Abstract
Melaleuca leucadendron L. has been used as a tranquilizing, sedating, evil-dispelling and pain-relieving agent. We examined the effects of M. leucadendron L. extracts on oxidative stress and inflammation. M. leucadendron L. was extracted with methanol (MeOH) and then fractionated with chloroform (CHCl3) and butanol (BuOH). Antioxidant activity of the MeOH extract and BuOH fraction were higher than that of both α-tocopherol and butyrated hydroxytoluene (BHT). Total phenol content in the extracts of M. leucadendron L., especially the BuOH fraction, well correlated with the antioxidant activity. The anti-inflammatory activity of BuOH extracts were investigated by lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production, and cyclooxygenase-2 (COX-2) expression in RAW 264.7 macrophages. The BuOH fraction significantly inhibited LPS-induced NO and PGE2 production. Furthermore, BuOH extract of M. leucadendron L. inhibited the expression of COX-2 and iNOS protein without an appreciable cytotoxic effect on RAW264.7 cells. The extract of M. leucadendron L. also suppressed the phosphorylation of inhibitor κBα (IκBα) and its degradation associated with nuclear factor-κB (NF-κB) activation. Furthermore, BuOH fraction inhibited LPS-induced NF-κB transcriptional activity in a dose-dependent manner. These results suggested that M. leucadendron L. could be useful as a natural anti-oxidant and anti-inflammatory resource.
Keywords: Melaleuca leucadendron L., antioxidant activity, inducible nitric oxide synthase, cyclooxygenase-2, NF-κB
INTRODUCTION
Oxidative stresses suggestively correlate strongly with the aging process and certain degenerative diseases (1). The reactive oxygen species (ROSs) are involved in up-regulating inflammatory gene expressions by causing redox-based activation of nuclear factor-κB (NF-κB) and the COX-2 signaling pathways (2).
Prostaglandins (PGs) and nitric oxide (NO) are involved in various pathophysiological processes including inflammation and carcinogenesis. Prostaglandins (PGs) are lipid mediators involved in many processes, including inflammation, and are produced by many cell types. More notably, prostaglandin E2 (PGE2) affects cell proliferation and tumor growth and suppresses the immune response to malignant cells (3). NO plays an important role in the regulation of many physiological functions, such as host defense, neurotoxicity, and vasodilation (4). However, the excess productions of NO have been implicated with immunological and inflammatory diseases including septic shock, rheumatoid arthritis, graft rejection, and diabetes (5). The inducible isoform of cyclooxygenase, COX-2, and nitric oxide synthase (iNOS) are mainly responsible for the production of large amounts of PGE2 and NO (6). Inhibition of PGE2 and NO production is an important therapeutic consideration in the development of anti-inflammatory agents.
Macrophages play an important role in the host defense mechanism against bacterial and viral infections. When macrophages are activated by various stimuli, such as lipopolysaccharide (LPS) and interferon-γ (IFN-γ), they inhibit the growth of a wide variety of tumor cells and microorganisms by releasing factors such as NO, cytokines, and eicosanoid mediators of the immune response (7). PGE2 production by COX-2 and NO production by iNOS are mainly regulated at the transcriptional level (6). NF-κB is a transcription factor that regulates the expression of multiple immune and inflammatory genes (8). LPS activates NF-κB in macrophages, which induces the expression of iNOS and COX-2 (6).
Plants have always been among the common sources of medicines, either processed as traditional preparations or used to extract pure active principles. Because of the large chemical diversity among natural products, many research groups screen plant extracts in their search for new promising therapeutic candidates for various diseases (9).
Melaleuca leucadendron L., known as the paper-bark tree (9), is widely distributed throughout Taiwan. The bark and leaves are used in folk medicine as tranquilizing, sedating, evil-dispelling, and pain-relieving agents (10,11). However, to date, no scientific data supports these activites and the mechanims through which the extract may be functioning are still unknown. Thus, this study examined the antioxidant effects of all the methanol extract fractions of M. leucadendron L. and evaluated the anti-inflammatory effect of its extracts in LPS-stimulated RAW264.7 macrophages.
MATERIALS AND METHODS
Plant material
Melaleuca lucadendron L. was obtained from the Bio-products Research Center, Yonsei University in Seoul, Korea. The plant material was shade dried and grounded to a powder. A voucher specimen is deposited at 4°C.
