Abstract
This study was designed to investigate whether Sargassum ringgoldianum extract may inhibit α-glucosidase and α-amylase activities, and alleviate postprandial hyperglycemia in streptozotocin-induced diabetic mice. The IC50 values of Sargassum ringgoldianum extract against α-glucosidase and α-amylase were 0.12 mg/mL and 0.18 mg/mL, respectively, which evidenced higher activities than those of acarbose. The blood glucose levels of the Sargassum ringgoldianum extract administered group were significantly lower compared to the control group in the streptozotocin-induced diabetic mice. Moreover, the area under the two-hour blood glucose response curve was significantly reduced and the absorption of dietary carbohydrates was delayed after administration of Sargassum ringgoldianum extract in the diabetic mice. Therefore, these results indicated that Sargassum ringgoldianum extract may help decrease the postprandial blood glucose level via inhibiting α-glucosidase.
Keywords: Sargassum ringgoldianum extract, α-glucosidase, hypoglycemic effect, diabetic mice
INTRODUCTION
The prevalence of diabetes is increasing worldwide, and many diagnosed with this disease will die or become disabled due to complications (1,2). Postprandial blood glucose levels may elevate while fasting plasma glucose levels remain normal, which some have called “postprandial diabetes” (3). This state not only initiates the development of early microvascular and macrovascular complications, but it also can contribute to a more rapid progression to symptomatic diabetes by causing glucose toxicity in muscles and pancreatic beta cells. The control of postprandial hyperglycemia, therefore, offers the potential for early intervention and prevention of diabetes complications (4).
α-Glucosidase and α-amylase play a significant role in the digestive process of dietary complex carbohydrates; inhibition of both enzymes can retard the digestion of carbohydrates, delay glucose absorption, and reduce plasma glucose levels, resulting in a decrease in postprandial hyperglycemia (5). Therefore, reducing postprandial hyperglycemia levels has been considered one of the most effective therapeutic approaches, with fewer disadvantages than earlier diabetic treatments (6–8). Continuous use of synthetic agents, such as gliclazide, metformin and voglibose, should be limited because they may cause flatulence, abdominal cramps, vomiting, diarrhea, and other side effects (9). With respect to suppression of glucose production from carbohydrates and glucose absorption from the intestine, increasing efforts are being made to find and investigate potential inhibitors of α-glucosidase and α-amylase in natural products showing no side effects (6–8).
Marine macroalgae, or seaweeds, are one of nature’s most biologically active resources and possess a wealth of bioactive compounds. Seaweed extracts have demonstrated various biological activities, such as antioxidant potential (10,11), anti-inflammatory properties (12), and anti-coagulant (13) and apoptotic activities (14). Sargassum ringgoldianum, belonging to the Sargassaceae family, is regarded as an edible brown alga and grows on the coast of Jeju Island, Korea. S. ringgoldianum extract (SRE) is rich in minerals, water-soluble polysaccharides and phenolic compounds (15). The SRE contained the highest amount of phenolic compounds among seaweeds screened for antioxidative activities. The SRE also had the strongest scavenging activity against the superoxide anion radical and hydroxyl radical among seaweeds (16). The biological benefits of SRE, including antioxidant (15–18), anti-tumor (18), anti-coagulant (19), anti-hyper-lipidemic, anti-hypertensive and anti-arteriosclerosis activities (20,21), have been shown in several studies. However, the hypoglycemic effect of SRE has yet to be elucidated. Therefore, this study was designed to investigate whether or not SRE inhibits α-glucosidase and α-amylase activities, and alleviates postprandial hyper-glycemia in streptozotocin (STZ)-induced diabetic mice.
