) Samples contained chemical additives per 100 g of fresh ginger paste. The chemical additives included 0.2% L-cystein (browning inhibitor), 2% NaCl, 0.1% sodium benzoate (gassing inhibitor), and 0.1% xanthan gum (anti-liquid separation inhibitor). 100 g of control and pre-treatment samples were packaged in aluminum laminated nylon bags. The samples were examined at room temperature, 10°C, and −20°C according to the storage periods.
pH and color value
The ginger paste (10 g) and 10 mL of distilled water were placed in a flask and blended with a vortex mixer (G560E, Scientific Inc., New York, NY, USA). The pH of the blended samples was measured using a pH meter (Model 827, Metrohm., Zofinyen, Switzerland). To measure the color value, the ginger paste was put in a plastic petridish (5 cm diameter, 5 mm height) and placed on 5 sheets of white paper. The L, a, b, and ΔE values were calculated using a colorimeter (CE-310, Macbeth, Minolta, Koyto, Japan). The tests were repeated more than 3 times to obtain a mean value.
Free sugar and organic acid
The ginger paste (2 g) was mixed with 40 mL of 80% ethanol and blended with a vortex mixer (G560E, Scientific Inc., New York, NY, USA) for 2 min. The extract was then filtered through a 0.2 μm filter (Millex-HN, Millipore, Bedford, MA, USA). Total free sugar contents corresponded to the sum of fructose, glucose, and sucrose contents analyzed by high performance liquid chromatography (HPLC) (JASCO., Tokyo, Japan). Chromatographic analysis was performed on a SUPELCOGEL AG2 (5 μm, 300×7.8 mm i.d. Supelco, Milford, MA, USA) column with an isocratic mobile phase (100% water). The HPLC operating parameters were as follows: injection volume, 20 μL; column flow rate, 0.5 mL/min; chromatographic run time, 20 min; model 830-RI detector. In the organic acid analysis, the ginger paste (1 g) was diluted with 15 mL of distilled water and sonicated for 10 min using a sonicator (JAC Co., Hwaseong-si, Korea). The samples were then filtered through a 0.2 μm filter (Millex-HN, Millipore). Organic acid components were acetic, citric, fumaric, lactic, malic, malonic, oxalic succinic, and tartaric acid and analyzed by HPLC (JASCO). Chromatographic analysis was performed on a Aminex HPX-87H (5 μm, 300×7.8 mm i.d., Biorad, Hercules, CA, USA) column with isocratic mobile phase. The mobile phase for analysis was 0.008 N H2SO4 with HPLC parameters as follows: flow rate, 0.6 mL/min; injection volume, 20 μL; Ultraviolet (UV) detector 210 nm; oven temperature at 50°C.
Gingerol analysis
The ginger paste (2 g) and 10 mL of ethanol were placed in a test tube and subjected to ultrasonication (JAC Co.) for one hr. After filtering, samples were concentrated and dissolved in 10 mL of methanol. Samples filtered with a 0.2 μm filter (Millex-HN, Millipore) were used for HPLC analysis (JASCO). Chromatographic analysis was performed on a XTerra ™RP18 (5 μm, 150×4.6 mm i.d., Waters, Milford, MA, USA) column with a gradient mobile phase. The mobile phase for analysis was water with 2% acetic acid (A) and 100% methanol (B). The HPLC operating parameters were as follows: injection volume, 20 μL; column flow rate, 0.8 mL/min; chromatographic run time, 20 min; UV 282 nm. The gingerol content of each sample was converted from standard curves of 6-gingerol (Sigma-Aldrich Co., Milwaukee, WI, USA), 8-gingerol (Sigma-Aldrich Co.), 10-gingerol (Sigma-Aldrich Co.) and shogaol (Sigma-Aldrich Co.). The tests were repeated more than 3 times to obtain a mean value.
Statistical analysis
Chemical analysis of samples was conducted in triplicate and significant differences between samples’ means were determined using Duncan’s multiple range tests (p<0.05). Duncan's multiple range tests were performed to separate the means of individual sensory scores between samples (SAS V.8 2000, SAS Institute, Cary, NC, USA).
