Abstract
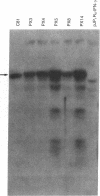
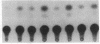
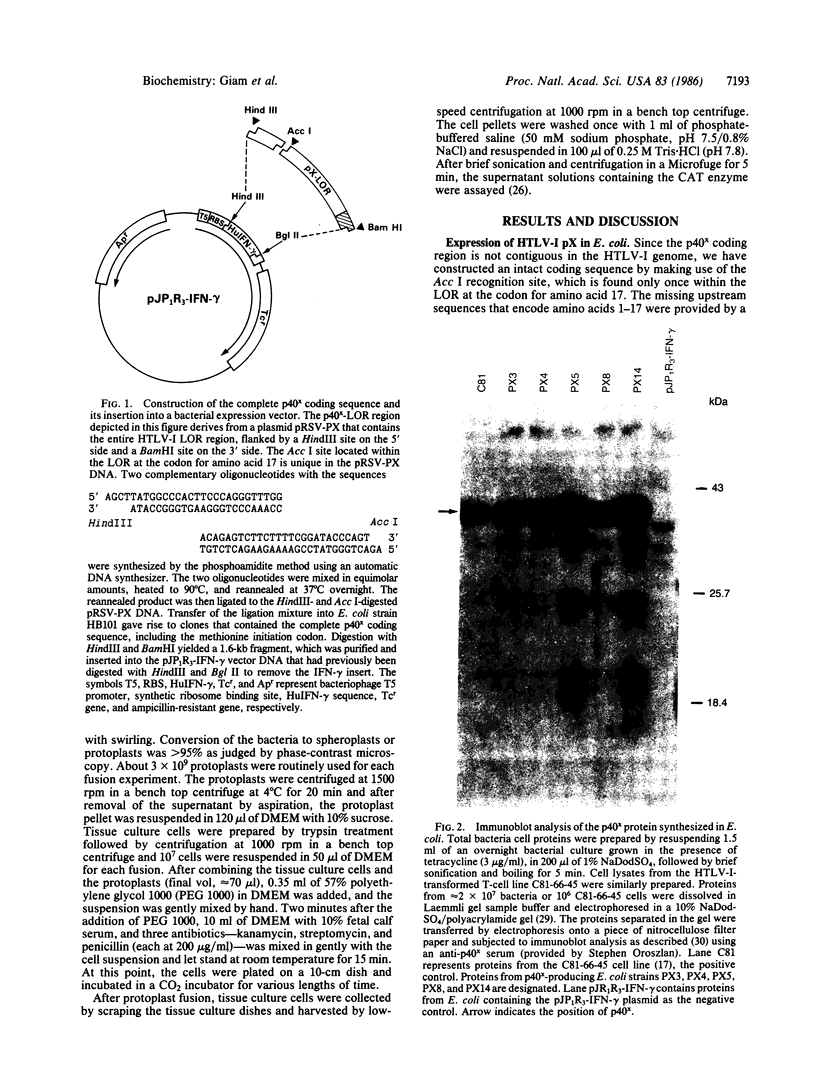
Human T-cell leukemia virus type I (HTLV-I), a virus associated with adult T-cell leukemia, contains a long open reading frame (LOR) in the 3' end of its genome between the env region and the 3' long terminal repeat (LTR). This open reading frame encodes a 40-kDa protein (designated p40x) that has been implicated as a positive control element for transcription from the HTLV-I LTR in a phenomenon known as trans-activation. We now report the expression of the complete p40x coding sequence as a 40-kDa protein in Escherichia coli. The p40x protein produced in bacteria is shown, using the protoplast fusion technique, to possess biological activity by its ability to trans-activate a HTLV-I LTR-chloramphenicol acetyltransferase plasmid that is stably integrated into the genome of mouse L cells. This stimulatory activity could be detected within 2 hr after fusion, suggesting the possibility of a direct role for p40x in trans-activation of the HTLV-I LTR. The production of p40x in large quantities in E. coli, together with the rapid protoplast fusion assay for its biological activity, should facilitate the analysis of p40x mutants and the elucidation of the molecular mechanism of trans-activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Essex M., Haseltine W. A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985 Mar 8;227(4691):1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J., Patarca R., Briggs D., Perkins D., Wong-Staal F. Structure of 3' terminal region of type II human T lymphotropic virus: evidence for new coding region. Science. 1984 Jul 27;225(4660):419–421. doi: 10.1126/science.6330894. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Jay E., Rommens J., Pomeroy-Cloney L., MacKnight D., Lutze-Wallace C., Wishart P., Harrison D., Lui W. Y., Asundi V., Dawood M. High-level expression of a chemically synthesized gene for human interferon-gamma using a prokaryotic expression vector. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2290–2294. doi: 10.1073/pnas.81.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Sharp P. A. Transcription control by oncogenes. Cell. 1985 May;41(1):3–5. doi: 10.1016/0092-8674(85)90049-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Litzkas P., Jha K. K., Ozer H. L. Efficient transfer of cloned DNA into human diploid cells: protoplast fusion in suspension. Mol Cell Biol. 1984 Nov;4(11):2549–2552. doi: 10.1128/mcb.4.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Goldin A. L., Levine M., Glorioso J. High-efficiency transfer of DNA into eukaryotic cells by protoplast fusion. Methods Enzymol. 1983;101:402–411. doi: 10.1016/0076-6879(83)01030-7. [DOI] [PubMed] [Google Scholar]
- Saxinger W., Blattner W. A., Levine P. H., Clark J., Biggar R., Hoh M., Moghissi J., Jacobs P., Wilson L., Jacobson R. Human T-cell leukemia virus (HTLV-I) antibodies in Africa. Science. 1984 Sep 28;225(4669):1473–1476. doi: 10.1126/science.6089348. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Setoyama C., de Crombrugghe B. Regulation of a collagen gene promoter by the product of viral mos oncogene. Nature. 1985 Mar 21;314(6008):286–289. doi: 10.1038/314286a0. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Kalyanaraman V. S., Sarngadharan M. G., Blattner W. A., Gallo R. C. Antibodies against three purified proteins of the human type C retrovirus, human T-cell leukemia-lymphoma virus, in adult T-cell leukemia-lymphoma patients and healthy Blacks from the Caribbean. Cancer Res. 1983 Feb;43(2):886–891. [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Press M. F., Souza L. M., Murdock D. C., Cline M. J., Golde D. W., Gasson J. C., Chen I. S. Studies of the putative transforming protein of the type I human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1427–1430. doi: 10.1126/science.2990027. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Goh W. C., Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman W., Golde D. W., Temple P. A., Orr E. C., Clark S. C., Chen I. S. HTLV x-gene product: requirement for the env methionine initiation codon. Science. 1985 Jun 28;228(4707):1534–1537. doi: 10.1126/science.2990032. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]