MATERIALS AND METHODS
Materials and preparation of S. ringgoldianum extract
S. ringgoldianum algae was collected from the coast of Jeju Island, South Korea. The sample was first washed 3 times with tap water to remove salt, epiphytes, and sand attached to the surface, and then carefully rinsed with fresh water. Thereafter, the sample was lyophilized using a vacuum freeze dryer (Samwon Freezing Engineering Co., Busan, Korea) and homogenized with a grinder (Shinhan Science & Technology Co., Kyunggi, Korea). The S. ringgoldianum powder was extracted 3 times with 80% methanol, filtered through Whatman No. 1 filter paper, and evaporated under a vacuum using a rotary evaporator (BUCHI Co., Flawil, Switzerland). The extract was thoroughly dried for removal of solvents and stored in a deep freezer (−80°C) (22). 2.8 g of extract per 13.0 g of powdered S. ringgoldianum was obtained.
In vitro inhibition assay for α-glucosidase activity
The α-glucosidase inhibitory assay was conducted using the chromogenic method developed by Watanabe et al. (23). Briefly, yeast α-glucosidase (0.7 U, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 100 mM phosphate buffer (pH 7.0), containing 2 g/L bovine serum albumin and 0.2 g/L NaN3, and used as the enzyme solution. A 5 mM p-nitrophenyl-α-D-glucopyranoside (pNGP) in the same buffer (pH 7.0) was used as the substrate solution. 50 μL of enzyme solution and 10 μL of sample dissolved in dimethyl sulfoxide at a 5 mg/mL concentration were mixed in a well and absorbance was measured at 405 nm using a microplate reader. After incubation for 5 min, substrate solution (50 μL) was added and incubated for another 5 min at room temperature. The increase in absorbance from zero time was measured. Inhibitory activity was expressed as 100 minus relative absorbance difference (%) of test compounds to absorbance change of the control where test solution was replaced by carrier solvent. The measurements were performed in triplicate and the IC50 value, i.e., the concentration of the extracts that results in 50% inhibition of maximal activity, was determined.
In vitro inhibition assay for α-amylase activity
α-Amylase inhibitory activity was assayed in the same way (23) as described previously for α-glucosidase inhibitory assay, except that porcine pancreatic amylase (100 U, Sigma-Aldrich) and blocked p-nitrophenyl-α-D-maltopentoglycoside (Sigma-Aldrich) were used as enzyme and substrate, respectively.
Experimental animals
Four-week old male ICR mice (Orient Inc., Seoul, Korea) were kept under a 12 hr light/ 12 hr dark cycle with controlled room temperature. The animals were maintained with a 5L79 diet (Labdiet, Richmond, IN, USA), while tap water was available ad libitum. After an adjustment period of 2 weeks, diabetes was induced by intraperitoneal injection of STZ (60 mg/kg) freshly dissolved in a citrate buffer (0.1 M, pH 4.5) for the fasted (18 hr) animals. After 7 days, tail bleeds were performed and animals with a blood glucose concentration above 250 mg/dL were considered diabetic. The mice were administered orally soluble starch (2 g/kg BW) alone (control) or with SRE (300 mg/kg BW) or acarbose (50 mg/kg BW) dissolved in 0.2 mL of water.
Measurement of blood glucose level
Both normal mice and STZ-induced diabetic mice fasted overnight were randomly divided into 3 groups of 7 mice. Fasted animals were deprived of food for at least 12 hr but allowed free access to water. After overnight fasting, the mice were orally administered either soluble starch (2 g/kg BW) alone (control) or starch with SRE (300 mg/kg BW). Blood samples were taken from the tail vein at 0, 30, 60, and 120 min. Blood glucose was measured using a glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Areas under the curve (AUC) were calculated using the trapezoidal rule (24).