Extraction and isolation
Dried Melaleuca lucadendron L. (1 kg) were grounded and extracted twice with 75% methanol (4 L, v/v) for 24 hr at room temperature. The extract was concentrated, frozen, and lyophilized (22.2 g). The methanol extract was further fractionated successively with chloroform, n-butanol, and water. Each fraction was evaporated and dried under reduced pressure (11.2 g chloroform fraction, 2 g butanol fraction, 8.8 g water fraction).
Total phenolic contents
Total phenolic contents in the extract were determined according to the method of Gutfinger (12). One mL of each 1 mg/mL extract concentration was mixed with 1 mL of 2% Na2CO3 followed by standing for 3 min. Then, 0.2 mL of 50% Folin-Ciocalteau reagent was added to the mixture. After standing for 30 min, the solution was centrifuged at 13,400×g for 5 min. The absorbance was measured at 750 nm using a spectrophotometer (Shimadzu UV-1601, Tokyo, Japan) and total phenolic contents were expressed as gallic acid equivalents (GAE).
DPPH radical scavenging activity
The scavenging activities of M. leucadendron L. extracts were determined using the stable free radical 1,1-diphenyl-2-picryl-hydrazil (DPPH), according to a modified method of Shirwaikar and Somashekar (13). DPPH solution was prepared as a 0.2 mM ethanol concentration. 2 mL of the sample was vortex-mixed with 1 mL of DPPH solution, and incubated at room temperature for 30 min. The absorbance was measured at 517 nm against a blank and the activity was expressed as DPPH scavenging activity (% inhibition)={(Acontrol–A sample)/Acontrol}×100%, where Asample is the absorbance of the sample and Acontrol is the absorbance of the control. The scavenging activity of DPPH radicals was expressed as IC50.
Superoxide anion radical scavenging activity
The scavenging activity for superoxide anion radical was analyzed via a hypoxanthine/xanthine oxidase generating system coupled with reducing nitroblue tetrazolium (NBT), following the method of Kirby and Schmidt (14). The reaction mixture contained 125 μL of buffer (50 mM K2HPO4/KOH, pH 7.4) and 20 μL of 15 mM Na2EDTA, 30 μL of 3 mM hypoxanthine, 50 μL of xanthine oxidase (1unit per 10 mL buffer) and 25 μL of plant extract (a diluted sonicated solution of 10 μg per 250 μL buffer). The absorbance of the solution was measured at 540 nm.
Antioxidant activity on linoleic acid oxidation
The oxidation test was conducted by using the linoleic acid model system. A 0.2 mL sample solution and 0.5 mL of 0.2 M sodium phosphate buffer (pH 7.0) were mixed with 0.5mL of 2.5% linoleic acid in ethanol. The peroxidation was initiated by adding 50 μL of 0.1 M 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) and carried out at 37°C for 200 min in the dark. The degree of oxidation of peroxides was examined, according to the thiocyanate method, by reading the absorbance at 500 nm after coloring with FeCl2 and ammonium thiocyanate (15). A control test was performed with linoleic acid without sample solution.
Cell culture
RAW264.7 murine marcrophages (KCLB No. 40071) were purchased from the Korea Cell Line Bank (Seoul, Korea). The macrophages were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco/BRL, Grand Island, NY, USA) containing 10% fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin (Gibco/BRL). Macrophages were then incubated in 24 well tissue plates at a density of 1×106 cells/mL for 24 hr at 37°C. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Treatment of macrophages with LPS
Macrophages were incubated with 10 μg/mL of LPS to stimulate COX-2 and iNOS gene expression. Each sample was dissolved in dimethylsulfoxide (DMSO) and added to the incubation medium 1 hr prior to addition of LPS. The final concentration of DMSO was adjusted to 0.1% (v/v).
MTT assay for cell viability
Cell viability was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reagent (16). After culturing on 96 well plates for 24 hr, the cells were washed twice and incubated with 110 μL of 0.5 mg/mL MTT for 2 hr at 37°C. The medium was discarded and 100 μL DMSO was then added. After 30 min incubation, the absorbance at 570 nm was measured using a microplate reader (Molecular Devices Co., Downingtown, PA, USA).
