Abstract
Energy balance is regulated, in part, by orexigenic signaling pathways of the vagus nerve. Fasting-induced modifications in the expression of orexigenic signaling systems have been observed in vagal afferents of lean animals. Altered basal cannabinoid (CB)1 receptor expression in the nodose ganglia in obesity has been reported. Whether altered body weight or a high fat diet modifies independent or additive changes in CB1 expression is unknown. We investigated the expression of CB1 and orexin 1 receptor (OX-1R) in nodose ganglia of rats fed ad libitum or food deprived (24h), maintained on low or high fat diets (HFD), with differing body weights. Male Wistar rats were fed chow or HFD (diet-induced obese: DIO or diet-resistant: DR) or were body weight matched to the DR group but fed chow (wmDR). CB1 and OX-1R immunoreactivity were investigated and CB1 mRNA density was determined using in situ hybridization. CB1 immunoreactivity was measured in fasted rats after sulfated cholecystokinin octapeptide (CCK8s) administration. In chow rats, fasting did not modify the level of CB1 mRNA. More CB1 immunoreactive cells were measured in fed DIO, DR and wmDR rats than chow rats; levels increased after fasting in chow and wmDR rats but not in DIO or DR rats. In HFD fasted rats CCK8s did not reduce CB1 immunoreactivity. OX-1R immunoreactivity was modified by fasting only in DR rats. These data suggest that body weight contributes to the proportion of neurons expressing CB1 immunoreactivity in the nodose ganglion, while HFD blunts fasting-induced increases, and CCK-induced suppression of, CB1-immunoreactivity.
Keywords: gut-brain axis, diet-induced obesity, energy balance, cannabinoid
Introduction
Energy balance is regulated by the complex interaction of numerous pathways (Berthoud et al. 2011). In the periphery the gastrointestinal tract, liver, pancreas and adipose tissue signal the nutritive status of the body to the central nervous system (CNS) through neuronal pathways and circulating hormones. The CNS and gastrointestinal tract are linked by the bidirectional, hormonal and neuronal pathway termed the gut-brain axis. Information from enteroendocrine cells and the enteric nervous system is relayed to the brain stem via vagal afferent neurons, whose cell bodies lie in the nodose ganglia. Plasticity of the neurochemistry of vagal afferents during feeding implicates this pathway in the control of food intake (Dockray & Burdyga 2011). Expression in the nodose ganglia of the orexin-1 receptor (OX-1R) that mediates orexigenic signaling, is not modified by fasting (Burdyga et al. 2004) whereas the expression of another orexigenic receptor, the cannabinoid CB1 receptor, is increased when lean animals are food-deprived and the fluctuation in expression is inhibited by exogenous and endogenous cholecystokinin (CCK) (Burdyga et al. 2004).
The endocannabinoid system plays a role in the control of food intake through orexigenic actions mediated by cannabinoid CB1 receptor activation (Sharkey & Pittman 2005); indeed the administration of a CB1 receptor agonist induces feeding in rodents (Jarbe & DiPatrizio 2005) and humans (Foltin et al. 1986) while antagonism of CB1 inhibits food intake (Salamone et al. 2007). Furthermore, CB1 receptor gene deficient mice are leaner than their wildtype counterparts and do not develop diet-induced obesity (DIO) (Ravinet Trillou et al. 2004). Components of the endocannabinoid system, including endogenous ligands and their synthetic and catabolic enzymes and receptors, are located in nervous and endocrine systems known to be involved in the regulation of energy balance; including the central nervous system, gastrointestinal tract, liver and adipose tissue (Richard et al. 2009; Sharkey & Pittman 2005; Ueda & Yamamoto 2000). An important role for peripheral CB1 receptors in controlling food intake has been shown by experiments where food intake was inhibited following intraperitoneal, but not intracerebroventricular, administration of the CB1 receptor antagonist/inverse agonist, rimonabant (Gómez et al. 2002). The peripheral endocannabinoid system was further implicated in the regulation of energy balance by experiments using CB1 receptor antagonists which do not pass the blood brain barrier. With their actions restricted to the periphery, URB447 (LoVerme et al. 2009) and AM6545 (Cluny et al. 2010; Randall et al. 2010) have been shown to inhibit food intake in rodents. Furthermore, elevated endocannabinoid levels in the duodenum, liver and pancreas have been found in obese rodents (Izzo et al. 2009). Also, levels of CB1 receptor mRNA are elevated in the stomach of DIO mice (Di Marzo et al. 2008) and nucleus of the solitary tract of obese rats (Bello et al. 2012), and the fasting of lean rats elevates anandamide levels in the duodenum (Gómez et al. 2002).
Recently, it has been reported that vagal afferent CB1 expression in ad libitium fed DIO-leptin resistant and leptin receptor gene-deficient, obese Zucker rats is elevated compared to lean controls and that this is due to the leptin-deficient status of these models (De Lartigue et al. 2012). In other studies, anandamide levels in small intestine of DIO mice were significantly lower than in their lean counterparts while anandamide levels in duodenum of obese Zucker rats was increased compared to lean wild-type rats (Izzo et al. 2009). This implies that increased body weight alone may not be responsible for modified endocannabinoid levels in small intestine and that in obese rodents the ingestion of a high fat diet may have differing actions to those seen with genetic leptin signaling deficiency.
We hypothesized that body weight and a high fat diet would have differing effects on CB1 receptor expression in vagal afferents. The changes in level of expression of cannabinoid CB1 protein and mRNA were determined in the nodose ganglia of rats in differing states of obesity during ad libitum fed and fasted conditions. We also investigated the effects on the protein expression of another orexigenic-mediating receptor, OX-1R.
Materials and Methods
Animals
Male Wistar rats, purchased from Charles River (St-Constant, Quebec, Canada), were singly housed, under a 12 h light-dark cycle (lights off 19:00), in sawdust floor, plastic cages and allowed free access to tap water and either standard laboratory chow (5P14 – Prolab RMH 2500, 12 % kcal from fat, 60 % kcal from carbohydrate and 28 % kcal from protein; Lab Diet, St. Louis, MO, USA) or a high fat diet (D12451, 45 % kcal from fat, 35 % kcal from carbohydrate and 20% kcal from protein; Research Diets Inc., New Brunswick, NJ, USA) unless otherwise stated. Rats used in in situ hybridization studies were singly housed in wire-bottom cages suspended above absorbent paper and fed standard laboratory chow. Male Wistar rats (210–230 g, 7–8 weeks old) used in the fasting time-course studies and CCK8s studies were fed standard laboratory chow or a high fat diet (824053, 45 % kcal from fat, 35 % kcal from carbohydrate and 20% kcal from protein; Special Diet Services, UK). All animal care and experimental procedures were in accordance with the guidelines of the Canadian Council on Animal Care or were conducted under UK Home Office authority, with approval from the appropriate institutional authorities.
