Abstract
The inhibitory effect of polyphenol extracts (Seapolynol™, SPN) of the marine brown algae Ecklonia cava and dieckol, a major component of SPN, on hyperlipidemia was investigated in ICR mice fed a high-fat diet (HFD) for five weeks. For analysis of the anti-hyperlipidemic effects of SPN and dieckol, these two agents were given orally on a daily basis to HFD-fed mice for four weeks, starting one week after the beginning of HFD feeding. Groups administered with SPN as well as dieckol showed lower body weight gains than the HFD only group. Administration of SPN and dieckol also resulted in a significant reduction of the level of total cholesterol (TCHO), triglyceride (TG), and low-density lipoprotein (LDL) cholesterol in the serum of HFD-fed mice. In Oil Red O staining using 3T3-L1 preadipocytes, it was shown that both SPN and dieckol markedly inhibited lipid accumulation of 3T3-L1 cells. Furthermore, SPN and dieckol (50 μg/mL) significantly inhibited 3-hydroxyl-methyl glutaryl coenzyme A (HMGCoA) reductase activity in vitro. Taken together, these results suggest that polyphenols of Ecklonia cava (SPN) and dieckol reduce body weight gain and fat accumulation in HFD-induced obese mice, and that their hypolipidemic effect is related to the inhibition of adipogenesis of adipocytes and HMGCoA reductase activity.
Keywords: hyperlipidemia, Ecklonia cava, Seapolynol™, polyphenols, dieckol
INTRODUCTION
It is well known that hyperlipidemia is one of important risk factors involved in the development of cardiovascular disease (1). Treatment of hyperlipidemia involves diet control, exercise, and the use of lipid-lowering diets and drugs (2). However, some patients cannot tolerate the adverse effects of these oral drugs (3); therefore, there continues to be a high demand for new oral anti-hyperlipidemic drugs.
Marine algae are widely distributed and abundant throughout the coastal areas of many countries and are a rich source of useful substances such as agar, carra-genean and alginate (4). Furthermore, secondary metabolites produced in marine algae are known to be sources of natural compounds with interesting pharmaceutical properties. In particular, interest in polyphenol compounds from marine algae is increasing as we learn more about their pharmacological actions (5,6).
Brown algae have long been used traditionally as foodstuffs and folk medicines in Asian countries. Among many brown algae, Ecklonia cava contains unique polyphenols called “phlorotannins”. Although these species contain biologically active polysaccharides and lipids such as fucoidan, laminaran, fucoxanthin and fucosterol, an increasing number of reports indicate that many of the medicinal properties of these species are derived from their polyphenols. Recently, phlorotannins have been reported to possess antioxidant (7), antibacterial (8), anti-inflammatory (9), plasmin activating (10), vasodilatory (11), anti-matrix metalloproteinase (MMP) (6), anti-HIV (12) and anticarcinogenic (13) activities in both in vitro and in vivo models. In addition, phlorotannins isolated from Ecklonia stolonifera, have been reported to show anti-diabetic (14) effects in genetically diabetic KK-A(y) mice. Since those reported activities of the phlorotannins are highly relevant in normalizing metabolic functions, phlorotannins are considered to be highly promising nutrients in the moderation of metabolic syndromes. However, there is yet no study reporting anti-hyperlipidemic effect of Ecklonia cava polyphenols in animal models. In this study, we investigated the inhibitory effect of purified polyphenols from Ecklonia cava (Seapolynol™, SPN) and dieckol, a major component of SPN (average content of 8.2%), on hyperlipidemia induced by high-fat diet (HFD) in mice, and on adipogenesis in 3T3-L1 cells.
MATERIALS AND METHODS
Materials
Polyphenol extract from Ecklonia cava (Seapolynol™, SPN) and dieckol were kindly supplied by Botamedi, Inc. (Jeju, Korea). The total polyphenol content of SPN as phloroglucinol equivalent was 98.5%. Notable compounds in SNP identified by HPLC are dieckol, 8,8′-bieckol, 6,6′-bieckol, and phlorofurofucoeckol A (Waters, Milford, MA, USA) column: CAPCELL PAK ODS column (4.6×250 mm) (Fig. 1); eluent: 30% aqueous MeOH; flow rate: 0.8 mL/min. Dieckol was isolated and characterized as described previously (15). The other chemicals used in this study were purchased from Sigma (St. Louis, MO, USA).
Fig. 1.
Chemical structures of phlorotannins.