Statistical analysis
The data are represented as the mean±standard deviation (SD) of triplicate experiments. The statistical analysis was performed using SAS software. The Student’s t-test was used for comparisons between control and sample groups. The values were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple range tests, and p-values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Inhibitory effect of SRE on α-glucosidase and α-amylase in vitro
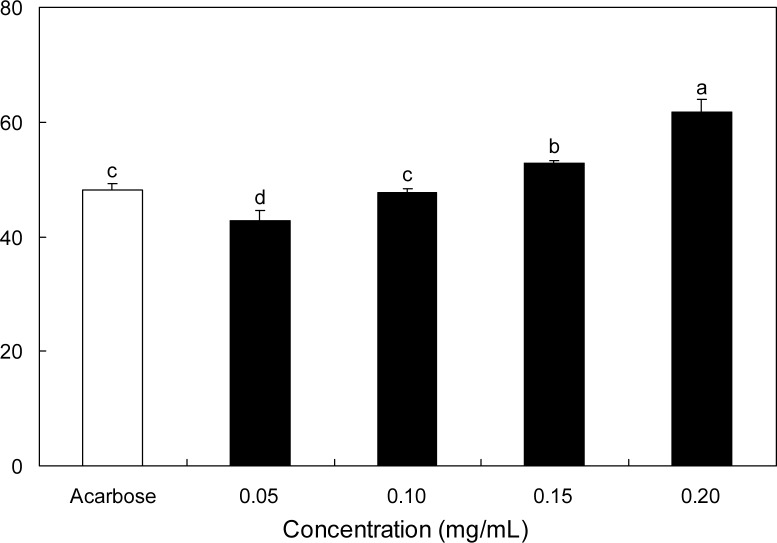
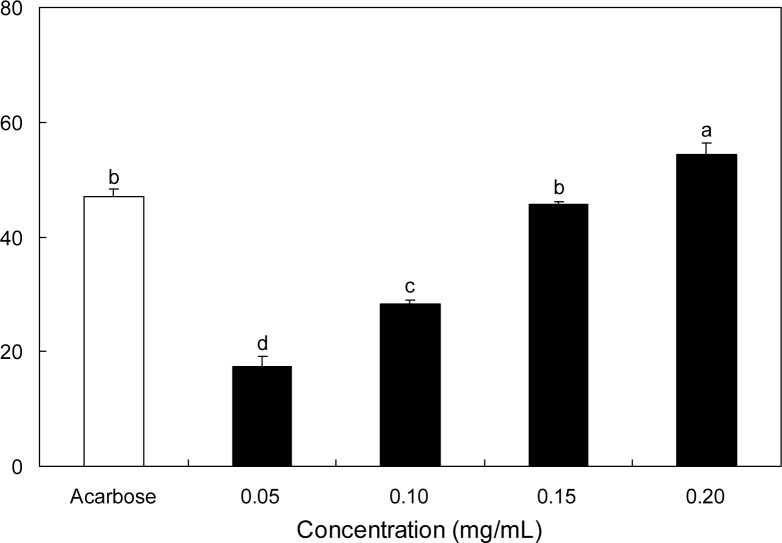
The inhibitory effect of SRE against α-glucosidase was determined using p-nitrophenyl-α-glucopyranoside (pNPG) as the substrate. SRE inhibited α-glucosidase activity in a dose-dependent manner by 42.88, 47.63, 52.85, and 61.79% at 0.05, 0.10, 0.15, and 0.20 mg/mL concentrations, respectively (Fig. 1). Acarbose, the α-glucosidase inhibitor used as an oral hypoglycemic agent, at 0.20 mg/mL, inhibited the enzyme activity by 48.04%. Thus, 0.20 mg/mL of SRE inhibited the α-glucosidase activity significantly higher than the same concentration of acarbose. The dose-depedent α-amylase inhibitory effect of SRE was illustrated using p-nitrophenyl-α-maltopenotoglycoside (pNPM) as a substrate (Fig. 2). The α-amylase inhibitory activity of SRE at the concentration of 0.20 mg/mL was significantly higher than that of acar-bose at the same concentration. IC50 values of SRE against α-glucosidase and α-amylase were 0.12 and 0.18 mg/mL, respectively (Table 1).
Fig. 1.