Experimental groups
Age-matched rats (202.4 ± 1.8 g, 7 weeks old) were randomly assigned to be fed either a standard laboratory chow (n = 6; body weight 201.3 ± 5.6 g) or a high fat diet (n = 20; body weight 202.7 ± 1.8 g; Research Diets Inc. diet). It has been shown that Wistar rats fed a high fat diet (45 % calories from fat) for 6 weeks display characteristics of obesity including a greater body weight, insulin resistance, increased fasting plasma insulin, glucose and leptin levels and increased triglycerides in skeletal muscle and plasma (Buettner et al. 2000). The rats in the current study were fed the high fat diet for 11 weeks in order for the DIO and DR phenotypes to develop. The body weight of each rat was measured weekly. After 11 weeks of ingesting a high fat diet the rats were designated as being diet-induced obese (DIO) or diet-resistant (DR) based on their body weight. Rats weighing ≤ 590 g were classed as DR (n = 9) and those weighing ≥ 612 g were classed as DIO (n = 11). Other rats were body weight matched (but not age-matched) to the DR group and fed only a chow diet (wmDR; n = 6).
Tissue collection for immunohistochemistry
Rats were fed ad libitum or fasted for 24 h before being deeply anesthetized with sodium pentobarbital (>65 mg/kg) administered intraperitoneally (i.p.). Rats were then perfused intracardially with ice-cold phosphate buffered saline (PBS; 1 l/kg rat), to remove the blood, followed by ice-cold 4 % paraformaldehyde (PFA; 1 l/kg rat), to fix the nodose ganglia. Nodose ganglia were microdissected and immersion fixed in 4 % PFA for 1 h at room temperature. Tissues were washed in PBS 3 times at 10 min intervals before being cryoprotected overnight in 20 % sucrose at 4°C. Nodose ganglia were then embedded in optimal cutting temperature medium and exhaustively cut (7 µm) longitudinally using a cryostat. Sections were sequentially placed on 10 slides. In other experiments, a time-course of fasting was investigated: rats (210–230 g) were fed a high fat diet (Special Diet Services diet) for 4 weeks before being killed by a rising concentration of CO2 after a fasting period of 0, 6, 12, 24, 36 and 48 h (n = 4–5 per time point). The mid and caudal regions of nodose ganglia were dissected and fixed in 4 % PFA.
CCK8s administration
Rats fed chow or high fat diet (Special Diet Services diet) for 4 weeks were fasted for up to 24 h. Animals received an i.p. injection of sulfated cholecystokinin octapeptide (CCK8s; 10 nmol dissolved in saline; Bachem, St. Helens, Merseyside, UK) at the end of the fasting period. Rats (n = 3 per group) were killed by a rising concentration of CO2 0, 1, 4 and 8h after receiving CCK8s. The mid and caudal regions of nodose ganglia were dissected and fixed in 4% PFA.
Immunohistochemistry
Nodose ganglia sections from chow, DIO, DR and wmDR rats were washed in PBS containing 0.1 % Triton X-100 (PBS-T; 3 × 10 min) and then incubated in normal donkey serum (1:10; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 30 min at room temperature. Sections were incubated in either rabbit anti-CB1-C-terminal (CB1-CT; 1:1000 (Hajos et al. 2000)), rabbit anti-CB1-N-terminal (CB1-NT; 1:1000) (Berghuis et al. 2007; Tsou et al. 1998) or rabbit anti-OX-1R (1:1000; OX1R11-A; Alpha Diagnostic International, San Antonio, TX) primary antibodies for 48 h at 4°C. Sections were washed in PBS-T (3 × 10 min) and incubated, at room temperature for 1 h in the secondary antibody donkey anti-rabbit CY3 (1:100; #711-165-152, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). Sections were washed in PBS (3 × 10 min) and mounted in bicarbonate-buffered glycerol before being viewed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Jena, Germany) with images captured using a Retiga 2000R digital camera (QImaging, Surrey, BC, Canada). Four images were captured at 40x magnification from one representative section of the nodose ganglion per slide. The number of immunoreactive cells was determined for each image using Image Pro-Plus software version 7.0 (MediaCybernetics, Silver Spring, MD, USA). For each antibody, the range of intensity was manually determined in preliminary experiments to detect positively stained cells and to exclude background staining. For all analyses, regardless of the immunoreactivity under investigation, the area of intensity detected as a region of immunoreactivity was set to include cell bodies only. The number of immunoreactive cells per nodose ganglion was calculated as a percent of the total cell count. Specificity of the CB1-NT and OX-1R antibodies were tested using immunizing peptides (OX-1R: OX1R11-P, Alpha Diagnostic International). Immunoreactivity to CB1-NT (Tsou et al. 1998) or OX-1R was not observed following incubation with the corresponding peptide. Specificity of the CB1-NT and CB1-CT antibodies has previously been demonstrated by a lack of immunoreactivity in brain tissue of CB1 receptor gene-deficient mice (Hu et al. 2012).
Frozen cryostat sections of nodose ganglia from rats involved in the fasting time-course study and the CCK8s study were rinsed in PBS, permeabilized in ethanol, and processed for immunofluorescence as described previously (Burdyga et al. 2004). For detection of CB1 both affinity-purified goat and rabbit polyclonal antibodies were used (1:90; Santa Cruz Biotechnology, Santa Cruz, CA (Burdyga et al. 2004)). The secondary antibodies, FITC-conjugated (Jackson ImmunoResearch Laboratories, West Grove, PA) donkey anti-goat or antirabbit IgG, were used as appropriate. Sections were examined using an Axioplan Universal microscope, and images were processed using the Axio Vision 3.0 Imaging system with deconvolution options (Carl Zeiss Vision). The quantification of neurons expressing CB1 was made by counting 400–600 immunoreactive cell profiles in five sections per ganglion, selecting sections separated by 90 µm that passed through the full length of the caudal and mid regions of the ganglia.