Animals and diets
Male ICR mice (7 weeks of age, 10 mice per group) were purchased from Damul Sci. (Daejeon, Korea) and allowed free access to commercial chow (normal diet) for 7 days. Thereafter, the mice were maintained for 5 weeks on a normal diet or HFD (Table 1). Various doses of SPN (1.25, 2.5 and 5 mg/mouse) and dieckol (0.5, 1 and 2 mg/mouse) were administered orally into HFD-fed mice daily for 4 weeks from 1 week after the beginning of HFD feeding. Fenofibrate, a drug of the fibrate class having a potent hypolipidemic effect (16), was used as a positive control. All animal experiments were carried out in accordance with the Guidelines for Institutional Animal Care and Use Committee of Konyang University.
Table 1.
Composition of experimental diets (g/kg diet)
| Normal | HFD1) | |
|---|---|---|
| Casein (from milk) | 200 | 200 |
| Corn starch | 350 | 155 |
| Sucrose | 300 | 50 |
| Dextrose | 50 | 132 |
| Cellulose | 50 | 50 |
| Soybean oil | – | 25 |
| Lard | – | 175 |
| Mineral mixture | 35 | 35 |
| Vitamin mixture | 10 | 10 |
| TBHQ2) | – | 0.014 |
| L-cystein | 3 | 3 |
| Choline bitartrate | 2 | 2.5 |
High-fat diet.
Tertiary butylhydroquinone.
Cell culture and adipocyte differentiation
Mouse fibroblast line 3T3-L1 preadipocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in humidified atmosphere of 5% CO2/95% air at 37°C to confluence. 3T3-L1 cells (1×105/well) were plated in 6-well plates, and cultured for three days. Thereafter, induction of adipocyte differentiation was initiated by treatment of the cells with the differentiation medium containing 1 μM insulin, 1 μM dexamethasone (Dex), and 0.5 mM isobutyl methyl xanthine (IBMX) for 3 days, followed by 3 days of treatment with the medium containing 1 μM insulin alone. The cells were maintained in DMEM complete medium for the following 4 days. SPN and dieckol (∼200 μg/mL) were added into 3T3-L1 cells during the induction of adipocyte differentiation. Differentiation of preadipocytes to mature adipocytes was confirmed by observation using microscope and by Oil Red O staining of lipid vesicles (17).
Oil Red O staining and measurement of TG contents
Four days after the induction of differentiation, 3T3-L1 cells were rinsed twice with phosphate-buffered saline (PBS), fixed with 10% formalin for 1 hr, and washed three times with PBS. Cells were stained with filtered Oil Red O (0.3%) working solution for 20 min, and then the stained cells were washed three times with distilled water. The stained TG droplets were photographed using an Olympus CKX41SF (Tokyo, Japan) inverted microscope with a digital imaging device. Cells at the designated stage of induction of differentiation were washed twice with PBS and lysed by dimethyl sulfoxide (DMSO). Absorbance of TG in the cell lysates was measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader (BioTek, Winooski, VT, USA) (18).
Serum analysis
The levels of TCHO, TG, HDL-cholesterol, glucose, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), creatinine (CRE) and in the serum of mice were determined using DryChem 3500i (Fuji, Tokyo, Japan)the manufacturer’s directionstotal.
Measurement of HMGCoA reductase inhibition
Inhibitory effect of SPN and dieckol on enzyme activity HMGCoA reductase was measured using a HMGCoA reductase assay kit (Sigma) according to the manufacturer’s directions. As a positive inhibitor for HMGCoA reductase in this assay, the pravastatin included in the assay kit was used.