RESULTS AND DISCUSSION
pH and color value
The pH changes of four ginger paste groups were examined at different storage temperatures (Fig. 1). For room temperature storage, the pH values of the control (A), pasteurized sample (B), fermented sample (C) and samples containing chemical additives (D) were pH 6.19, 6.23, 5.50 and 6.02, respectively, at the initial storage time. After ten days, the pH value of each sample had not significantly changed. During the initial storage at a refrigerated temperature (10°C), the pH of the pasteurized and fermented samples were 6.30 and 5.79, respectively, and remained constant during storage. On the other hand, the pH of the sample containing chemical additives decreased from pH 6.21 to 4.87 after twenty weeks of storage whereas the control increased from pH 6.9 to 7.0 after ten weeks of storage. For frozen (−20°C) storage, the pH values of groups A∼D were pH 6.90, 6.69, 5.93 and 6.65, respectively, at the initial storage time. After five months, the pH of the samples containing the chemical additives decreased to pH 6.07∼6.09 and no further changes were observed for up to ten months of storage. Generally, the pH values of all samples remained relatively constant when stored at room temperature. At the refrigerated temperature, the pH of the control group increased with an increase in the storage period while the pH of the fermented and pasteurized samples remained constant. For samples stored at −20°C, the pH value changed after five months and remained constant for up to ten months of storage. Choi et al. reported that pH of various ginger pastes showed decreasing trends according to increasing storage periods at 5°C (6). In this study, the pH value increased depending on the pre-treatment method and storage temperature at the initial storage time, but remained stable at the mid storage time of 5 months.
Fig. 1.
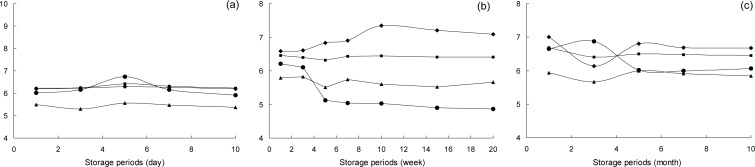
Changes in the pH of pre-treatment ginger paste according to storage temperature and period.
 control,
control,
 pasteurized ginger paste,
pasteurized ginger paste,
 fermented ginger paste,
fermented ginger paste,
 chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
The color value of the surface of the ginger paste was measured at different storage temperatures (Table 1). At room temperature, the L value (lightness) of the control was 49.29∼55.02, which decreased with storage time. However, no changes were observed for the fermented sample and sample containing chemical additives. The a value, which is a measure of the red-green color, was 0.90∼0.99 in the control and pasteurized sample at the initial storage time. The a value of the fermented sample and sample containing chemical additives ranged from −3.42∼−2.40. The a value of samples decreased or increased with an increase in storage time regardless of pre-treatment method. The b value, measuring the yellow-blue color, for the control and pasteurized samples, were between 18.77∼19.06 and for the fermented sample and sample containing chemical additives were between 20.2∼20.70. The b value of the samples slightly decreased with an increase in storage time. For refrigerated storage (10°C), the L value of the control decreased with storage time, but remained constant for the other samples. Regardless of pre-treatment, the a value of the samples increased with an increase in storage periods. In contrast, the b value of the samples did not change after ten weeks of storage. For frozen storage (−20°C), the a value of the control was −0.94 at initial storage time and increased to 3.17 after three months, which did not change any further for up to ten months of storage. However, the a value of the pasteurized sample, fermented sample and sample containing chemical additives remained the same color value throughout storage. Kim and Lee reported that the a (redness) and b (yellowness) of ginger paste increased with an increase in storage time at room temperature (17). Similar results were observed in this study; the a value of most of the samples increased with an increase in storage time at room temperature. Fig. 2 shows the ΔE value, which was calculated based on the L, a, and b values of ginger paste, according to storage time. Based on this analysis, during room temperature storage, the control and chemical additives samples showed more color change than the fermented and pasteurized samples after ten days of storage. During refrigerated storage, a larger color change was observed for the sample containing chemical additives than the other samples, but the color changes of the control and samples containing the chemical additives were similar during room temperature storage. In contrast, no change in the color value was observed for frozen samples after ten months of storage regardless of pre-treatment method.
Table 1.