Determination of PGE2 production
Macrophages were pretreated with aspirin (250 μM) for 2 hr to inactivate the COX-1 enzyme prior to the COX-2 activity assay. The cells were first washed three times with serum-free DMEM before adding LPS (10 μg/mL) to induce COX-2 expression. The culture supernatants were collected 20 hr later for measuring PGE2 concentrations using ELISA kits (R&D System, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Determination of nitrite production
The nitrite concentration in the medium was measured as an indicator of NO production according to the Griess method as described previously (17). An amount of 100 μL of each culture supernatant was mixed with the same volume of Griess reagent (1% sulfanilamide in 5% phosporic acid, and 0.1% naphthylethylenediamine dihydrochloride in water) and the absorbance of the mixture at 550 nm was measured using a microplate reader. Nitrite concentrations were calculated from a standard curve of sodium nitrite prepared in the culture medium.
SDS-polyacrylamide gel electrophoresis and Western blot analysis
RAW264.7 cells (1×106 cells/mL), grown in 60 mm dishes to confluence, were incubated with or without LPS in the absence or presence of tested samples for 24 hr, respectively. Cells were washed with ice-cold phosphate-buffered saline (PBS) and stored at −70°C until further analysis. Macrophages were collected and an equal amount of protein (30 μg/lane) was loaded and electrophoresed on an 8% (for iNOS and COX-2) or 10% SDS-polyacrylamide gel (for phospho-IκBα and IκBα). Gels were then transferred to polyvinylidene difluoride (PVDF; Millipore Co., Billerica, MA, USA) membranes. Membranes were blocked and incubated for 1 hr at room temperature with 1:1000 dilutions of primary antibodies: rabbit IκBα polyclonal, mouse phospho-IκBα monoclonal, rabbit iNOS polyclonal, and goat COX-2 polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA). α-tubulin (Santa Cruz Biotechnology) was used as an internal control. The immunoreactive protein was detected using a chemiluminescent system (ECL kit; Amersham Pharmarcia Co., Piscataway, NY, USA). After exposure to X-ray film, band intensities were calculated from the optical density using an image analyzer (Vilber Co., Mame-la-Vallee Cedex, France).
NF-κB-driven reporter gene assay
An NF-κB reporter construct, consisting of the firefly luciferase gene under the control of the consensus NF-κB site, was used to quantify NF-κB transcriptional activity. A Renilla luciferase reporter was used as an internal control to normalize the reporter gene activity. RAW264.7 cells, seeded (1×105 cells/mL) in 60 mm dishes, were transiently transfected with the reporter construct (3 μg) using the Fugene 6 reagent (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. After 3 hr post transfection, the cultures were treated with various concentrations of compounds tested and stimulated with 10 μg/mL of LPS. After 24 hr incubation, each 60 mm culture dish was washed twice with cold PBS, harvested, and subjected to dual luciferase assays. Dual-luciferase assays were performed with the Dual-Luciferase Reporter Assays System (Promega, Madison, WI, USA), according to the manufacturer’s instructions, and luciferase activities were determined with a luminometer (FLUO-star; BMG Labtechnologies, Ortenberg, Germany).
Statistical analysis
Each experiment was performed at least in triplicate. Results are expressed as the means±standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s t-test for multiple comparisons. Statistical significance is expressed as *p<0.05.
RESULTS AND DISCUSSION
Total phenolic contents
Phenolic compounds have potential antioxidant activities (18). Previous studies have established phenolic compounds as major antioxidants in medicinal plants, fruits and vegetables (19,20). Total phenolic contents of methanol extracts and solvent fractions of M. leucadendron L. are shown in Table 1. When investigating total phenolics, the butanol (BuOH) extract showed highest levels of phenolics (508.43±2.22 μg GAE/g extract) among all the four extracts (Table 1). Phenolic compounds are widely distributed throughout the plant system demonstrating variable biological activities including antioxidant, anti-inflammatory and anti-carcinogenic (21,22).
Table 1.
Total phenolic contents of M. leucadendron L. extracts
| Solvent fractions | Yield (%) | Total phenolic contents (μg GAE/g extract) |
|---|---|---|
| MeOH | 2.22 | 289.23±5.21 |
| CHCl3 | 1.12 | 107.36± 1.88 |
| BuOH | 0.20 | 508.43± 2.33 |
| H2O | 0.88 | 122.72±3.71 |
Data representative the mean±SD of three separate experiments.