Statistical analysis
Results were expressed as mean±standard error, and compared using Student’s t-test. The value of p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Body weight gains
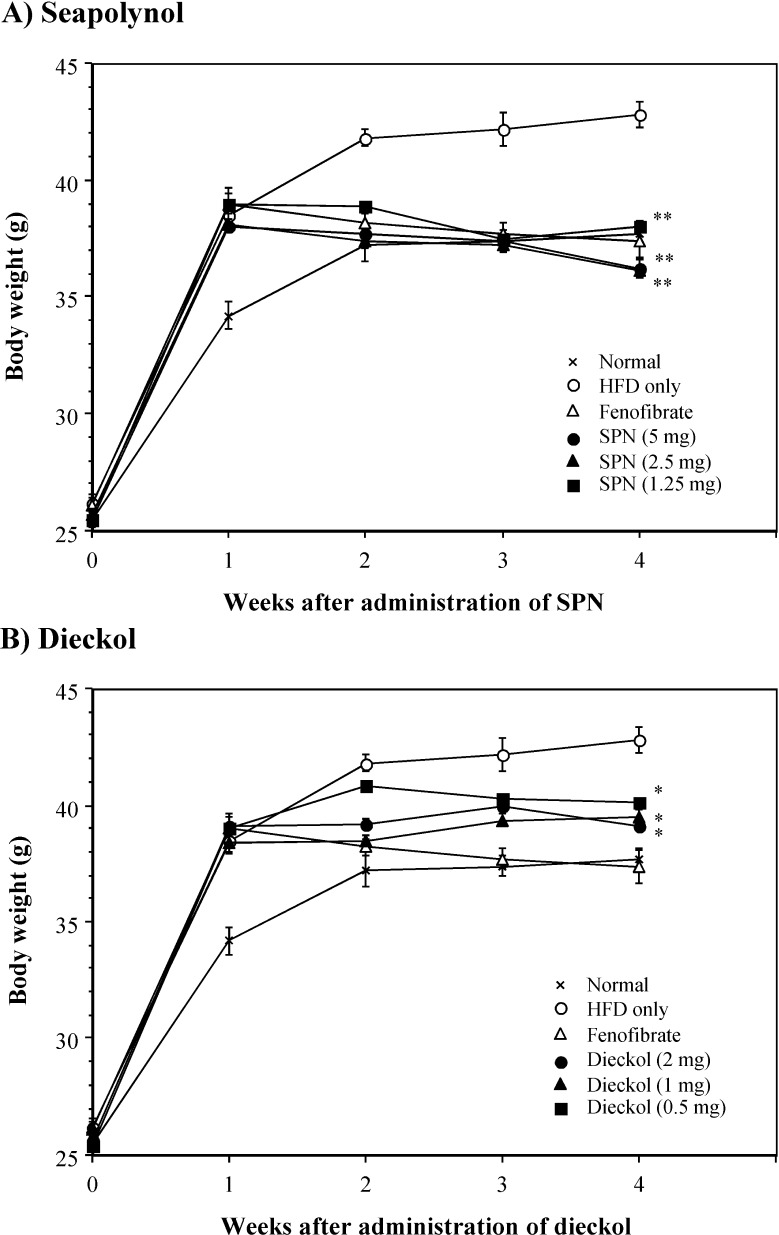
The mice fed an HFD for five weeks increased the body weight significantly compared to those fed a normal diet. To examine the inhibitory effect of SPN and dieckol on HFD-induced hyperlipidemia, both compounds were orally administered daily for 4 weeks from 1 week after the beginning of HFD feeding. SPN was administered at the dosage from 1.25 to 5 mg per mouse, but dieckol was administered at lower dosage (0.5 to 2 mg/mouse) than that of SPN because it is a single compound derived from SPN. Oral administration of SPN, as well as dieckol, significantly decreased the body weight gains of HFD-fed mice (Fig. 2). In particular, SPN at the dose of 1.25 mg/mouse showed almost the same activity with the positive control, fenofibrate, at the dose of 4 mg/mouse.
Fig. 2.
Inhibitory effect of SPN and dieckol on body weight gains of HFD-fed mice. Ten mice per group were fed an HFD for 5 weeks. The indicated doses of SPN or dieckol were administered orally into HFD-fed mice daily for 4 weeks from 1 week after the beginning of HFD feeding. Body weight of the mice was measured every week. *p<0.01; **p<0.001, compared with the HFD-fed control group by Student’s t-test.
