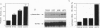Abstract
The insulin-like growth factor I receptor (IGF-I-R) plays a critical role in transformation events. It is highly overexpressed in most malignant tissues where it functions as an anti-apoptotic agent by enhancing cell survival. Tumor suppressor p53 is a nuclear transcription factor that blocks cell cycle progression and induces apoptosis. p53 is the most frequently mutated gene in human cancer. Cotransfection of Saos-2 (os-teosarcoma-derived cells) and RD (rhabdomyosarcoma-derived cells) cells with IGF-I-R promoter constructs driving luciferase reporter genes and with wild-type p53 expression vectors suppressed promoter activity in a dose-dependent manner. This effect of p53 is mediated at the level of transcription and it involves interaction with TBP, the TATA box-binding component of TFIID. On the other hand, three tumor-derived mutant forms of p53 (mut 143, mut 248, and mut 273) stimulated the activity of the IGF-I-R promoter and increased the levels of IGF-I-R/luciferase fusion mRNA. These results suggest that wild-type p53 has the potential to suppress the IGF-I-R promoter in the postmitotic, fully differentiated cell, thus resulting in low levels of receptor gene expression in adult tissues. Mutant versions of p53 protein, usually associated with malignant states, can derepress the IGF-I-R promoter, with ensuing mitogenic activation by locally produced or circulating IGFs.
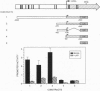
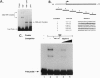
Full text
PDF

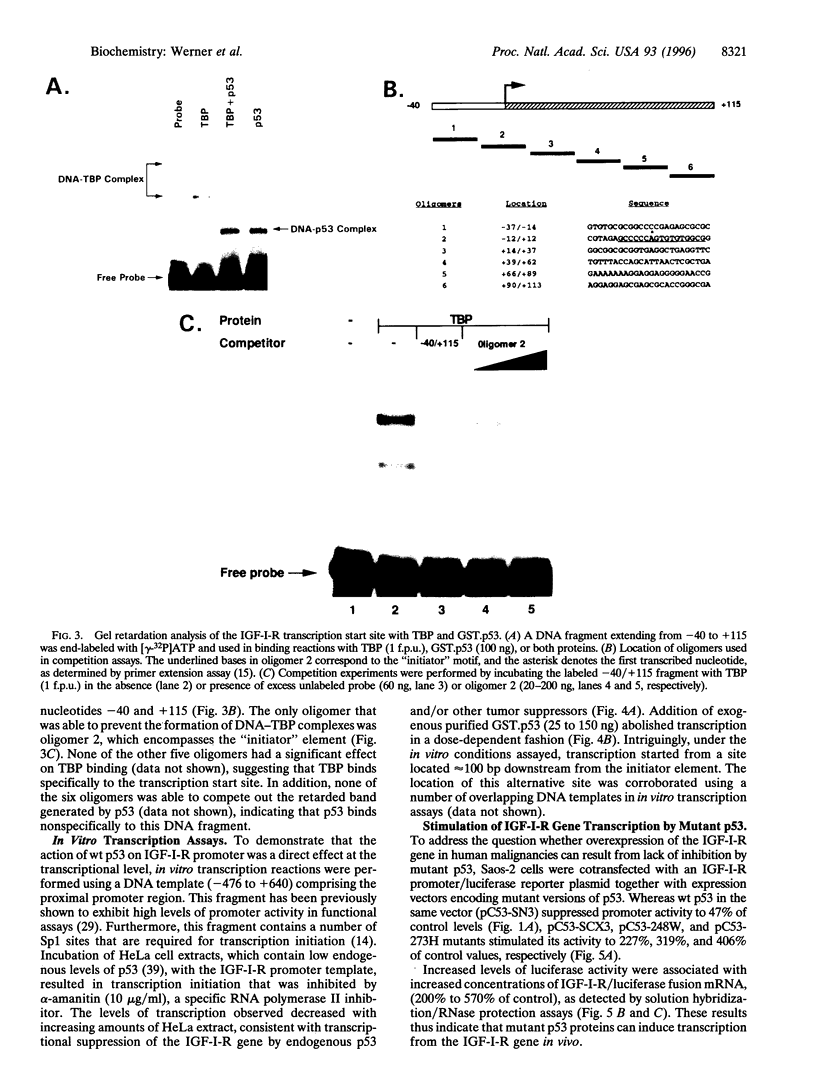
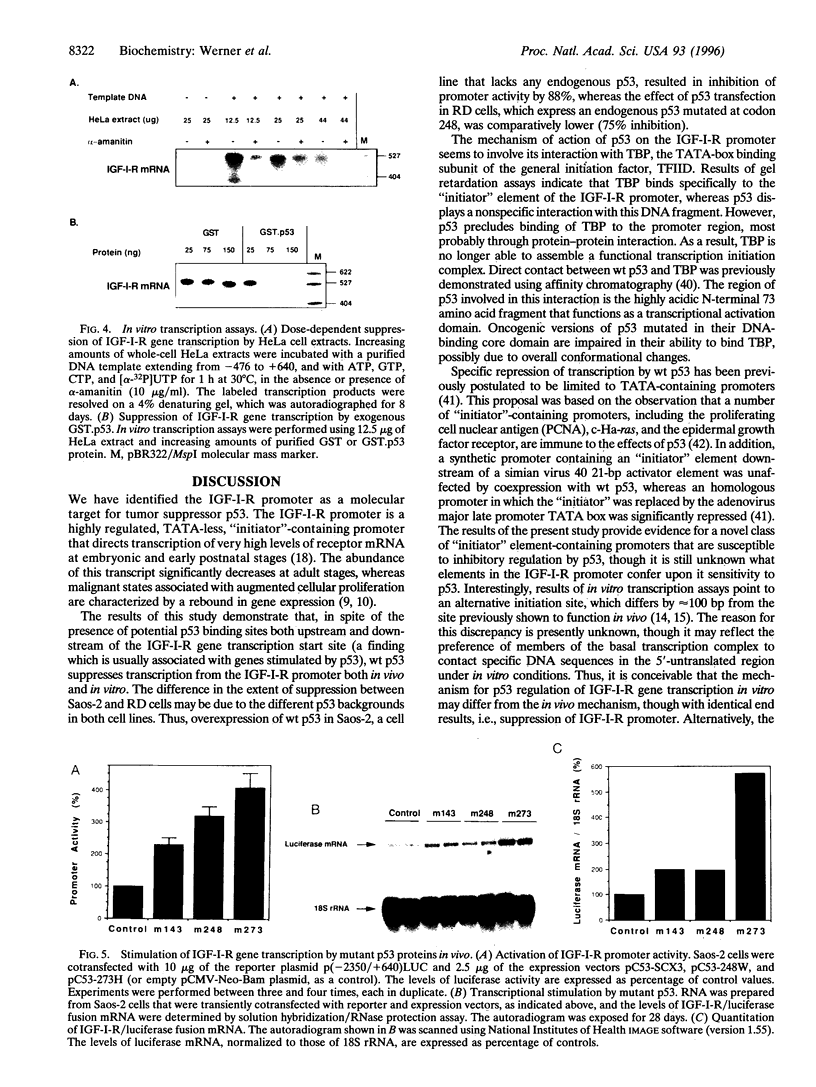

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993 Oct 8;75(1):73–82. [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994 Dec 16;79(6):927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995 Jan 15;55(2):249–252. [PubMed] [Google Scholar]
- Bohan C. A., Kashanchi F., Ensoli B., Buonaguro L., Boris-Lawrie K. A., Brady J. N. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr. 1992;2(4):391–407. [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B. R., Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995 Oct 19;377(6550):646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooke D. W., Bankert L. A., Roberts C. T., Jr, LeRoith D., Casella S. J. Analysis of the human type I insulin-like growth factor receptor promoter region. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1113–1120. doi: 10.1016/0006-291x(91)90654-p. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989 Feb;10(1):68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Felix C. A., Kappel C. C., Mitsudomi T., Nau M. M., Tsokos M., Crouch G. D., Nisen P. D., Winick N. J., Helman L. J. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992 Apr 15;52(8):2243–2247. [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993 Oct 28;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Jones J. I., Clemmons D. R. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995 Feb;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Werner H., Beitner-Johnson D., Roberts C. T., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995 Apr;16(2):143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Mack D. H., Vartikar J., Pipas J. M., Laimins L. A. Specific repression of TATA-mediated but not initiator-mediated transcription by wild-type p53. Nature. 1993 May 20;363(6426):281–283. doi: 10.1038/363281a0. [DOI] [PubMed] [Google Scholar]
- Mamula P. W., Goldfine I. D. Cloning and characterization of the human insulin-like growth factor-I receptor gene 5'-flanking region. DNA Cell Biol. 1992 Jan-Feb;11(1):43–50. doi: 10.1089/dna.1992.11.43. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashewski G., Banks L., Pim D., Crawford L. Analysis of human p53 proteins and mRNA levels in normal and transformed cells. Eur J Biochem. 1986 Feb 3;154(3):665–672. doi: 10.1111/j.1432-1033.1986.tb09449.x. [DOI] [PubMed] [Google Scholar]
- Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992 Oct;6(13):3169–3176. doi: 10.1096/fasebj.6.13.1397838. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Pietenpol J. A., Tokino T., Thiagalingam S., el-Deiry W. S., Kinzler K. W., Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Resnicoff M., Abraham D., Yutanawiboonchai W., Rotman H. L., Kajstura J., Rubin R., Zoltick P., Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995 Jun 1;55(11):2463–2469. [PubMed] [Google Scholar]
- Sell C., Baserga R., Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res. 1995 Jan 15;55(2):303–306. [PubMed] [Google Scholar]
- Sell C., Dumenil G., Deveaud C., Miura M., Coppola D., DeAngelis T., Rubin R., Efstratiadis A., Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994 Jun;14(6):3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell C., Rubini M., Rubin R., Liu J. P., Efstratiadis A., Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Truant R., Xiao H., Ingles C. J., Greenblatt J. Direct interaction between the transcriptional activation domain of human p53 and the TATA box-binding protein. J Biol Chem. 1993 Feb 5;268(4):2284–2287. [PubMed] [Google Scholar]