Tissue collection for in situ hybridization
Rats were fed ad libitum (n = 4) or fasted for 24 h (n = 4) before being deeply anaesthetized with ketamine (60mg/kg) / xylazine (7.5mg/kg, i.p.). They were perfused intracardially with 200 ml of ice-cold isotonic saline followed by 500 ml of 4 % PFA. Right nodose ganglia were microdissected and immersion fixed in paraformaldehyde for 7 days. Ganglia were cryoprotected and stored at −30°C until paraffin inclusion. Fifteen µm thick longitudinal sections were cut and placed on microscopic slides.
In situ hybridization histochemistry
The protocol for in situ hybridization was largely adapted from the previously described technique (Simmons et al. 1989). Deparaffinized sections were successively fixed for 20 min in 4 % PFA: this second fixation step improves the hybridization signal, especially when there are low amounts of targeted mRNA. Sections were then digested for 30 min at 37°C with proteinase K (10 µg/ml in 100 mM Tris-HCl containing 50 mM EDTA, pH 8.0), acetylated with acetic anhydride (0.25 % in 0.1 M triethanolamine, pH 8.0), and dehydrated through graded concentrations (50, 70, 95, and 100 %) of alcohol. After vacuum drying for at least 2 h, 90 µl of the hybridization mixture, containing an antisense 35S-labeled cRNA probe (107 cpm/ml), was spotted on each slide. Coverslips were mounted on the slides and incubated overnight at 60°C in a slide warmer. The next day, coverslips were removed and the slides were rinsed 4 times with 4x saline-sodium citrate (SSC: 0.6 M NaCl, 60 mM trisodium citrate buffer, pH 7.0) containing 1 mM 1,4-dithiothreitol (DTT), digested for 30 min at 37°C with RNase A (10 mg/ml; Roche Diagnostics, Indianapolis, IN, USA), washed in descending concentrations of SSC (2x for 10 min, 1x for 5 min, 0.5x for 5 min, 0.1x for 30 min at 60°C) containing 1 mM DTT, and dehydrated through graded concentrations of alcohol. After 2 h of vacuum drying, the slides were exposed on an X-Ray film (Eastman Kodak, Rochester, NY, USA) for 24 h. Once removed from the autoradiography cassettes, the slides were defatted in toluene and dipped in NTB2 nuclear emulsion (Kodak). The slides were exposed for 7 d before being developed in D19 developer (Kodak) for 3.5 min at 14–15°C and fixed in rapid fixer (Kodak) for 5 min. Finally, tissues were rinsed in running distilled water for 1–2 h, counterstained with thionin (0.25 %), dehydrated through graded concentrations of alcohol, and cleared in toluene, and coverslips were applied with dibutylphtalate-xylol mounting medium.
35S-labeled cRNA probe
The CB1 cRNA probe was generated as described previously (Timofeeva et al. 2009). Radioactive riboprobes were synthesized by incubation of 250 ng linearized plasmid in 10 mM NaCl, 10 mM DTT, 6 mM MgCl, 40 mM Tris (pH 7.9), 0.2 mM ATP/GTP/CTP, 100 µCi α-35S-UTP (Perkin Elmer, Montreal, QC, Canada), 40 U RNase inhibitor (Roche Diagnostics, Indianapolis, IN, USA), and 20 U of SP6 and T7 RNA polymerase for, respectively, antisense and sense probes for 60 min at 37°C. The DNA templates were treated with 100 µl of DNAse solution containing 0.1 U/ml DNAse (Roche Diagnostics), 0.25 mg/ml of tRNA and 50 mM Tris/10 mM MgCl2. The riboprobes were purified using RNeasy Mini Kit (Qiagen, Missisauga, ON, Canada), eluted in 150 µl of 10 mM Tris-1 mM EDTA buffer, and incorporated in a hybridation solution containing (per ml) 107 cpm of 35S probe, 52 % formamide, 330 mM NaCl, 10 mM Tris (pH 8), 1 mM EDTA (pH 8), 1X Denhart’s solution, 10 % dextran sulphate, 0.5 mg/ml of tRNA, 10mM DTT, and diethyl pyrocarbonate water. This solution was mixed and heated at 65°C and then spotted on slides. The specificity of the probe was confirmed by the absence of positive signal in sections hybridized with sense probe.
Quantitative analysis of the hybridization signal
The hybridization signals revealed on NTB2-dipped nuclear emulsion slides were examined under dark-field microscopy using an Olympus BX60 microscope (Olympus America, Melville, NY, USA). Images were acquired with a camera (RT Slider, model 2.3.0, Diagnostic Instruments) and analyzed with ImagePro-Plus software version 4.5.1.23. Saturation of the hybridization signal was avoided by adjusting the exposure time for the image with the strongest hybridization signal. The luminosity of system was set to the maximum, and the saturation warning option was used to visualize saturated regions in the image preview. Thereafter, according to the pixel distribution histogram, the exposure time was adjusted in order to reduce to zero the number of saturated (pure white) pixels. The same exposure time was used throughout the analysis of the entire series. The entire area of the nodose section was outlined; the pixel density of the hybridization signal was measured and then corrected by subtracting background readings taken from areas immediately surrounding the nodose. Four to seven sections were analyzed for each nodose and the average pixel density was considered for statistical analysis.
Data analysis
Data are expressed as the mean ± s.e.mean and were analyzed using either an unpaired t test or one way analysis of variance followed by Bonferroni’s multiple comparison test where appropriate. p < 0.05 was considered significant.