Lipids and glucose profiles in serum
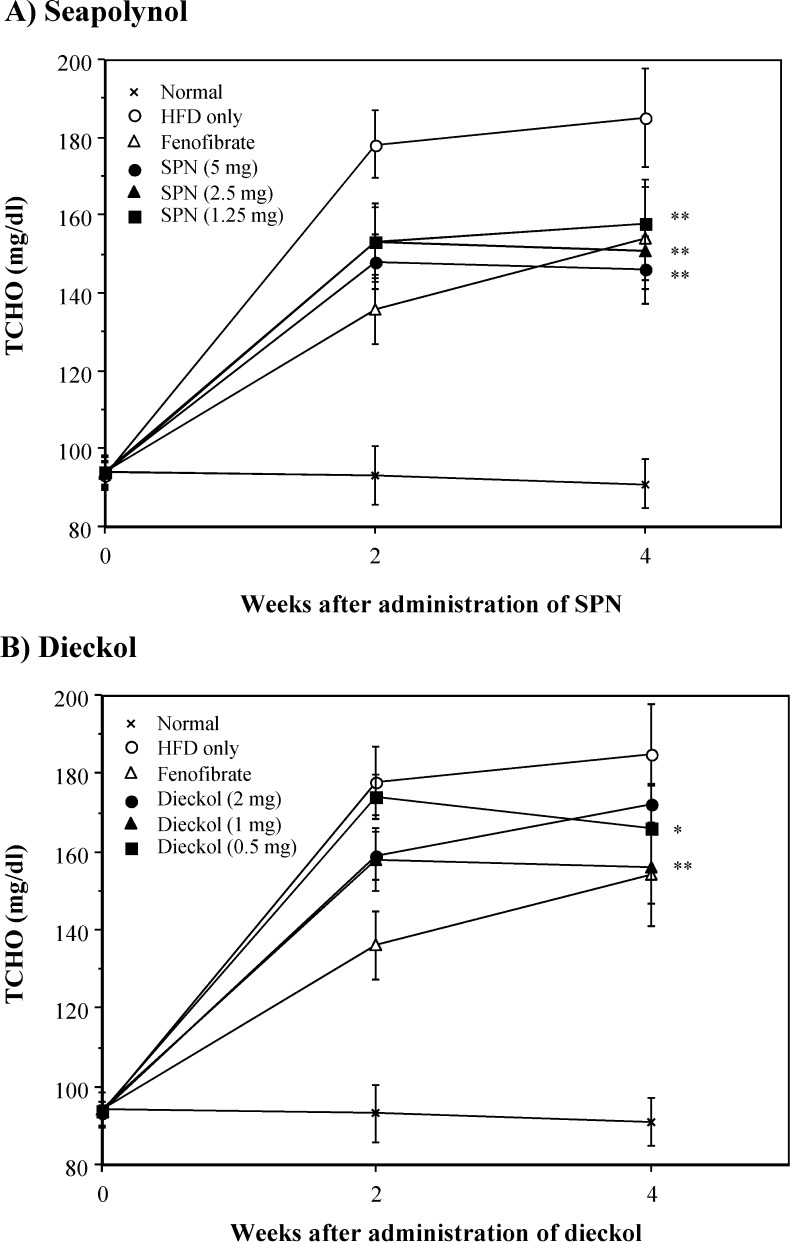
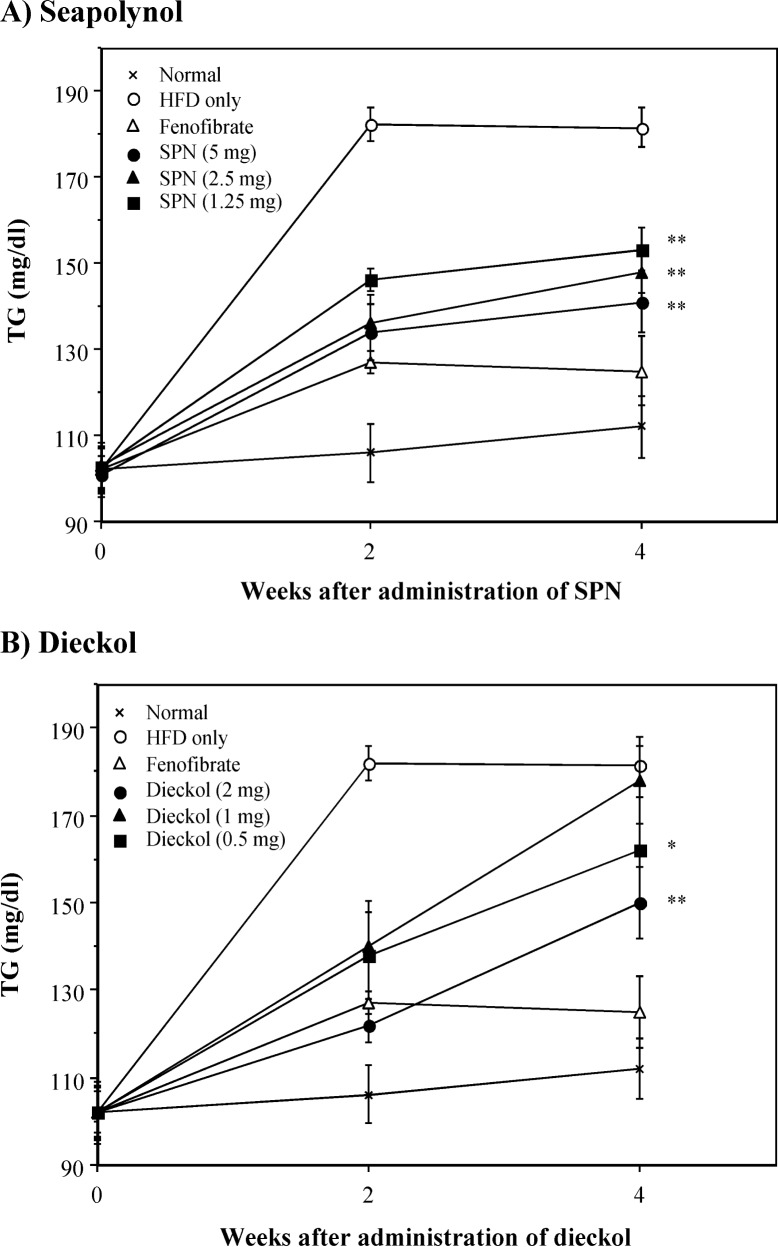
In the case of obesity induced by high fat intake, hyperlipidemia, which presents as an abnormally high concentration of lipids in blood, is usually observed (19). In general, this abnormally high concentration of lipids in blood appears to increase total cholesterol or triglyceride levels in blood (20). To explore whether the reduction of body weight gains by SPN and dieckol correlated with changes in serum levels of lipids and glucose, blood samples were obtained from each group of mice. TCHO and TG levels in the HFD-fed group were prominently increased compared with that in the normal group (Fig. 3 and Fig. 4). Administration of SPN significantly decreased the increased levels of both TCHO and TG in HFD-fed mice. The inhibitory effect of SPN on the increased TCHO and TG levels in serum was observed from 2 weeks after SPN administration. In addition, treatment with SPN significantly reduced the elevated level of LDL-cholesterol in serum of HFD-fed mice on week 4 (Table 2). Similarly, treatment with dieckol significantly reduced the elevated levels of TCHO, TG and LDL-cholesterol in the serum of HFD-fed mice, even though its inhibitory activity was lower than that of SPN. However, both SPN and dieckol did not affect the level of HDL-cholesterol and glucose in the serum (Table 2). In this study, it was shown that the HFD feeding increased the level of HDL-cholesterol in the serum. It is likely that the increase of HDL-cholesterol in serum of HFD-fed mice is related to increase of TCHO in serum by feeding HFD (Fig. 3); however, it is not clear why the feeding of HFD resulted in increase of the level of HDL-cholesterol in serum. It has been well recognized that the increase of cholesterol level in serum is followed by hypercholesterolemia, and it is a major