Changes in the color value of pre-treatment ginger paste according to storage temperature and period
| Storage condition (periods) | Control | Pasteurized sample | Fermented sample | Chemical additives addition sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| L | a | b | L | a | b | L | a | b | L | a | b | ||
| Room temp. (days) | 1 | 54.16a | 0.99b | 19.06a | 51.13a | 0.90ab | 18.77a | 56.45a | −2.40a | 20.70a | 56.63ab | −3.42a | 20.25a |
| 3 | 55.02a | −0.56d | 19.40a | 51.18a | 0.41c | 17.22a | 56.50a | −2.10a | 19.35a | 56.99ab | −2.92b | 20.52a | |
| 5 | 52.42a | 0.87c | 17.86b | 49.21b | 0.89ab | 16.64b | 54.94b | −1.40b | 19.05a | 58.31b | −1.71c | 21.83a | |
| 7 | 53.52a | −0.01e | 19.18a | 49.57b | 0.96a | 16.70b | 56.70a | −2.53a | 19.18a | 55.43b | −0.97d | 20.52a | |
| 10 | 49.29a | 1.66a | 16.77b | 52.01a | 0.89ab | 17.57a | 56.43a | −2.14a | 16.77b | 55.43b | −0.16e | 18.87b | |
|
| |||||||||||||
| 10°C (weeks) | 1 | 53.97a | 0.98c | 19.94a | 52.04a | 0.83b | 18.67b | 54.15a | −2.32c | 18.77b | 54.85b | −3.53d | 20.05b |
| 3 | 50.84b | 2.64a | 18.06b | 52.92a | −0.68d | 19.28a | 55.53a | −1.63b | 19.95a | 58.11a | −3.23d | 21.14b | |
| 5 | 52.06a | 2.65a | 18.85b | 53.15a | 0.67c | 20.05a | 53.71ab | −1.60b | 19.23a | 58.48a | −3.17d | 23.46a | |
| 7 | 51.42a | 2.22a | 18.93ab | 52.34a | −0.69d | 18.96b | 53.87ab | −2.53c | 17.92c | 58.81a | −2.52c | 23.02a | |
| 10 | 52.21a | 1.93ab | 17.62c | 53.69a | 1.65a | 18.36b | 55.74a | −2.09b | 21.29a | 58.06a | −2.25c | 20.23b | |
| 15 | 48.26c | 2.01b | 18.64ab | 50.75b | 1.46a | 17.40c | 54.75a | −1.37b | 18.78b | 51.31c | −1.13a | 20.41b | |
| 20 | 49.05c | 2.08b | 17.29c | 51.31b | 1.13ab | 18.41b | 53.78ab | −0.46a | 18.64b | 58.40a | −1.69b | 21.6b | |
|
| |||||||||||||
| −20°C (months) | 1 | 53.67a | 0.94b | 20.99a | 56.10a | −0.85b | 21.21a | 57.40a | −2.36c | 20.55a | 57.69ab | −3.78a | 21.55a |
| 3 | 53.11a | 3.17a | 20.50a | 53.64b | −0.39a | 19.89b | 55.81b | −1.54b | 19.56a | 55.86b | −3.64a | 20.01a | |
| 5 | 51.35b | 3.39a | 19.91b | 52.37b | −0.23a | 19.88b | 51.62c | −0.66a | 17.80b | 55.00b | −3.46a | 18.98b | |
| 7 | 52.86a | 3.76a | 21.08a | 52.87b | −0.92b | 20.15a | 54.82b | −1.39b | 19.73a | 56.99ab | −3.48a | 20.49a | |
| 10 | 51.19b | 3.43a | 19.95b | 52.88b | −0.65a | 19.71b | 55.79b | −1.89b | 19.75a | 59.70a | −3.75a | 20.81a | |
Mean values a row not sharing a superscript letter are significantly different (p<0.05, Duncan’s multiple range test)
Fig. 2.
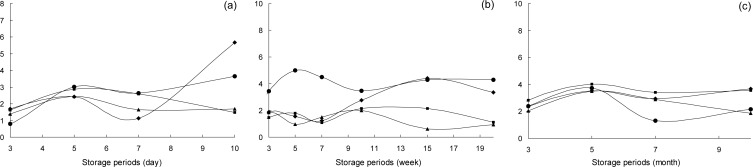
Changes in the ΔE of pre-treatment ginger paste according to storage temperature and period.