Results
Body weight
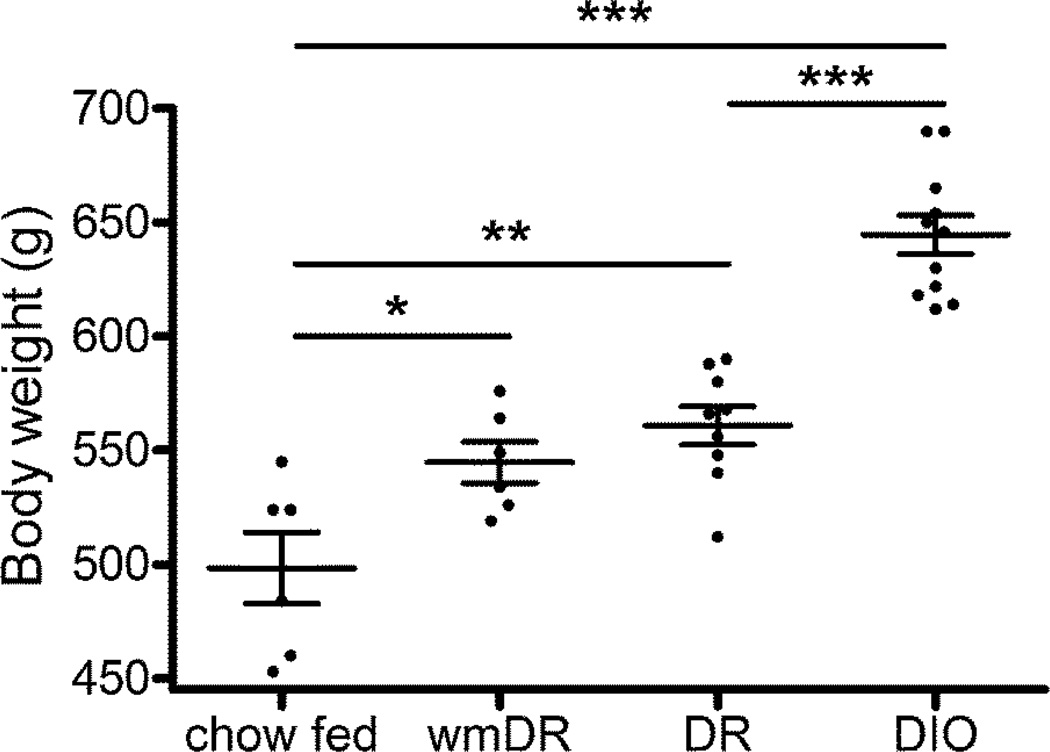
Both groups of rats fed the high fat diet for 11 weeks (DIO: 644.6 ± 8.6 g; DR: 560.9 ± 8.4 g) had a significantly greater (p < 0.01) body weight than the age-matched, chow-fed animals (chow: 498.3 ± 15.5 g; Figure 1). Furthermore, the body weight of DIO rats was significantly greater (p < 0.001) than DR rats (Figure 1). DR rats and rats that were body weight matched to DR but fed a chow diet (wmDR; 544.7 ± 9.1 g) had comparable body weights (p > 0.05) and both had a greater body weight (p < 0.05) than the chow-fed control rats (Figure 1).
Figure 1.
Body weight of male Wistar rats after 11 weeks on a chow (chow) or high fat diet (diet resistant: DR; diet-induced obese: DIO) or fed a chow diet until body weight matched to the DR group (wmDR). Lines and error bars represent the mean ± s.e.mean, n = 6–9 per group. * (p < 0.05), ** (p < 0.01) and *** (p < 0.001) denote a significant difference between indicated groups analyzed using 1 way ANOVA followed by Bonferroni’s multiple comparison test (ns denotes no significant difference).
CB1 receptor expression in chow-fed rats
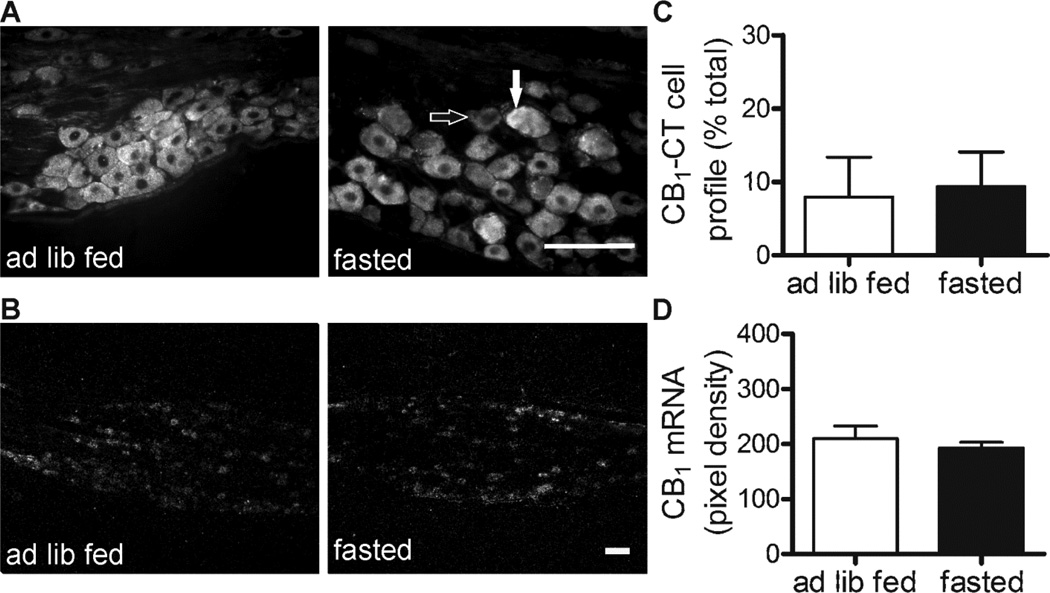
The proportion of cells that were immunoreactive to an antibody raised against the C-terminal of the CB1 receptor was comparable between rats who were ad libitum fed and those who were fasted for 24 h (p > 0.05; Figure 2A and C). However, the proportion of CB1-NT immunoreactive cells in 24 h fasted rats (CB1-NT cell profile % of the total number of cells ± s.e.mean: 21.5 ± 0.7 %) was significantly higher than in ad libitum fed chow-fed rats (1.1 ± 0.7 %; p < 0.001; Figure 3). There was no difference in CB1 mRNA density in nodose ganglia of ad libitum fed vs 24 fasted rats (p > 0.05; Figure 2B and D).
Figure 2.
CB1-CT immunoreactivity (A) and CB1 mRNA expression (B) in nodose ganglia from chow fed rats ad libitum fed (ad lib fed) or fasted for 24 h (fasted). Scale bars: 100 µm. An example of an immunopositive cell (closed arrow) and an immunonegative cell (open arrow) is indicated. Quantification of CB1-CT immunoreactivity (C) and CB1 mRNA levels (D). Bars represent the mean ± s.e.mean, n = 3 per group.