risk factor for atherosclerosis (21). An increased LDL-cholesterol level compared to the HDL-cholesterol level is thought to be associated with high risk for the development of cardiovascular disease and atherosclerosis (22). Therefore, these results suggest that both SPN and dieckol have a hypocholesterolemic effect in HFD-fed mice.
Fig. 3.
Inhibitory effect of SPN and dieckol on TCHO levels in serum of HFD-fed mice. Experimental procedure was same with that of Fig. 2. The level of TCHO in serum was measured on the indicated weeks. *p<0.05; **p<0.01, compared with the HFD-fed control group by Student’s t-test.
Fig. 4.
Inhibitory effect of SPN and dieckol on TG levels in serum of HFD-fed mice. Sera obtained from the experiment of Fig. 3 were used in measurement of TG level. *p<0.05; **p<0.01, compared with the HFD-fed control group by Student’s t-test.
Table 2.
Effect of SPN and dieckol on lipid profiles and glucose in serum of HFD-fed mice
| Groups | Variables (mg/dL)
|
||||
|---|---|---|---|---|---|
| Triglyceride | Total cholesterol | HDL-cholesterol | LDL-cholesterol | Glucose | |
| Normal | 110±7.8 | 106±2.7 | 68±5.2 | 16±2.3 | 101±9.8 |
| HFD only | 180±12.4 | 184±10.3 | 106±8.5 | 42±3.7 | 123±14 |
| + SPN 5 mg | 134±9.2*** | 145±11.7* | 110±6.5 | 14.2±2.8*** | 116±11 |
| + SPN 2.5 mg | 146±14** | 150±10.4* | 103±4.8 | 17.8±3.9*** | 116±13 |
| + SPN 1.25 mg | 148±12* | 156±13* | 110±7.4 | 16.4±2.2*** | 115±10 |
| + Dieckol 2 mg | 144±9.8** | 167±14 | 110±8.7 | 28.2±4.5* | 120±16 |
| + Dieckol 1 mg | 156±12.7 | 164±11* | 108±5.4 | 24.8±3.7** | 123±13 |
| + Dieckol 0.5 mg | 159±11.4 | 164±13 | 110±6.9 | 22.2±1.8** | 108±12 |
The level of the indicated lipids and glucose in serum of HFD-fed mice was determined by DryChem-3500i 4 weeks after oral administration of SPN and dieckol.
p<0.05,
p<0.01,
p<0.001, compared with the HFD-fed control group by Student’s t-test.