Inhibitory activity of S. ringgoldianum extract on α-glucosidase. Each value is expressed as mean±SD in triplicate experiments.a–dValues with different alphabets on bars are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as a positive control was 0.20 mg/mL.
Fig. 2.
Inhibitory activity of S. ringgoldianum extract on α-amylase. Each value is expressed as mean±SD in triplicate experiments. a–dValues with different alphabets on bars are significantly different at p<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as a positive control was 0.20 mg/mL.
Table 1.
IC50 value of S. ringgoldianum extract (SRE) on α-glucosidase and α-amylase
| Sample | IC50 (mg/dL)1)
|
|
|---|---|---|
| α-Glucosidase | α-Amylase | |
| SRE | 0.12±0.02* | 0.18±0.01* |
| Acarbose | 0.26±0.04 | 0.23±0.02 |
IC50 value is the concentration of sample required for 50% inhibition. Each value is expressed as mean±SD (n=3).
Significantly different from control at p<0.05.
Postprandial hyperglycemia is a prominent and early defect in the development of diabetes. The treatment goal for patients with diabetes is to maintain near-normal levels of glycemic control, both in the fasting and post-prandial states (4). While breaks down starch, glycogen and oligosaccharides by catalyzing the hydrolysis of α-1,4-glucosidic linkages, further breaks down the disaccharides into simpler sugars, readily available for intestinal absorption (8). Inhibitors of both α-amylase and α-glucosidase delay and prolong overall carbohydrate digestion, causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise (25). According to our data, SRE had significantly higher inhibitory activities than acarbose on α-glucosidase and α-amylase, suggesting SRE has proven to be useful in terms of diabetic control.
SRE contained a mixture of phlorotannins, high molecular weight polyphenols found to be polymerized bifuhalol (16). Polyphenolic compounds, such as tannins from terrestrial plants and phlorotannins from marine algae, are known to be associated with a variety of proteins to form complexes (26). The hydroxyl groups in phlorotannin derivatives may play an important role in promoting inhibitory activities. For example, o-quinones derived from catechols are covalently bound to amino acids and thiol groups; phlorotannins seem to be bound to active or binding sites of these enzymes, resulting in inhibition of their enzyme activities (27). Thus, SRE could be an effective inhibitor of intestinal α-glucosidase and α-amylase activities, which may prove to be synergistic in their potential therapeutic effects against the post-prandial plasma glucose rise.