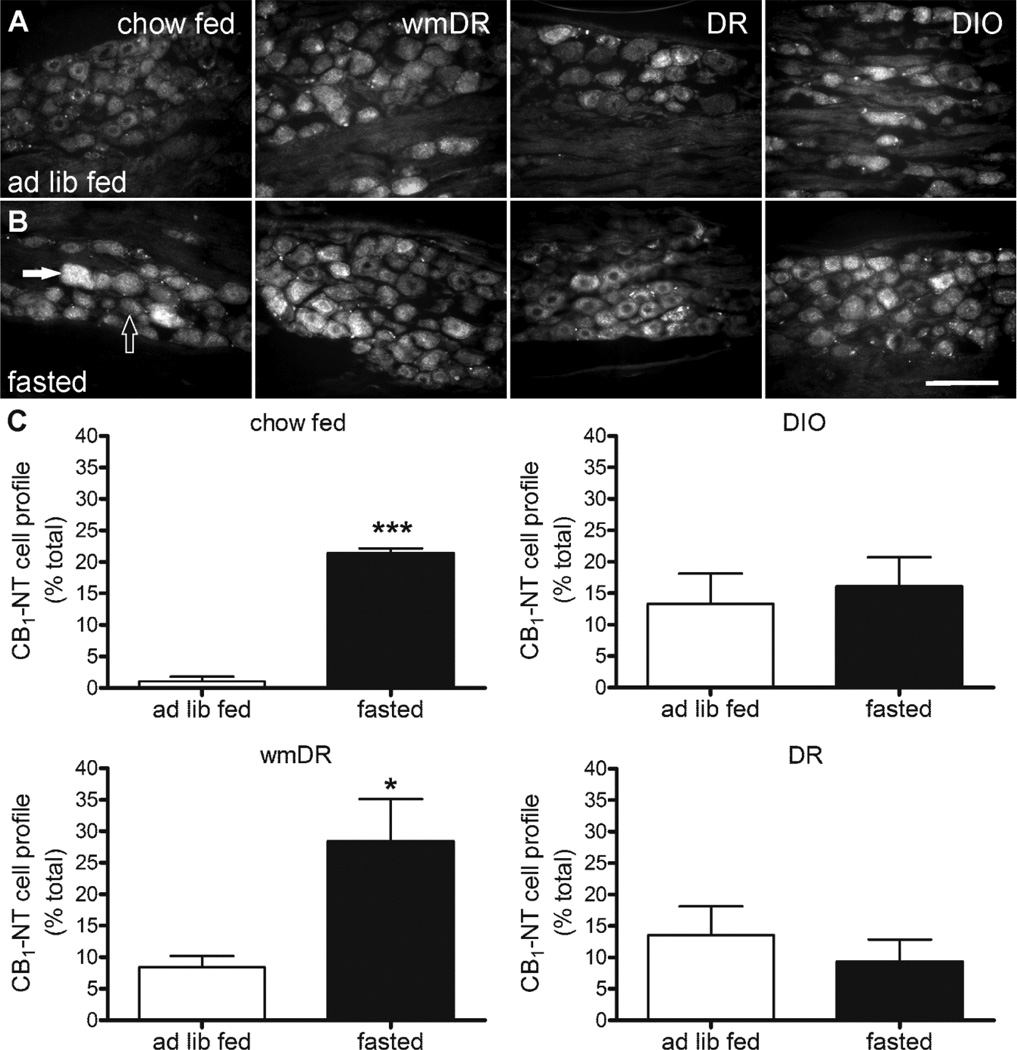
Figure 3.
CB1-NT immunoreactivity in nodose ganglia from chow fed, weight matched to diet resistant (wmDR), diet resistant (DR) and diet-induced obese (DIO) rats ad libitum fed (ad lib fed; A) or fasted for 24 h (fasted; B). Scale bar: 100 µm. An example of an immunopositive cell (closed arrow) and an immunonegative cell (open arrow) is indicated. Quantitative analysis of CB1-NT immunoreactivity (C) in nodose ganglia from chow fed, weight matched to diet resistant (wmDR), diet-induced obese (DIO), and diet resistant (DR) rats ad libitum fed or fasted for 24 h. Bars represent the mean ± s.e.mean, n = 3–5 per group.* (p < 0.05) and *** (p < 0.001) denote a significant difference to the corresponding ad lib fed group analyzed using unpaired t-test.
CB1 receptor immunoreactivity
In animals that were ad libitum fed the percentage of CB1-NT immunoreactive cells was significantly higher in DIO (13.3 ± 4.8 %; p < 0.05), DR (13.6 ± 4.6 %; p < 0.05) and wmDR (8.4 ± 1.8 %; p < 0.01) rats compared to chow-fed rats (1.1 ± 0.7 %). In DIO and DR rats the percentage of cells that were CB1-NT immunoreactive following a 24 h fast was comparable to the level in the fed ad libitum state (Figure 3). A time-course of fasting was investigated and, in rats fed a high fat diet, fasting up to 48 h did not modify the percentage of CB1-NT immunoreactive cells (fed ad libitum: 8 ± 2 %; 6 h fast: 10 ± 4 %; 12 h fast: 13 ± 2 %; 24 h fast: 13 ± 2 %; 36 h fast: 9 ± 1 %; 48 h fast: 12 ±1 %). Rats fed a chow diet yet matched in body weight to DR rats (wmDR) showed an increase (p < 0.05) in the proportion of CB1-NT immunoreactive cells in response to a 24 h fast (Figure 3).