Toxicity to organs
To investigate whether long-term administration of SPN and dieckol caused any toxicity to HFD-fed mice, we examined the influence on the livers and the kidneys of mice that received SPN or dieckol for 4 weeks. Toxicity to the livers was tested by measuring the level of GOT and GPT in serum, and for toxicity to the kidneys the level of CRE and BUN in serum was determined. Oral administration of SPN or dieckol daily for 4 weeks did not induce any toxicity to these organs (Table 3). These results indicate that both SPN and dieckol are safe for at least four-week oral administration in HFD-fed mice.
Table 3.
Changes in the level of GOT, GPT, CRE and BUN in serum of HFD-fed mice by administration of SPN and dieckol
| Treatment | Variables (IU/L)
|
|||
|---|---|---|---|---|
| GOT | GPT | CRE | BUN | |
| Normal | 78±3.4 | 31±1.4 | 0.42±0.03 | 28±1.4 |
| HFD only | 81±9.5 | 29±0.8 | 0.39±0.02 | 28±0.8 |
| + SPN 5 mg | 71±5.2 | 32±2.4 | 0.40±0.02 | 26±2.0 |
| + SPN 2.5 mg | 75±8.1 | 28±3.5 | 0.41±0.04 | 24±2.4 |
| + SPN 1.25 mg | 75±2.8 | 30±1.2 | 0.43±0.03 | 27±1.0 |
| + Dieckol 2 mg | 78±6.4 | 28±3.2 | 0.38±0.02 | 30±0.6 |
| + Dieckol 1 mg | 74±3.8 | 29±1.0 | 0.39±0.05 | 28±1.7 |
| + Dieckol 0.5 mg | 76±8.6 | 33±2.7 | 0.41±0.02 | 31±1.2 |
The level of the indicated GOT, GPT, CRE and BUN in serum of HFD-fed mice was determined by DryChem-3500i 4 weeks after oral administration of SPN and dieckol.
Anti-adipogenic effect on 3T3-L1 cells
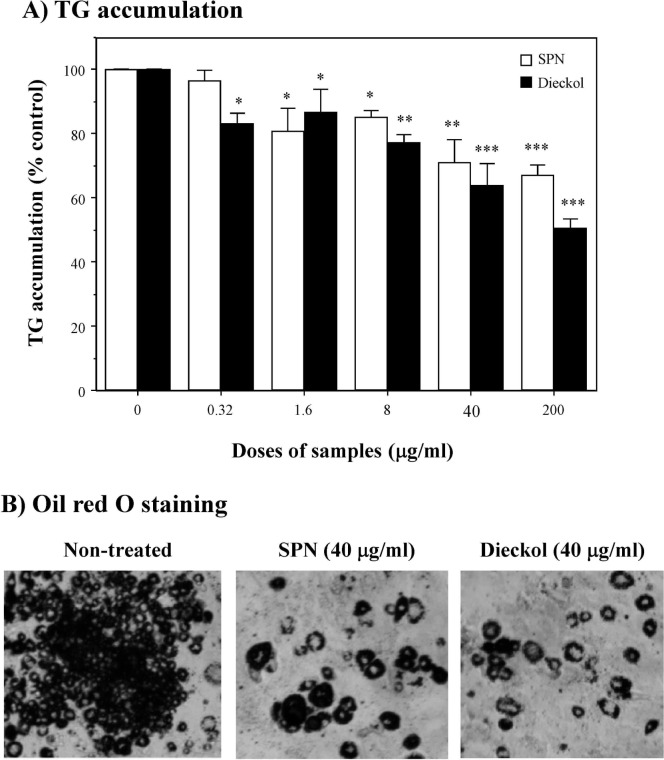
To evaluate anti-adipogeniuc effect of SPN and dieckol on 3T3-L1 adipocyte differentiation, post-confluent 3T3-L1 preadipocytes were maintained in adipocyte-induction media and exposed to various doses (0∼200 μg/mL) of SPN or dieckol. Morphological changes were observed due to accumulation of lipids in the preadipocytes. Subsequently, intracellular lipid drops were measured by Oil Red O staining. As shown in Fig. 5A, both SPN and dieckol effectively inhibited adipocyte differentiation in a dose-dependent manner. Oil Red O staining also revealed that the lipid accumulation in the cells treated with SPN as well as dieckol was significantly lower than the lipid accumulation in the control cells (Fig. 5B). Therefore, we concluded that the reduction of Oil Red O staining is mainly a result of an anti-adipogenic effect of SPN and dieckol leading to an inhibition in 3T3-L1 adipocyte differentiation. These in vitro data suggest that the inhibitory effect of SPN and dieckol on elevated level of TG in serum of HFD-fed mice as shown in Fig. 4 is associated with their activity to inhibit the formation of intracellular lipid drops in 3T3-L1 cells, and differentiation of these preadipocytes into adipocytes.