Radical scavenging activities
Among the four M. leucadendron L. extracts, the BuOH fraction showed the most potent radical scavenging activity in each assay (Table 2), showing IC50 at 4.8 μg/mL in the DPPH free radical scavenging assay and 10.1 μg/mL in the superoxide anion radical scavenging assay. Many studies suggested that the antioxidant activity of plants was likely related to redox properties of their phenolics’ behavior (23). In this study, positive correlations were found between total phenolic content in the M. leucadendron L. extracts and their antioxidant activities. Furthermore, the BuOH extract of M. leucadendron L. has the most potent antioxidant activity among all the four extracts.
Table 2.
IC501) values of radical scavenging activities and anti-oxidant activities of M. leucadendron L. extracts
| Solvent fractions | IC50 (μg/mL)
|
||
|---|---|---|---|
| DPPH radical scavenging | Superoxide anion radical scavenging | Antioxidant activity on linoleic acid oxidation | |
| MeOH | 5.1 | 14.5 | 6.2 |
| CHCl3 | 55.7 | 50.3 | 18.1 |
| BuOH | 4.8 | 10.1 | 3.2 |
| H2O | 60.0 | 51.0 | 20.4 |
| α-tocopherol | 3.9 | 5.7 | 3.7 |
| BHT | 2.3 | 4.2 | 2.88 |
BHT and α-tocopherol were used as positive controls.
IC50 value is the concentration of 50% inhibition.
Cell viability
The BuOH extract was used for anti-inflammatory activity because antioxidant capacity data showed that this extract has potential antioxidant activity. First, the effects of BuOH fractions on RAW264.7 cell viability were determined by an MTT assay. The BuOH fraction did not exhibit cytotoxicity at concentrations less than 1 μg/mL compared to an LPS-treated control (data not shown).
Inhibition of LPS-induced PGE2 and nitrite production
To evaluate the anti-inflammatory activity of BuOH extract, the effects of the extract on NO and PGE2 productions stimulated by LPS in RAW264.7 cells were investigated. As shown in Table 3, LPS increased NO production by approximately 7.6 fold; however, when the BuOH extract was treated at 0.01, 0.1 and 1 μg/mL for 24 hr, NO production decreased by 66.1, 51.4, and 45.6%, respectively. In addition, LPS increased PGE2 production by approximately 15 fold compared to the control, and PGE2 production decreased by 60.3, 44.1, and 33.8%, respectively.
Table 3.
Effects on PGE2 and nitrite production in LPS-stimulated RAW264.7 cells after in-vitro exposure to BuOH extract of M. leucadendron L.
| Treatment | PGE2 | % Control | Nitrite | % Control | |
|---|---|---|---|---|---|
| Control | 12.2±0.653)* | 6.83 | 1.7±0.10* | 13.1 | |
| LPS alone | 178.5±9.39 | 100 | 13.1±1.27 | 100 | |
| LPS+BuOH ex. | 0.01 μg/mL | 107.7±1.78* | 60.3 | 8.7±0.73* | 66.1 |
| 0.1 μg/mL | 78.8±3.69* | 44.1 | 6.7±0.18* | 51.4 | |
| 1 μg/mL | 69.1±2.69* | 33.8 | 5.9±0.16* | 45.6 | |
| LPS+Indometacin1) | 0.01 μg/mL | 21.7±0.90* | 21.6 | – | – |
| 0.1 μg/mL | 5.9±1.2* | 5.9 | – | – | |
| 1 μg/mL | 6.1±0.52* | 6.1 | – | – | |
| LPS+L-NAME2) | 0.01 μg/mL | – | – | 6.5±0.19* | 49.6 |
| 0.1 μg/mL | – | – | 4.3±0.17* | 32.9 | |
| 1 μg/mL | – | – | 3.3±0.11* | 25.5 |
Positive control for PGE2 and nitrite production, respectively.
Data representative as mean±SD of three separate experiments.
p<0.05, significantly different compared to LPS alone.
iNOS and COX-2 have been found to be highly induced at inflammatory sites in animals as well as patients with inflammatory diseases (3,6). NO and PGE2 are produced through the actions of iNOS and COX-2, respectively, and participate in diverse biological effects such as the regulation of vascular inflammation, neuro-transmission, and apoptosis (3,4). Numerous studies have revelaed that excessive NO and PGE2 production is improtant in the pathogenesis of inflammation and can lead to tissue damage (5). In this study, BuOH extract significantly inhibited NO and PGE2 production of activated macrophages, suggesting that this extract could be useful as an anti-inflammatory resource.