 control,
control,
 pasteurized ginger paste,
pasteurized ginger paste,
 fermented ginger paste,
fermented ginger paste,
 chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
Free sugar and organic acid
Changes in glucose, fructose, sucrose and total free sugar for ginger paste samples prepared by pre-treatment were analyzed as a function of storage temperature and period (Table 2). For room temperature storage, the fructose content in the sample containing chemical additives was 66.5 mg% after one day of storage and no free sugars were detected after seven days. For refrigerated storage, the total free sugar contents of the control, pasteurized sample and sample containing chemical additives were 162.5, 140.5 and 202.5 mg% after one day of storage while no free sugars were detected in the fermented ginger pastes. During frozen storage, the total free sugar content in pasteurized sample and sample containing chemical additives were 188.3∼201.7 mg% after one month of storage and 136.90∼170.5 mg% after ten months of storage. No free sugars were detected in the fermented samples and the decreasing of sugar content occurred more slowly than in room temperature and refrigerated storage. In addition, no free sugars were initially detected in the fermented ginger paste, but low levels of sucrose were observed after three months of storage. Kim and Lee reported that the major sugars of ginger were fructose, glucose, and sucrose and the sucrose content at initial periods of storage decreased, while the fructose and glucose content increased with storage (17).
Table 2.
Changes in the free sugar of pre-treatment ginger paste according to storage temperature and period (unit: mg%)
| Storage condition (periods) | Control | Pasteurized sample | Fermented sample | Chemical additives addition sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||
| Sucrose | Glucose | Fructose | Total | Sucrose | Glucose | Fructose | Total | Sucrose | Glucose | Fructose | Total | Sucrose | Glucose | Fructose | Total | ||
| Room temp (days) | 1 | – | 43.0 | 109.5 | 152.5 | – | 35.0 | 100.1 | 135.1 | – | – | – | – | – | – | 66.5 | 66.5 |
| 3 | – | 16.0 | 141.0 | 157.0 | – | 21.0 | 105.5 | 126.5 | – | – | – | – | – | – | 30.0 | 30.0 | |
| 5 | – | – | 97.5 | 97.5 | – | 17.5 | 96.5 | 114.0 | – | – | – | – | – | – | 23.0 | 23.0 | |
| 7 | – | – | 51.0 | 51.0 | – | 9.0 | 97.0 | 106.0 | – | – | – | – | – | – | – | – | |
| 10 | – | 4.0 | 5.5 | 9.5 | – | – | – | – | – | – | – | – | – | – | – | – | |
|
| |||||||||||||||||
| 10°C (weeks) | 1 | – | 43.0 | 119.5 | 162.5 | – | 59.0 | 81.5 | 140.5 | – | – | – | – | – | 92.0 | 110.5 | 202.5 |
| 3 | – | 35.0 | 104.5 | 139.5 | – | 78.0 | 106.0 | 184.0 | – | – | – | – | – | 53.5 | 115.5 | 169.0 | |
| 5 | – | 51.0 | 106.5 | 167.5 | – | 71.5 | 86.0 | 157.5 | – | – | – | – | – | 30.5 | 65.5 | 96.0 | |
| 7 | – | 43.0 | 103.4 | 146.4 | – | 84.3 | 74.8 | 159.1 | – | – | – | – | – | 20.3 | 39.9 | 60.3 | |
| 10 | – | 39.4 | 73.7 | 113.0 | – | 86.5 | 75.5 | 161.9 | – | – | – | – | – | 22.7 | 35.8 | 58.5 | |
| 15 | – | 56.2 | 77.3 | 133.5 | – | 98.7 | 78.9 | 177.6 | – | – | – | – | – | – | 35.8 | 35.8 | |
| 20 | – | 38.4 | 77.3 | 115.7 | – | 80.7 | 68.4 | 149.1 | – | – | – | – | – | – | – | – | |
|
| |||||||||||||||||
| −20°C (months) | 1 | – | 86.0 | 114.0 | 200.0 | 58.7 | 59.5 | 83.5 | 201.7 | – | – | – | – | – | 58.8 | 129.5 | 188.3 |
| 3 | – | 73.9 | 90.6 | 164.6 | 68.6 | 61.2 | 54.7 | 184.5 | 40.9 | – | – | 40.9 | – | 90.7 | 86.3 | 176.9 | |
| 5 | – | 73.9 | 90.6 | 181.9 | 65.6 | 61.3 | 56.8 | 183.7 | 64.9 | – | – | 64.9 | – | 84.4 | 80.8 | 165.2 | |
| 7 | – | 75.4 | 79.6 | 154.9 | 76.6 | 57.7 | 54.8 | 189.1 | 39.8 | – | – | 39.8 | – | 83.8 | 81.1 | 180.8 | |
| 10 | – | 58.8 | 78.2 | 136.9 | 72.5 | 53.1 | 44.9 | 170.5 | 24.8 | – | – | 24.8 | – | 80.8 | 77.9 | 158.8 | |