Fig. 5.
Effect of SPN and dieckol on cellular lipid droplets of 3T3-L1 adipocytes. Differentiating 3T3-L1 cells were treated every 3 days with the indicated doses of SPN or dieckol for 9 days in adipocyte-induction media. Oil Red O dye was dissolved in DMSO, and the accumulation of lipid contents in the cells was determined by optical density detected at 450 nm as described in Materials and Methods. This data is the representative of three individual experiments. *p<0.05; **p<0.01; ***p<0.001, compared with the untreated group by Student’s t-test.
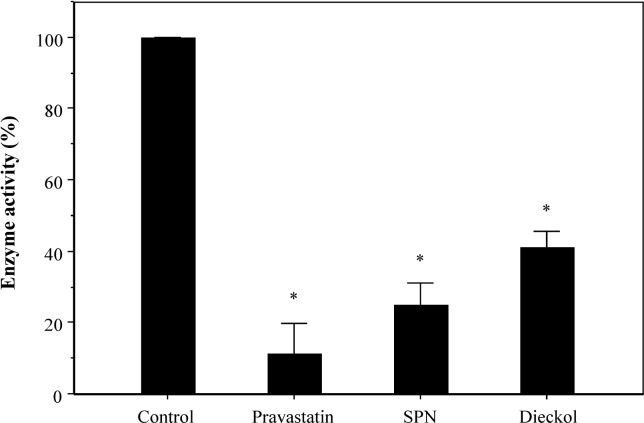
Inhibition of HMGCoA reductase activity
HMGCoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids (16). The in vitro inhibitory effect of SPN and dieckol on HMGCoA reductase was measured using an HMGCoA reductase assay kit. Both SPN and dieckol at a final concentration of 50 μg/mL significantly inhibited HMGCoA reductase activity by approximately 78% and 61%, respectively (Fig. 6). These results established the possibility that the anti-hyperlipidemic effect of SPN and dieckol in vivo correlated with inhibition of the activity of HMGCoA reductase.
Fig. 6.
Inhibitory effect of SPN and dieckol on HMGCoA re-ductase activity in vitro. Effect of SPN or dieckol on enzyme activity HMGCoA reductase was measured using a HMGCoA reductase assay kit (Sigma) according to manufacturer’s suggestion. *p<0.001, compared with the control group by Student’s t-test.
In the present study, we demonstrated that oral administration of SPN and dieckol effectively suppressed body weight gain, and decreased TCHO and TG levels in the serum of mice fed an HFD. Moreover, we evaluated the anti-obesity effect of SPN and dieckol on adipocyte differentiation in 3T3-L1 cells, and confirmed our findings in obese animal models fed an HFD. However, it is unclear whether there is a difference in anti-hyperlipidemic effect between SPN and dieckol. In conclusion, this study suggests that a polyphenol extract (Seapolynol™, SPN) of Ecklonia cava and dieckol, a major component of SPN, reduces body weight gain and fat accumulation in HFD-fed mice. Further study to clarify mechanisms underlying the anti-hyperlipidemic and anti-obesity effect of SPN and dieckol is required.
Acknowledgments
This study was financially supported by the Ministry of Knowledge Economy (MKE) and Korea Institute for Advancement of Technology (KIAT) through Jeju Leading Industry Development for Economic Region. And this study was partly supported by the grant of Myunggok Institute for Medical Science in Konyang University.