CB1 receptor immunoreactivity following CCK8s administration
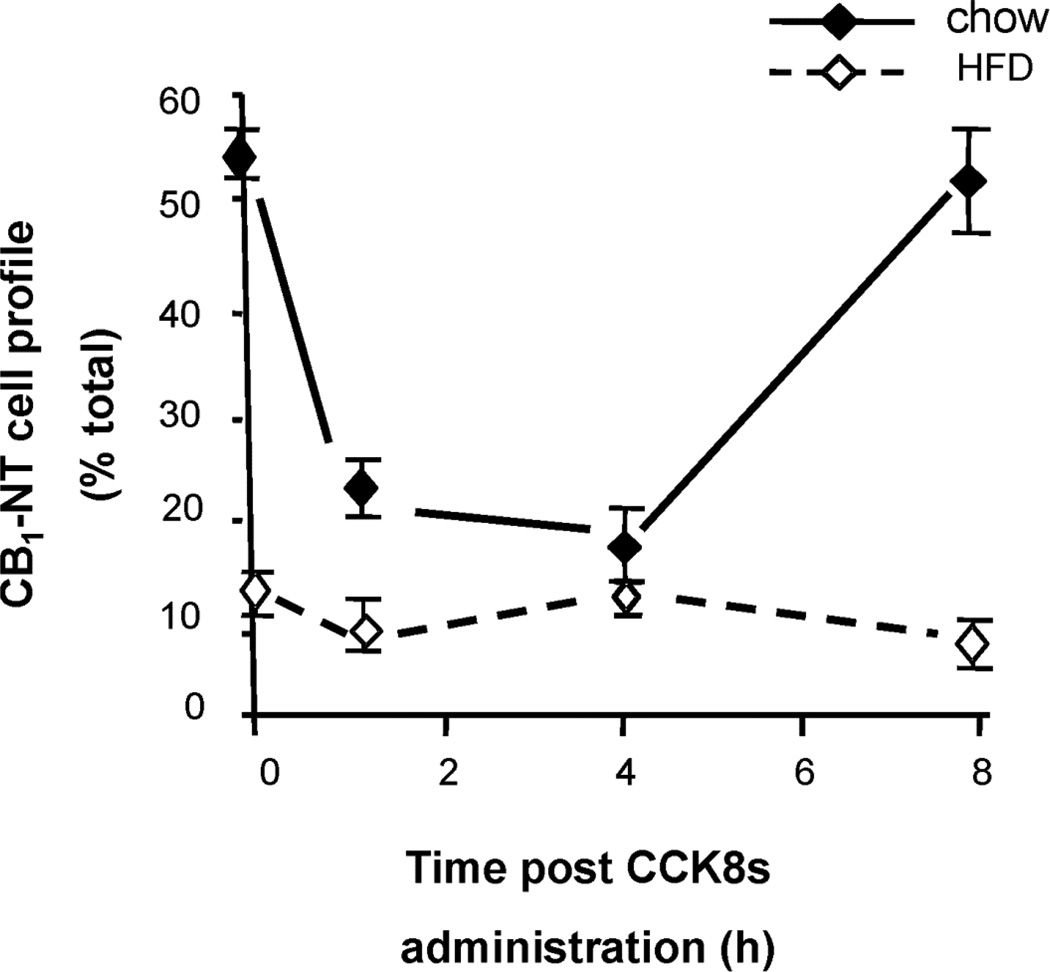
In order to examine the effect of a high fat diet on CCK-induced changes in CB1 receptor immunoreactivity rats were maintained on a high fat diet for 4 weeks only which is not long enough for the phenotypes of DIO and DR to develop. In rats fed a high fat diet for 4 weeks body weight increased from 230 ± 2 g to 432 ± 6, compared with 233 ± 5 g increasing to 390 ± 9 g in chow-fed controls (p < 0.05). The administration of CCK8s to 24 h fasted, chow-fed rats induced a reduction in CB1 receptor cell profile 1 and 4 h post injection (Figure 4). Eight h after the CCK8s injection the percentage of CB1 immunoreactive cells in the nodose ganglia of chow-fed rats fasted for 24 h had increased back to a comparable level as was observed prior to CCK8s administration (Figure 4). CCK8s administration had no effect on the CB1 cell profile in the nodose ganglia in rats that were fed a high fat diet and fasted for 24 h (Figure 4).
Figure 4.
The effect of CCK8s (10 nmol, i.p.) administration on CB1-NT immunoreactivity in nodose ganglia from 24 h fasted rats fed chow or high fat diet. Data points represent the mean ± s.e.mean, n = 3 per group.
OX-1R immunoreactivity
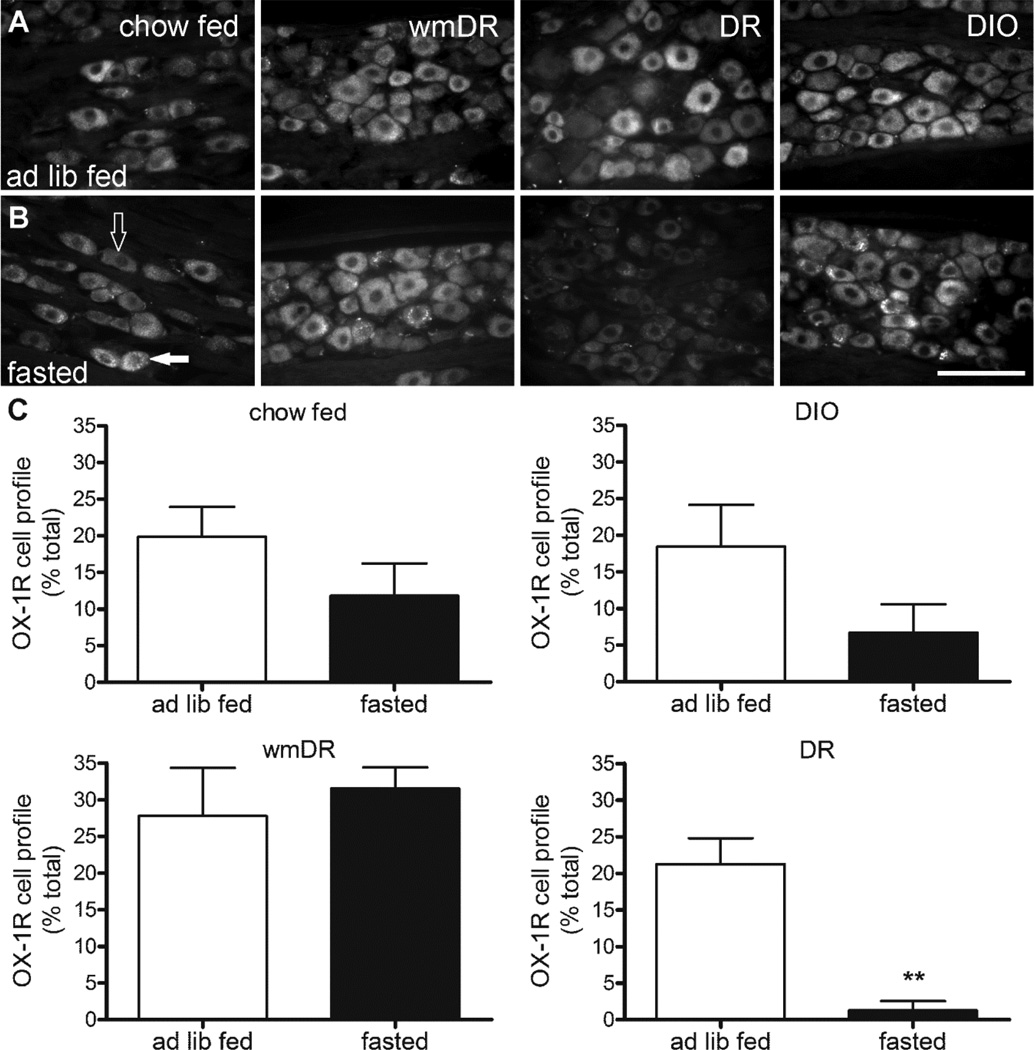
The proportion of OX-1R immunoreactive cells in nodose ganglia from ad libitum fed DIO (18.4 ± 5.7 %), DR (21.3 ± 3.5 %) and wmDR (27.8 ± 2.6 %) rats was comparable to that in the chow fed animals (19.8 ± 4.1 %; p > 0.05; Figure 5). There was a significant reduction (p < 0.01) in the percentage of OX-1R immunoreactive cells in fasted DR rats (1.3 ± 1.3 %), compared to those fed ad libitum (21.3 ± 3.5 %; Figure 5C). Fasting did not modify the proportion of OX-1R immunoreactive cells in chow, DIO or wmDR rats (Figure 5).