These results demonstrated the effects of pre-treatment and storage temperatures on the free sugar content in ginger paste. Fig. 3 shows the organic acid content of ginger paste as a function of storage temperature and periods. For room temperature storage, the total organic acid content for the control, pasteurized, fermented, and chemical additives samples were 2.16∼2.35%, 2.39∼ 2.79%, 1.95∼2.31% and 2.35∼3.02%, respectively, at the initial storage time. After storage for ten days, the total organic acid content of the pasteurized sample and sample containing chemical additives was 0.40∼0.58% and 0.63∼0.83%, respectively. However, the total organic acid content of fermented samples was 3.44∼3.88%.After one week of refrigerated storage, the total organic acid content of the pasteurized sample and sample containing chemical additives was 2.50∼2.71%, 4.51∼5.08 %, 3.62∼4.96% and 2.28∼2.68%, respectively. The total organic acid content decreased with storage time, 1.72 ∼1.73%, 1.98∼3.29%, and 1.42∼1.65% for the pasteurized, fermented, and chemical additives samples, respectively, after twenty weeks of storage. These results agree with studies conducted by Choi et al. who reported that the total organic acid content of ginger paste decreased with storage time (18,19). On the other hand, for frozen storage, the organic acid content did not change after ten months of storage. Generally, the total organic acid content decreased depending on pre-treatment method.
Fig. 3.
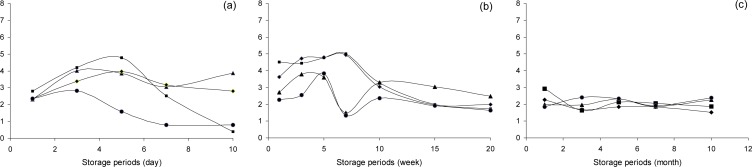
Changes in the total organic acid of pre-treatment ginger paste according to storage temperature and period.
 control,
control,
 pasteurized ginger paste,
pasteurized ginger paste,
 fermented ginger paste,
fermented ginger paste,
 chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
chemical additives addition samples. (a) storage at room temperature, (b) 10°C, (c) −20°C.
Gingerol content
Gingerols, the main pungency components of fresh ginger, contain the following major constituents: 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol. The total gingerol content in ginger paste samples prepared by pre-treatment were analyzed as a function of storage temperature and time (Table 3). Shogaol was not detected in the control and other pre-treatment gingers. At room temperature, the total gingerol content of fermented samples did not change for up to ten days of storage. The total gingerol content of the other samples was lower than the fermented samples and did not change significantly with storage time. During refrigerated storage, the total gingerol content of the control and pasteurized samples showed decreasing trends with an increase in storage time, but the fermented sample and sample containing chemical additives did not change from one to ten weeks of storage. After one month of frozen storage, the total gingerol content was 102. 9 mg% for the control and 112.1 mg% for the pasteurized samples, which decreased to 83.3 mg% and 55.88 mg%, respectively, after longer storage times. Meanwhile, the total gingerol content of the fermented sample and sample containing chemical additives decreased to 109.7 mg% and 90.6 mg%, respectively, after ten months of storage. According to previous studies, the zingeron content can be altered by high temperatures, acidic and alkali conditions, and storage periods (20). This study confirms that the pH, color value, total organic acid and gingerol contents of the samples was retained, increased or decreased with an increase in storage time depending on storage temperature and pre-treatment. More studies are needed to examine the pre-treatment effect of ginger paste according to storage temperature and periods.
Table 3.