REFERENCES
- 1.Frishman WH. Biologic markers as predictors of cardiovascular disease. Am J Med. 1998;104:18S–27S. doi: 10.1016/s0002-9343(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ. Lipid management: current diet and drug treatment options. Am J Med. 1996;101:40S–49S. doi: 10.1016/s0002-9343(96)00319-1. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar D. Lipid-lowering drugs in the management of hyperlipidemia. Pharmacol Therapeut. 1998;79:205–230. doi: 10.1016/s0163-7258(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 4.Taskin E, Ozturuk M, Kurt O. Turkiye’nin yararlanilabilir denizel alg potansiyeli. 2001. pp. 225–232. XI. Ulusal Su Urunleri Sempozyumu.
- 5.Joe MJ, Kim SN, Choi HY, Shin WS, Park GM, Kang DW, Kim YK. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblast. Biol Pharm Bull. 2006;29:1735–1739. doi: 10.1248/bpb.29.1735. [DOI] [PubMed] [Google Scholar]
- 6.Heo SJ, Kim JP, Jung WK, Lee NH, Kang HS, Jun EM, Park SH, Kang SM, Lee YJ, Park PJ, Jeon YJ. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol. 2008;18:676–681. [PubMed] [Google Scholar]
- 7.Kang K, Park Y, Hwang HJ, Kim SH, Lee JG, Shin HC. Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch Pharm Res. 2003;26:286–293. doi: 10.1007/BF02976957. [DOI] [PubMed] [Google Scholar]
- 8.Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother. 2002;50:889–893. doi: 10.1093/jac/dkf222. [DOI] [PubMed] [Google Scholar]
- 9.Shin HC, Hwang HJ, Kang KJ, Lee BH. An anti-oxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharm Res. 2006;29:165–171. doi: 10.1007/BF02974279. [DOI] [PubMed] [Google Scholar]
- 10.Fukuyama Y, Kodama M, Miura I, Kinzyo Z, Mori H, Nakayama Y, Takahashi M. Anti-plasmin inhibitor. V. Structures of novel dimeric eckols isolated from the brown alga Ecklonia kurome OKAMURA. Chem Pharm Bull. 1989;37:2438–2440. doi: 10.1248/cpb.37.2438. [DOI] [PubMed] [Google Scholar]
- 11.Jung HA, Hyun SK, Kim HR, Choi JS. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fisheries Sci. 2006;72:1292–1299. [Google Scholar]
- 12.Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem. 2008;16:7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 13.Hwang HJ, Chen T, Nines RG, Shin HC, Stoner GD. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int J Cancer. 2006;119:2742–2749. doi: 10.1002/ijc.22147. [DOI] [PubMed] [Google Scholar]
- 14.Iwai K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A(y) mice. Plant Foods Hum Nutr. 2008;63:163–169. doi: 10.1007/s11130-008-0098-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee MH, Lee KB, Oh SM, Lee BH, Chee HY. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J Korean Soc Appl Biol Chem. 2010;53:504–507. [Google Scholar]
- 16.Xie W, Wang W, Su H, Xing D, Cai G, Du L. Hypolipidemic mechanisms of Ananas comosus L. leaves in mice: different from fibrate but similar to statins. J Pharmacol Sci. 2007;103:267–274. doi: 10.1254/jphs.fp0061244. [DOI] [PubMed] [Google Scholar]
- 17.Ji Z, Mei FC, Cheng X. Epac, not PKA catalytic subunit, is required for 3T3-L1 preadipocyte differentiation. Front Biosci. 2010;2:392–398. doi: 10.2741/e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CK, Kim M, Oh SD, Lee SM, Sun B, Choi GS, Kim SK, Bae H, Kang C, Min BI. Effects of Atractylodes macrocephala Koidzumi rhizome on 3T3-L1 adipogenesis and an animal model of obesity. J Ethnopharmacol. 2011;137:396–402. doi: 10.1016/j.jep.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 20.Drevon CA. Fatty acids and expression of adipokines. Biochim Biophys Acta. 2005;1740:287–292. doi: 10.1016/j.bbadis.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Wissler RW. Theories and new horizons in the pathogenesis of atherosclerosis and the mechanisms of clinical effects. Arch Pathol Lab Med. 1992;116:1281–1291. [PubMed] [Google Scholar]
- 22.Castelli WP. Epidemiology of triglycerides: a review from Framingham. J Cardiol. 1992;70:H3–H9. doi: 10.1016/0002-9149(92)91083-g. [DOI] [PubMed] [Google Scholar]