Effect of SRE on blood glucose levels in vivo
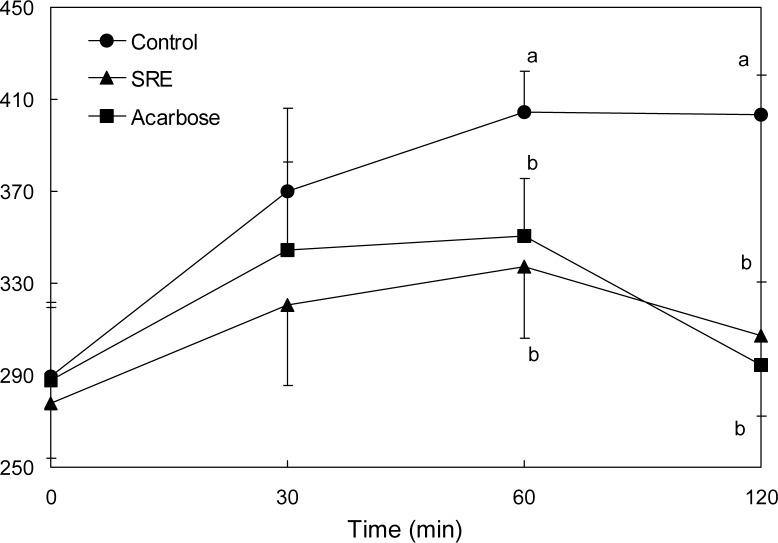
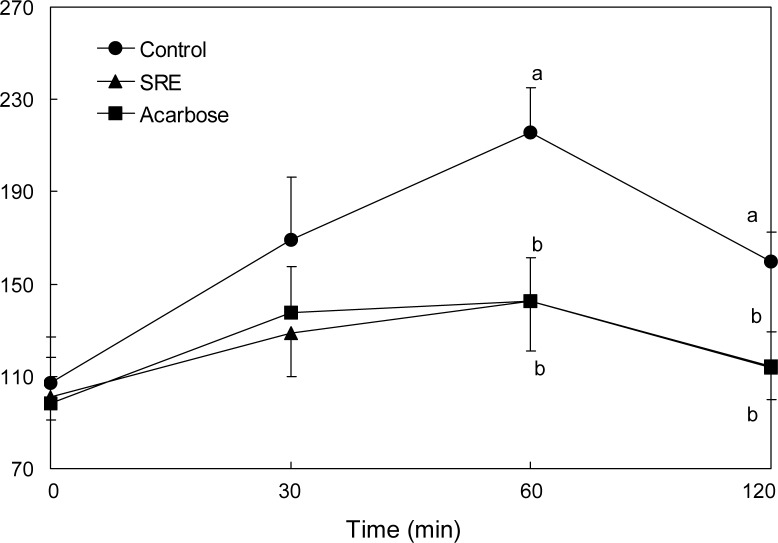
The effect of SRE on blood glucose levels after a meal was investigated in streptozotocin-induced diabetic and normal mice. Postprandial blood glucose levels of SRE-administered diabetic mice were significantly lower than control group mice (Fig. 3). The blood glucose levels of diabetic mice in the control group were 370.0, 404.5 and 403.5 mg/dL at 30, 60 and 120 min, respectively. However, the increase in postprandial blood glucose level was significantly reduced (p<0.05) in diabetic mice administered SRE (320.7, 337.3 and 307.0 mg/dL at similar respective time points). The postprandial blood glucose level was also significantly decreased when normal mice were orally administered starch with SRE (Fig. 4). In addition, the blood glucose levels of the SRE and acarbose administered groups were similar in both the streptozotocin-induced diabetic and the normal mice.
Fig. 3.
Blood glucose level after administration of S. ringgoldianum extract (SRE) in STZ-induced diabetic mice. Control (distilled water), SRE (300 mg/kg), and acarbose (50 mg/ kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of 7 mice (n=21). a,bValues with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test.
Fig. 4.
Blood glucose level after administration of S. ringgoldianum extract (SRE) in normal mice. Control (distilled water), SRE (300 mg/kg), and acarbose (50 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of 7 mice (n=21). a,bValues with different alphabets are significantly different at p<0.05 as analyzed by Duncan’s multiple range test.
The area under the two-hour blood glucose response curve (AUC) of the SRE administered group (636.3±64.3 mg·hr/dL) was significantly lower (p<0.05) than the control group (762.5±48.0 mg·hr/dL) in diabetic mice (Table 2). The AUC in normal mice corroborated with the hypoglycemic effect of SRE.
Table 2.
Area under the curve (AUC) of postprandial glucose responses of normal and streptozotocin-induced diabetic mice
| Group1) | AUC (mg·hr/dL)
|
|
|---|---|---|
| Normal mice | Diabetic mice | |
| Control | 353.1±39.5a | 762.5±48.0a |
| SRE | 253.5±34.9b | 636.3±64.3b |
| Acarbose | 257.6±36.3b | 654.5±63.8ab |
Control (distilled water), S. ringgoldianum extract (SRE, 300 mg/kg), and acarbose (50 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of 7 mice (n=42).
Values with different alphabets in a column are significantly different at p<0.05 as analyzed by Duncan’s multiple range test.