Inhibition of LPS-induced COX-2 and iNOS protein expression
LPS strongly upregulates iNOS and COX-2 levels in RAW264.7 cells (3). To determine whether the inhibitory effects of BuOH extract on NO and PGE2 productions were related to the modulation of iNOS and COX-2 expressions, western blotting was used. As shown in Fig. 1, expression of the COX-2 and iNOS protein were barely detectable in unstimulated cells, but markedly increased after LPS treatment. Treatment with BuOH extract (0.01, 0.1 and 1 μg/mL) for LPS-stimulated macrophages caused dose-dependent inhibition of COX-2 and iNOS protein expressions, consistent with PGE2 and nitrite production (Table 3). These results suggested that BuOH extract attenuates the production of NO and PGE2 by inhibiting the expressions of iNOS and COX-2 in LPS-stimulated macrophage cells. Thus, the anti-inflammatory effcts of the BuOH extract may be attributed to its suppressive activity on PGE2 and NO production by blocking iNOS and COX-2 gene and protein expressions.
Fig. 1.
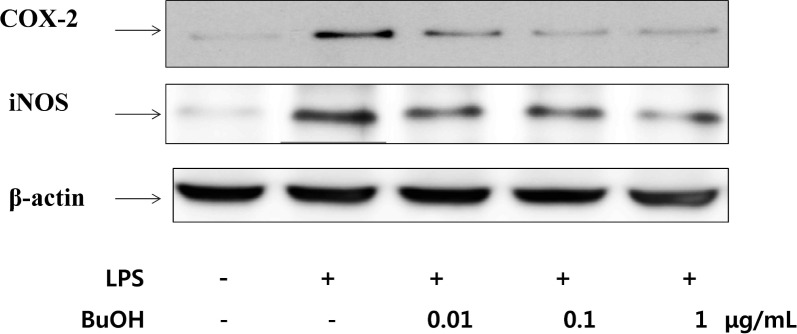
Inhibition of COX-2 and iNOS protein expression by BuOH extract of M. leucadendron L. in LPS-stimulated RAW264.7 cells. Cells were pretreated with the indicated concentrations of BuOH extract for 1 hr before being incubated with LPS (10 μg/mL) for 24 hr. Cell lysates were then prepared and subjected to western blotting using an antibody specific for COX-2 and iNOS. β-actin was used as an internal control.
Inhibition of LPS-induced phosphorylation and degradation of IκBα
In order to explore the mechanism underlying the anti-inflammatory effect of BuOH extract, our attention focused on the NF-κB signal pathway. Recent studies have reported that NF-κB regulates the expression of iNOS and COX-2. NF-κB is one of the most ubiquitous eukaryotic transcription factors, regulating gene expression of cytokines and enzymes involved in controlling inflammatory responses (6). Fuctionally active NF-κB exists as a heterodimer, but is usually in an inactive seqesterd complex bound to its endogenous inhibitor inhibitory kappa B-alpha (IκBα), in the cytoplasm. However, bound IκBα is rapidly phosphorylated by IκBα kinase in response to external stimuli, such as inflammatory cytokines and LPS, and is subsequently degraded by proteosomes. The free dimeric NF-κB, activated by the dissociation of IκBα, translocates into the nucleus and induces transciption of a wide variety of target genes that encode regulatory proteins, including cytokines and growth factors. The activation of these genes by NF-κB then leads to physiological responses such as inflammatory or immune responses (8).
The inhibitory effect of phosphorylation and degradation of IκBα in BuOH extracts in LPS-activated macrophages was examined (Fig. 2). The cytoplasmic levels of phospho-IκBα and IκBα were determined by western blotting. Phospho-IκBα protein concentration in RAW 264.7 cells increased after LPS treatment, but decreased after BuOH extract treatment. IκBα proteins also decreased to almost undetectable levels after LPS treatment and recovered after the BuOH extract treatment. Treatment of macrophages with 1 μg/mL of BuOH extract completely blocked LPS-induced IκBα degradation (Fig. 2). Yin et al. reported that the anti-inflammatory properties of aspirin have been linked to suppression of NF-κB activation through stabilization of IκB (24). Therefore, the phosphorylation and degradation of IκB proteins are critical in NF-κB activation.