Figure 5.
OX-1R immunoreactivity in nodose ganglia from chow fed, weight matched to diet resistant (wmDR), diet resistant (DR) and diet-induced obese (DIO) rats ad libitum fed (ad lib fed; A) or fasted for 24 h (fasted; B). Scale bar: 100 µm. An example of an immunopositive cell (closed arrow) and an immunonegative cell (open arrow) is indicated. Quantitative analysis of OX- immunoreactivity (C) in nodose ganglia from chow fed, weight matched to diet resistant (wmDR), diet-induced obese (DIO), and diet resistant (DR) rats ad libitum fed or fasted for 24 h. Bars represent the mean ± s.e.mean, n = 3 per group. ** (p < 0.01) denotes a significant difference to the corresponding ad lib fed group analyzed using unpaired t-test.
Discussion
The current study investigated the effects of a high fat diet and of body weight on the expression of orexigenic-mediating receptors in nodose ganglia of rats under ad libitum fed and 24 h fasted conditions. We present data showing that the proportion of N-terminal CB1 receptor immunoreactive cells in the nodose ganglia is dependent on feeding status. Observed differences in immunoreactivity to antibodies raised against differing terminals of the receptor may be indicative of changes in receptor activity as opposed to a change in protein expression. The data further suggest that N-terminal CB1 receptor immunoreactivity is enhanced in animals with a greater body weight and that high-fat feeding may blunt fasting-induced increases in receptor expression and CCK-induced reduction in receptor immunoreactivity. This was not observed for OX-1R.
We have confirmed the previous observation (Burdyga et al. 2004; De Lartigue et al. 2012) that immunoreactivity to an antibody raised against the N-terminal of the CB1 receptor is low in the nodose ganglia of ad libitum fed rats maintained on a standard chow diet, and elevated following a period of fasting. However, the present data suggest that the percentage of cells expressing immunoreactivity to an antibody raised against the carboxy tail of the receptor is not reduced when animals are ad libitum fed, and is not modified by fasting. The differences observed in N-terminal immunoreactivity may reflect a change in receptor activity, and that C-terminal immunoreactivity is unchanged may be indicative of unaltered CB1 receptor protein expression in differing feeding statuses.
The cannabinoid CB1 receptor is a class A, rhodopsin-like, G protein-coupled receptor (GPCR) consisting of an extracellular N-terminal, 7 transmembrane helices, with 3 extracellular loops, and an intracellular C-terminal. The C-terminal is involved in G protein binding and thus the induction of signal transduction during receptor activation (Mukhopadhyay & Howlett 2001; Nie & Lewis 2001). The N-terminal of CB1 receptor is not involved in ligand binding or receptor activation but has been suggested to be involved in speculated chaperone-mediated functions such as receptor synthesis and degradation (Andersson et al. 2003). However, upon CB1 receptor activation the N-terminal protein unfolds (Gupta et al. 2007). This suggests that activation-induced unfolding of the N-terminal results in the exposure of the epitope to which the CB1-NT antibody used in the current studies was raised. We show that the proportion of cells displaying immunoreactivity to the N-terminal of CB1 is very low when lean, chow-fed animals are ad libitum fed and that this is increased following a 24h fast. Such fluctuations were not seen with the degree of immunoreactivity to the C-terminal of the receptor. The specificity of the antibody used in the current studies has been confirmed by a lack of staining in CB1 receptor knockout mice suggesting that the possibility of non-specific staining is highly unlikely. Moreover, our findings suggest that CB1 mRNA expression in the nodose ganglia of chow fed rats is similarly not modified by feeding status. CB1 mRNA transcript was evident at comparable levels in cell bodies of vagal afferents in rats which were fed ad libitum and in those which were fasted for 24h. While these data are similar to those reported by Jelsing and colleagues (Jelsing et al. 2009) it has also been reported that CB1 mRNA was reduced by feeding and increased by fasting in the nodose ganglia of rats (Burdyga et al. 2004). Technical differences between the studies may account for the differing results. RNA probes were used in the present study, and also in that reported by Jelsing and colleagues (Jelsing et al. 2009), whereas a three-oligonucleotide probe was used in the study where feeding status-modifications in mRNA density were reported (Burdyga et al. 2004). RNA probes offer a high degree of sensitivity and stability (due to high specific activities, genetic complexities and hybridization energy between RNA-RNA hybrids). Furthermore, higher stringency of the RNA-RNA hybridization results in the detection of less abundant transcripts. The current findings suggest that neither mRNA nor overall protein expression are altered by feeding status, but rather our results using the N-terminal antibody may reflect activated and inactivated states of the receptor. Levels of the endocannabinoid 2-arachidonoylglycerol (2-AG) in rat hypothalamus (Kirkham et al. 2002) and in whole mouse brain (Hanus et al. 2003) have been shown to be significantly increased after a 24h period of fasting. Furthermore, fasting for 24h induced an increase in the levels of the major endocannabinoids, 2-AG and anandamide, in rat limbic forebrain (Kirkham et al. 2002), duodenum, pancreas and liver (Izzo et al. 2009) compared to non-food deprived animals. That there are low levels of endocannabinoids during fed states implies that there is little opportunity for CB1 receptor activation. This suggests that the conformation of the N-terminal of the receptor would be such that the antibody directed towards this region could not bind, resulting in low immunoreactivity in the ad libitum fed rat nodose ganglia in the current study. It is suggested that CB1 activation plays a role in the maintenance of fasting-induced neurochemical phenotypes in the nodose ganglia such as increased CB1 and reduced Y2R expression (Burdyga et al. 2010). Administration of a CB1 receptor antagonist/inverse agonist blocked fasting-induced changes in expression of CB1 and the anorexigenic-mediating receptor Y2 in the nodose ganglia (Burdyga et al. 2010). This suggests that CB1 receptors in the nodose ganglia, as opposed to the central or peripheral terminals of the vagal afferents are involved in this effect. In the current study, the percentage of cells that showed CB1 N-terminal immunoreactivity was increased in the nodose ganglia of fasted rats and this may reflect receptor activation by elevated endocannabinoid levels, leading to the maintenance of fasting-induced neurochemical phenotypes in this structure.