Controlling not only fasting, but also postprandial hyperglycemia, are important in maintaing blood glucose levels, the major target of diabetic therapy (28). According to a previous study, postprandial hyperglycemia might be more strongly correlated with cardiovascular morbidity and mortality than fasting hyperglycemia (29). Early identification of postprandial hyperglycemia and its effective control, therefore, offer the potential for early intervention and prevention of diabetes complications (4). Thus, we determined the anti-hyperglycemic effect by SRE in streptozotocin-induced diabetic and normal mice after consumption of starch. The increase in post-prandial blood glucose level was significantly suppressed by SRE administration in both streptozotocin-induced diabetic and normal mice groups. This result indicates that SRE may delay the absorption of dietary carbohydrates consumed, thereby suppressing an increase in post-prandial blood glucose levels. Acarbose which flattens postprandial blood glucose peak reduces the AUC of the blood glucose response curve (30). In this study, SRE decreased both blood glucose level at the peak time point and the AUC.
Type 2 diabetes is a chronic disease characterized by simultaneous insulin deficiency and insulin resistance events, with the resultant hyperglycemia leading to microvascular and macrovascular complications. A large number of intervention trials demonstrated that improving glycemic control achieves considerable reduction of these complications (31–33). α-Glucosidase and α-amylase play a significant role during dietary complex carbohydrate digestion. The main benefits attributable to α-glucosidase inhibitors are reductions both in postprandial glycemia levels and in the total range of postprandial glucose levels (5). Many synthetic compounds such as gliclazide, metformin and voglibose have been used in the treatment of diabetes; however, many have been associated with marked toxic or undesirable side effects (9). Therefore, marine algae have become good candidates for the source of natural anti-diabetic materials (26). In an earlier report, phloroglucinol derivatives isolated from Ecklonia cava have the potential to prevent diabetes mellitus because of their high α-glucosidase and α-amylsase inhibitory activities (27). Brown algae extract may have a beneficial effect on controlling post-prandial glucose levels in diabetic obese rats (34). Our current finding showed that SRE could potentially be developed as a novel natural nutraceutical to improve postprandial hyperglycemia and prevent diabetic complications because of its strong α-glucosidase inhibitory activity.
In conclusion, SRE inhibited α-glucosidase and α-amylase activities followed by a diminished rise in blood glucose, resulting in a reduction in postprandial hyper-glycemia. Furthermore, SRE may delay the absorption of dietary carbohydrates in the intestine, leading to suppression of an increased blood glucose level after a meal. Thus, we suggest that SRE could be a potential candidate in developing medicinal preparations and nutraceutical for diabetes and related symptoms.
Acknowledgments
This research was financially supported by the National research foundation of Korea through the project.
REFERENCES
- 1.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Cause-specific mortality in a population with diabetes. Diabetes Care. 2002;25:43–48. doi: 10.2337/diacare.25.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Fos CS, Coady S, Sorlie PD, D’Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the framingham heart study. Circulation. 2007;115:1544–1150. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD. Postprandial hyperglycaemia and a-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40(suppl):S51–S55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 4.Ratner RE. Controlling postprandial hyperglycemia. Am J Cardiol. 2001;8(suppl):26H–31H. doi: 10.1016/s0002-9149(01)01834-3. [DOI] [PubMed] [Google Scholar]
- 5.Lebovitz HE. Alpha-glucosidase inhibitors. Endocrinol Metabol Clin North Am. 1997;26:539–551. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 6.Fernando MR, Wickramasingle N, Thabrew MI, Ariyananda PL, Karunanayake EH. Effect of Artocarpus heterophyllus and Asteracanthus longifolia on glucose tolerance in normal human subjects and inmaturity-onset diabetic patients. J Ethnopharmacol. 1991;31:277–282. doi: 10.1016/0378-8741(91)90012-3. [DOI] [PubMed] [Google Scholar]