Fig. 2.
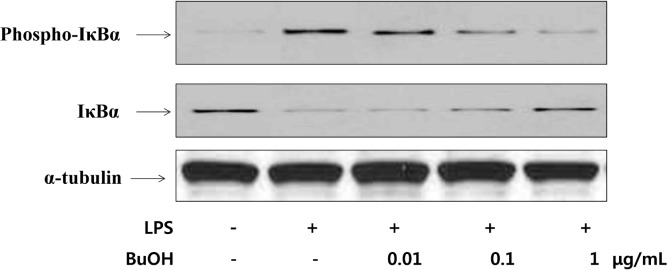
Effect of BuOH extract of M. leucadendron L. on the LPS-induced phosphorylation and degradation of IκBα in RAW264.7 cells. Cells were pretreated with the indicated concentrations of BuOH extract of M. leucadendron L. for 1 hr before being incubated with LPS (10 μg/mL) for 24 hr. Cell lysates were then prepared and subjected to western blotting using an antibody specific for phospho-IκBα and IκBα. α-tubulin was used as an internal control.
Recently, natural compounds such as curcumin, epigallocatechin gallate, and resveratrol have been reported to ameliorate LPS-induced inflammatory response by downregulating the activities of NF-κB (6,8). Our data indicated that the BuOH extract may inhibit NF-κB activation by suppressing the phosphorylation and degradation of IκBα in LPS-induced RAW264.7 cells. Taken together, BuOH extracts of M. leucadendron L. could be good candidates for the treatment of various human inflammatory disorders because its inhibitory effect on pro-inflammatory mediators is comparable to other previously characterized compounds, such as curcumin, epigallocatechin gallate, and resveratrol (6).
Blocking of NF-κB-regulated gene expression by M. leucadendron L. extract
We performed an NF-κB-driven luciferase reporter gene assay to show that BuOH extracts were able to block NF-κB-reglulated gene expressions (iNOS and COX-2). Incubation of RAW264.7 cells with LPS for 24 hr increased NF-κB transcription activity strongly, but the LPS-induction was markedly inhibited by the extracts.
The luciferase assay revealed that BuOH extracts suppress LPS-mediated gene expression mainly via inhibition of NF-κB activity. These results indicate that inhibition of COX-2 and iNOS gene expression by the BuOH extracts may be, at least in part, related to the NF-κB inhibition pathway.
M. leucadendron L. has been long used in traditional oriental medicine of South Asia for the treatment of inflammatory diseases; however, the activites and mechanisms through which the extract may be acting are unknown. This study supports the pharmacological basis of M. leucadendron L. used as a herbal medicine for the treatment of inflammation. Our study also indicates the possibility for using M. leucadendron L. extract in cancer chemoprevention or anticancer therapy.
In conclusion, our data suggest M. leucadenddron L. extract exhibited noticeable antioxidant activity and mediates inhibition of COX-2 enzyme activity, which can affect related gene and protein expressions. M. leucadendron L. functions by a mechanism of action similar to that of NSAIDs. Together, these results add a novel aspect to the biological profile of M. leucadendron L.
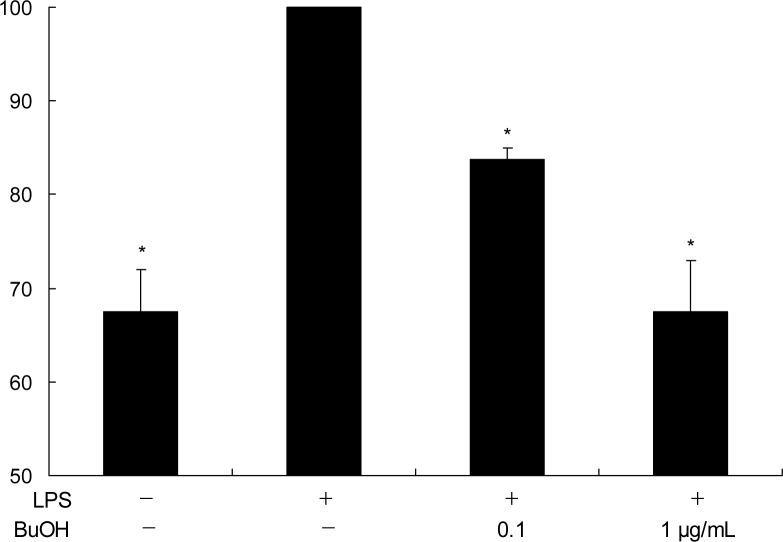
Fig. 3.