- Ueba T., Nosaka T., Takahashi J. A., Shibata F., Florkiewicz R. Z., Vogelstein B., Oda Y., Kikuchi H., Hatanaka M. Transcriptional regulation of basic fibroblast growth factor gene by p53 in human glioblastoma and hepatocellular carcinoma cells. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9009–9013. doi: 10.1073/pnas.91.19.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Adamo M., Roberts C. T., Jr, LeRoith D. Molecular and cellular aspects of insulin-like growth factor action. Vitam Horm. 1994;48:1–58. doi: 10.1016/s0083-6729(08)60495-1. [DOI] [PubMed] [Google Scholar]
- Werner H., Bach M. A., Stannard B., Roberts C. T., Jr, LeRoith D. Structural and functional analysis of the insulin-like growth factor I receptor gene promoter. Mol Endocrinol. 1992 Oct;6(10):1545–1558. doi: 10.1210/mend.6.10.1448110. [DOI] [PubMed] [Google Scholar]
- Werner H., LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv Cancer Res. 1996;68:183–223. doi: 10.1016/s0065-230x(08)60354-1. [DOI] [PubMed] [Google Scholar]
- Werner H., Rauscher F. J., 3rd, Sukhatme V. P., Drummond I. A., Roberts C. T., Jr, LeRoith D. Transcriptional repression of the insulin-like growth factor I receptor (IGF-I-R) gene by the tumor suppressor WT1 involves binding to sequences both upstream and downstream of the IGF-I-R gene transcription start site. J Biol Chem. 1994 Apr 29;269(17):12577–12582. [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Shen-Orr Z., Rauscher F. J., 3rd, Morris J. F., Roberts C. T., Jr, LeRoith D. Inhibition of cellular proliferation by the Wilms' tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Mol Cell Biol. 1995 Jul;15(7):3516–3522. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Stannard B., Bach M. A., LeRoith D., Roberts C. T., Jr Cloning and characterization of the proximal promoter region of the rat insulin-like growth factor I (IGF-I) receptor gene. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1021–1027. doi: 10.1016/0006-291x(90)91996-6. [DOI] [PubMed] [Google Scholar]
- Werner H., Woloschak M., Adamo M., Shen-Orr Z., Roberts C. T., Jr, LeRoith D. Developmental regulation of the rat insulin-like growth factor I receptor gene. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7451–7455. doi: 10.1073/pnas.86.19.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenzie-Gregory B., Khachi A., Garraway I. P., Smale S. T. Mechanism of initiator-mediated transcription: evidence for a functional interaction between the TATA-binding protein and DNA in the absence of a specific recognition sequence. Mol Cell Biol. 1993 Jul;13(7):3841–3849. doi: 10.1128/mcb.13.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Kashanchi F., Zhan Q., Zhan S., Brady J. N., Fornace A. J., Seth P., Helman L. J. Regulation of insulin-like growth factor II P3 promotor by p53: a potential mechanism for tumorigenesis. Cancer Res. 1996 Mar 15;56(6):1367–1373. [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]