- 7.Welsh PA, Lachance CA, Wasserman BP. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698–1704. doi: 10.1093/jn/119.11.1698. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari MR, Anurakkun NJ, Hong G, Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem. 2008;106:247–252. [Google Scholar]
- 9.Hanefeld M. The role of acarbose in the treatment of non-insulindependent diabetes mellitus. J Diabetes Complicat. 1998;12:228–237. doi: 10.1016/s1056-8727(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 10.Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Chandini SK, Ganesan P, Bhaskar N. In vitro anti-oxidant activities of three selected brown seaweeds of India. Food Chem. 2008;107:707–713. [Google Scholar]
- 12.Kang JY, Khan MNA, Park NH, Cho JY, Lee MC, Fujii H, Hong YK. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–190. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Pushpamali WA, Nikapitiya C, De Zoysa M, Whang I, Kim SJ, Lee J. Isolation and purification of an anticoagulant from fermented red seaweed Lomentaria catenata. Carbohydr Polym. 2008;73:274–279. [Google Scholar]
- 14.Kwon MJ, Nam TJ. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006;79:1956–1962. doi: 10.1016/j.lfs.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Kuda T, Ikemori T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009;112:575–581. [Google Scholar]
- 16.Nakai M, Kageyama N, Nakahara K, Miki W. Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Marine Biotechnology. 2006;8:409–414. doi: 10.1007/s10126-005-6168-9. [DOI] [PubMed] [Google Scholar]
- 17.Heo SJ, Park EJ, Lee KW, Jeon YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol. 2005;96:1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Yang HP. Antioxidant and antitumor activities of enzymatic extracts from Sargassum coreanum. 2007. PhD Dissertation, Cheju National University, Jeju, Korea.
- 19.Athukorala Y, Lee KW, Kim SK, Jeon YJ. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol. 2007;98:1711–1716. doi: 10.1016/j.biortech.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Athukorala Y, Jeon YJ. Screening for angiotensin 1-converting enzyme inhibitory activity of Ecklonia cava. J Food Sci Nutr. 2005;10:134–139. [Google Scholar]
- 21.Ren D, Noda H, Amano H, Nishino T, Nishizawa K. Study on antihypertensive and antihyperlipidemic effects of marine algae. Fisheries Science. 1994;60:83–88. [Google Scholar]
- 22.Min KH, Kim HJ, Jeon YJ, Han JS. Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/ KsJ-db/db mice. Diabetes Res Clin Pract. 2011;93:70–76. doi: 10.1016/j.diabres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of alpha-glucosidase inhibitors from tochucha (Eucommia ulmoides) Biosci Biotechnol Biochem. 1997;61:177–178. doi: 10.1271/bbb.61.177. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS. Effect of Rhemanniae radix on the hyper-glycemic mice induced with streptozotocin. J Korean Soc Food Sci Nutr. 2004;33:1133–1138. [Google Scholar]
- 25.Hasenah A, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes: with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Kim KY, Nama KA, Kurihara H, Kim SM. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69:2820–2825. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Li Y, Karadeniz F, Kim MM, Kim SK. α-Glucosidase and α-amylase inhibitory activities of phloroglucinol derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agric. 2009;89:1552–1558. [Google Scholar]
- 28.Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004;164:486–491. doi: 10.1001/archinte.164.5.486. [DOI] [PubMed] [Google Scholar]
- 29.Haller H. The clinical importance of postprandial glucose. Diabetes Res Clin Pract. 1998;40:S43–49. doi: 10.1016/s0168-8227(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 30.Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyper-proinsulinemia in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1997;36:143–151. doi: 10.1016/s0168-8227(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 31.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 32.Stettler C, Allemann S, Jü ni P, Cull CA, Holman RR, Egger M, Krähenbühl S, Diem P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152:27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 34.Nam JS, Lee WJ, Yoon IS, Kang MW, Jang HS, Youn JH, Kim BR, Kong HJ, Kim KH, Kim YH, Lee DS, Choi HJ. Effect of a brown algae extract on postprandial glucose control in neonatal diabetic and obese rats. J FASEB. 2007;21(845):2. [Google Scholar]