Changes in the gingerol content of pre-treatment ginger paste according to storage temperature and period (unit: mg%)
| Storage condition (periods) | Control | Pasteurized sample | Fermented sample | Chemical additives addition sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||
| 6-gingerol | 8-gingerol | 10-gingerol | Total | 6-gingerol | 8-gingerol | 10-gingerol | Total | 6-gingerol | 8-gingerol | 10-gingerol | Total | 6-gingerol | 8-gingerol | 10-gingerol | Total | ||
| Room temp (days) | 1 | 55.8ab | 15.7b | 17.9b | 89.4ab | 61.9ab | 17.7b | 19.2a | 98.7ab | 77.4a | 23.9a | 27.8a | 129.0a | 58.1a | 19.6a | 18.4a | 96.0a |
| 3 | 51.0c | 15.3b | 15.5b | 81.8c | 65.1a | 19.8a | 19.9a | 104.8a | 65.0b | 18.9c | 22.7b | 106.5ab | 51.1b | 17.1ab | 14.3b | 82.4b | |
| 5 | 60.4ab | 16.3ab | 19.2a | 95.8ab | 56.9c | 16.8ab | 17.5ab | 91.1b | 71.5ab | 20.8ab | 22.8b | 115.1ab | 50.1b | 17.9ab | 10.6c | 78.6bc | |
| 7 | 65.8a | 18.2a | 20.9a | 104.9a | 59.9ab | 16.9ab | 19.9a | 96.6ab | 75.9a | 17.2c | 26.6a | 119.7ab | 59.5a | 18.4ab | 18.5a | 96.3a | |
| 10 | 59.1b | 16.0ab | 16.9b | 91.9ab | 59.1ab | 16.4ab | 19.2a | 94.7b | 73.0ab | 19.8ab | 24.8ab | 117.6ab | 56.1a | 16.2b | 16.1ab | 88.3b | |
|
| |||||||||||||||||
| 10°C (weeks) | 1 | 55.8b | 15.7ab | 17.9b | 89.4ab | 61.9b | 17.7b | 19.2b | 98.7b | 77.4bc | 23.9a | 27.8a | 129.0a | 58.1c | 19.6b | 18.4b | 96.0b |
| 3 | 57.7ab | 16.3ab | 20.7ab | 94.6ab | 68.9a | 19.4a | 22.4a | 110.6a | 63.5e | 17.4b | 20.3d | 101.1d | 55.4cd | 19.9b | 17.7b | 92.9b | |
| 5 | 59.3ab | 16.9ab | 19.6ab | 95.8ab | 67.8a | 19.6a | 22.6a | 109.9a | 74.1bc | 20.7ab | 27.3a | 122.0a | 71.4ab | 23.6a | 22.8ab | 117.0a | |
| 7 | 65.3a | 19.1a | 23.3a | 107.7a | 62.5b | 19.1a | 20.3a | 101.8a | 68.2d | 19.4ab | 23.4c | 111.0c | 74.0a | 22.6a | 24.4a | 121.0a | |
| 10 | 53.4b | 12.2b | 14.9c | 80.5b | 60.8b | 10.6a | 13.4c | 84.7c | 70.2c | 13.1c | 25.8b | 109.1c | 61.3b | 11.7d | 15.3c | 88.2c | |
| 15 | 52.6b | 10.4bc | 14.9c | 77.8c | 59.5bc | 11.1c | 12.6c | 82.9c | 81.7ab | 13.9c | 28.2a | 123.8a | 61.5b | 10.5d | 13.8d | 85.8cd | |
| 20 | 59.9ab | 9.7c | 10.9d | 80.5b | 52.9c | 10.0c | 11.7c | 74.6d | 96.9a | 14.5c | 28.2a | 139.5b | 75.9a | 12.7c | 17.4b | 106.0a | |
|
| |||||||||||||||||
| −20°C (months) | 1 | 63.4ab | 18.3a | 21.3a | 102.9a | 68.9ab | 19.9a | 23.3ab | 112.1a | 74.1a | 21.2a | 23.7ab | 118.9a | 58.6ab | 18.0a | 18.5bc | 95.0a |
| 3 | 59.4c | 10.8c | 15.5b | 85.6c | 70.7a | 12.9b | 18.5b | 102.1ab | 66.4b | 12.6c | 17.6c | 96.5c | 60.5a | 12.1b | 15.6d | 88.1b | |
| 5 | 66.9a | 12.1b | 14.1b | 93.2ab | 60.5b | 11.3b | 21.7ab | 93.5b | 64.6b | 12.7c | 22.0ab | 99.2b | 59.3a | 11.6b | 21.6b | 92.4a | |
| 7 | 68.9a | 12.9b | 13.5c | 95.3ab | 60.1b | 12.8b | 26.4a | 99.3ab | 69.9ab | 15.4b | 28.0a | 113.3a | 61.9a | 10.2b | 22.7a | 94.8a | |
| 10 | 59.8c | 10.9c | 12.7c | 83.3c | 33.6c | 7.2c | 15.1c | 55.8d | 71.7ab | 13.6c | 24.4ab | 109.7a | 56.9c | 9.6c | 24.1a | 90.6ab | |
Mean values a row not sharing a superscript letter are significantly different (p<0.05, Duncan’s multiple range test).