Inhibition of NF-κB transcriptional activity by BuOH extract of M. leucadendron L. on LPS-stimulated RAW264.7 cells. The RAW264.7 cells transfected with an NF-κB reporter plasmid were treated with the indicated concentrations of BuOH extract of M. leucadendron L. and then stimulated with LPS (10 μg/mL). After 24 hr incubation, the activity of NF-κB was estimated by the luciferase assay. The relative NF-κB activity levels were expressed as the ratio of the activity of firefly luciferase over that of renilla luciferase. Data represent mean ±SD of three independent experiments performed in triplicate; *p<0.05 versus LPS alone.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0014061).
REFERENCES
- 1.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of aging. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Kabe Y, Ando Y, Hirao S, Yoshida M, Handa H. Redox regulation of inflammatory processes. Mem Inst Oswaldo Cruz. 2005;100:5–9. [Google Scholar]
- 3.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 5.Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343:1199–1206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 7.Hibbs JB, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role of L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 8.Baeuerle PA, Baichwal VR. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- 9.Yang TI. A list of palants in Taiwan. Natural publishing Co., Ltd.; Taipei, Taiwan: 1982. p. 982. [Google Scholar]
- 10.Gan WS. Manual of medicinal plants in Taiwan. Vol 3. National Research institute of Chinese Medicine Press; Taipei, Taiwan: 1965. p. 613. [Google Scholar]
- 11.Tsuruga T, Chun YT, Ebizuka Y, Sankawa U. Biologically active constituents of Melaleuca lecadendron: inhibitors of induced histamine release from rat mast cells. Chem Pharm Bull (Tokyo) 1991;39:3276–3278. doi: 10.1248/cpb.39.3276. [DOI] [PubMed] [Google Scholar]
- 12.Gutfinger T. Polyphenols in olive oil. J Am Oil Chem Soc. 1981;58:966–968. [Google Scholar]
- 13.Shirwaikar A, Somashekar AP. Anti-inflammatory activity and free radical scavenging studies of Aristolochia bracteolate Lam. Indian J Pharm Sci. 2003;65:68. [Google Scholar]
- 14.Kirby AJ, Schmidt RJ. The antioxidant activity of Chinese herbs for eczema and placebo herbs-I. J Ethnoparmacol. 1997;56:103–108. doi: 10.1016/s0378-8741(97)01510-9. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuda H, Yasumoto K, Iwama K. Antioxidative action of indole compounds during the autooxidation of linoleic acid. Eiyo Shokuryo. 1996;19:210–214. [Google Scholar]
- 16.Tubaro A, Florio C, Luxich E, Vertua R, Loggia RD, Yasumoto T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon. 1996;34:965–974. doi: 10.1016/0041-0101(96)00073-6. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt HHHW, Kelm M. Methods in Nitric Oxide Research. John Wiley & Sons Ltd; London, UK: 1996. Determination of nitrite and nitrate by the Griess reaction; pp. 491–497. [Google Scholar]
- 18.Kunyanga CN, Imungi JK, Okoth MW, Biesaiski HK, Vadivel V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci Technol. 2012;45:269–276. [Google Scholar]
- 19.Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch Pharm Res. 2006;29:849–858. doi: 10.1007/BF02973905. [DOI] [PubMed] [Google Scholar]
- 20.Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Seo SH, Kang EY, Kim SL, Park YJ, Ro HM, Chung IM. Phenolic compound concentration and anti-oxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 2008;56:7265–7270. doi: 10.1021/jf8008553. [DOI] [PubMed] [Google Scholar]
- 21.Kundu JK, Na HK, Surh YJ. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr. 2009;61:182–192. doi: 10.1159/000212750. [DOI] [PubMed] [Google Scholar]
- 22.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 23.Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans. 1996;24:790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 24.Yin MJ, Yamamoto Y, Graynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]