Acknowledgments
This study was supported by the research grant from Korea Research Foundation, Republic of Korea, which is gratefully appreciated.
REFERENCES
- 1.Connell DW. The pungent principles of ginger and their importance in certain ginger products. Food Technol Australia. 1969;21:570–575. [Google Scholar]
- 2.Lee YN. Flora of Korea. Kyohaksa; Seoul, Korea: 1996. pp. 1107–1109. [Google Scholar]
- 3.Lee CB. Illustrated Flora of Korea. Hyangmoon Publish Co.; Seoul, Korea: 1979. p. 231. [Google Scholar]
- 4.Kim JS, Koh MS, Kim YH, Kim MK, Hong JS. Volatile flavor components of Korean ginger. Korean J Food Sci Technol. 1991;23:141–149. [Google Scholar]
- 5.Enmaya H. Dictionary of Food Science. Dou Dan Publishing Co.; Tokyo, Japan: 1981. p. 300. [Google Scholar]
- 6.Choi MS, Kim DH, Lee KH, Lee YC. Effect of additives on quality attributes of minced ginger during refrigerated storage. Korean J Food Sci Technol. 2002;34:1048–1056. [Google Scholar]
- 7.Jeong MC. Flavor characteristics of ginger powder produced by enzymatic liquefaction process. 1997. PhD Dissertation. University of Chung-Ang, Seoul, Korea.
- 8.Chung TY, Lee SE, Jeong MC, Kim DC. Studies on the pretreatment effect of ginger on long-term storage. Korean J Food Sci Technol. 1996;28:458–463. [Google Scholar]
- 9.Chung HS, Lee HJ, Seong JH, Moon KD. Effect of heat pretreatment on the quality under storage of fresh ginger rhizomes. Korean J Food Preserv. 2009;16:623–628. [Google Scholar]
- 10.Jo KS, Kim JH, Shin HS. Major components affecting nonenzymatic browning in ginger paste during storage. Korean J Food Sci Technol. 1996;28:433–439. [Google Scholar]
- 11.Kim DH, Lee YC. Quality changes in minced ginger prepared with frozen ginger during storage. Korean J Food Sci Technol. 2004;36:943–951. [Google Scholar]
- 12.Jo KS, Kim JH, Shin HS. Storage stability of ginger paste. J Korean Soc Food Sci Nutr. 1997;26:1140–1146. [Google Scholar]
- 13.Jo KS, Chang YS, Shin HS. Inhibiting factors and kinetics of nonenzymatic browning in ginger paste model system. J Korean Soc Food Sci Nutr. 1997;26:1135–1139. [Google Scholar]
- 14.Sanford PA. Exocellular, microbial polysaccharides. Adv Carbohyd Chem Biochem. 1979;36:265–313. doi: 10.1016/s0065-2318(08)60238-3. [DOI] [PubMed] [Google Scholar]
- 15.Cheigh HS, Kim HY, Yeo KM, Kim BN. Fermentation aspects of fruit-vegetable juice by mixed cultures of lactic acid bacteria isolated from kimchi and yeast. J Korean Soc Food Sci Nutr. 1998;27:1059–1064. [Google Scholar]
- 16.Morichi T. Characteristic and utilization of lactic acid bacteria. Progress in recent researches. Milk Sci. 1997;46:1–20. [Google Scholar]
- 17.Kim DH, Lee YC. Quality changes in minced ginger prepared with frozen ginger during storage. Korean J Food Sci Technol. 2004;36:943–951. [Google Scholar]
- 18.Choi YH, Kim MS. Effects of CO2 absorbent in the PE film bag and styroform box during the ginger storage. Korean J Postharvest Sci Technol. 2001;8:286–290. [Google Scholar]
- 19.Choi YH, Lee SB, Kim MS. Improvement of quality and prolongation in chopped ginger storage. Agric Chem Biotechnol. 1996;40:123–127. [Google Scholar]
- 20.Aeschbach R, Loliger J, Scott BC, Murcis A, Buler J, Halliwell B, Agnoma OI. Antioxidant actions of thymol, carbacrol, 6-gingrerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]