We confirmed the development of two rat phenotypes in response to a high fat diet; those with a greater body weight, termed diet-induced obese (DIO) and those which were leaner termed diet-resistance (DR) (Levin et al. 1983). We used these models to examine whether feeding status-induced fluctuations in CB1 receptor N-terminal immunoreactivity in the nodose ganglia differed in response to high fat feeding and whether body weight influenced any observed modifications. In the present studies CB1 receptor immunoreactivity in the nodose ganglia of rats fed a high fat diet was greater than in chow fed rats, regardless of their DIO or DR status. In contrast, it has been reported that DIO, but not DR, rats have elevated levels of CB1 gene (Paulino et al. 2009) and protein expression (De Lartigue et al. 2012) in the nodose ganglia compared to chow controls. However, in the current study not only were the DR rats heavier than their chow fed controls but they also had a greater body weight than DR rats in the aforementioned studies (De Lartigue et al. 2012; Paulino et al. 2009); indicating that dietary fat content or increased body weight may have contributed to the increase in CB1 receptor immunoreactivity we report. To investigate this we used a group of rats which were body weight-matched to the DR group (wmDR) but only fed a chow diet.
In the wmDR animals the percentage of cells in the nodose ganglia which were immunopositive for CB1 when animals were ad libitum fed was higher than in the leaner, chow fed controls. This was also observed in the DR and DIO rats and thus may be related to a greater body weight, as opposed to dietary fat content. This suggests that in heavier animals there may be sustained cannabinoid-induced feeding, as evidenced by a higher proportion of activated CB1, even during states when animals would be expected to have low orexigenic stimulation. Interestingly, this appears to be independent of dietary fat composition. Our findings that the number of cells expressing immunoreactivity to the CB1 N-terminal antibody was higher in adlibitum fed animals that had a greater body weight, regardless of their diet implies that body weight may have additive, yet independent influences on the upregulation of the endocannabinoid system observed in obesity.
The present immunohistochemical data suggest that, in contrast to what is seen in chow fed rats, CB1 immunoreactivity is not increased in response to fasting when animals are fed a high fat diet. This effect is unlikely to be influenced by body weight as the number of cells that showed immunoreactivity to the CB1 N-terminal antibody was increased by fasting in the chow fed animals weight matched to DR rats. As described above there is an increase in endocannabinoid production in response to fasting but also there is evidence that the expression of the endocannabinoid system is modified in animals fed a high fat diet; such changes appear to be tissue- and endocannabinoid- specific. For example, in mice fed a high fat diet anandamide, but not 2-AG, levels were reduced in the stomach (Di Marzo et al. 2008) yet elevated in the liver (Osei-Hyiaman et al. 2005). Anandamide and 2-AG levels were increased in the pancreas of DIO mice compared to lean controls, and in visceral fat only 2-AG was increased in DIO mice (Matias et al. 2006). Increased 2-AG but reduced anandamide was observed in duodenum of mice fed a high fat diet (Izzo et al. 2009). A general increase in endocannabinoid levels was observed in skeletal muscle, brown adipose tissue, adrenal gland, heart and kidneys in mice fed high fat diets, with the time of onset and duration of the changes being dependent on the fatty acid content of the diet (Matias et al. 2008). CB1 protein expression in purified liver plasma membrane is increased in mice fed a high fat diet (Osei-Hyiaman et al. 2005). A high fat diet did not modify CB1 immunoreactivity in mouse pancreatic islets, visceral or subcutaneous adipose tissue (Starowicz et al. 2008). After being fed a high fat diet CB1 mRNA levels were reduced in mouse stomach antrum (Di Marzo et al. 2008) and in rat cingulate pre-frontal cortex, ventrolateral parvocellular paraventricular hypothalamic nucleus and ventromedial hypothalamic nucleus (Timofeeva et al. 2009). Furthermore, obese Zucker fa/fa rats demonstrated higher levels of endocannabinoids in the duodenum, liver and pancreas compared to lean rats and in the duodenum these levels were increased even further upon fasting (Izzo et al. 2009). This may suggest that the elevated level of endocannabinoid production in response to the two stimuli of high body weight and fasting is additive. As described above, high fat feeding is also a reported stimulus of endocannabinoid production and this may also act additively with fasting and a high body weight to induce endocannabinoid production. This may lead to downregulation of CB1 receptor and thus in the DIO and DR rats no further increase in activity was possible in response to fasting. It may be argued that fasting-induced increases in CB1-NT immunoreactivity in these rats is delayed however, the current studies suggest that even when the fasting period is increased to 48h immunoreactivity remains comparable to that in the ad libitum fed state.
There is growing evidence that in vagal afferent neurons the actions of CCK can influence the expression of receptors that are involved in the control of food intake, including CB1 (Dockray & Burdyga 2011). CB1 and CCK are colocalized in neurons in the rat nodose ganglia and CCK8 administration reduces CB1 expression in fasted chow-fed rats (Burdyga et al. 2004). However, the present study suggests that exogenous CCK has no effect on food deprived CB1 receptor immunoreactivity in heavy rats fed a high fat diet suggesting that control of CCK over this orexigenic signaling pathway may be lost following ingestion of high levels of fat and /or due to a heavier body weight. The level of CCK-1R mRNA in nodose ganglia is reported to be the same in weight-matched rats fed either chow or a high fat diet for 14 days (Broberger et al. 2001). However, mice fed a high fat diet for 15 days that had a greater body weight than chow fed mice had a lower level of CCK-1R mRNA in nodose ganglia and in these mice, exogenous CCK was less effective to reduce food intake than in chow fed mice (Nefti et al. 2009). This may suggest that a greater body weight, rather than a high fat diet plays a part in these differences. However, exogenous CCK is more efficacious to reduce food intake in chow fed rats that are obesity prone compared to chow fed rats that are significantly leaner (Swartz et al. 2010) and CCK1R mRNA is elevated in nodose ganglia of DIO rats yet is unchanged in rats who are resistant to the obesigenic effects of a high fat diet (Paulino et al. 2009) suggesting that body weight and fat content of the diet may both exert modifications in CCK signaling. The studies discussed above also highlight possible differences in response to CCK depending on the animal model used, with the hypophagic effect of exogenous CCK being less efficacious in mice (Nefti et al. 2009) and more efficacious in DIO rats (Swartz et al. 2010). It has been suggested that leptin signaling is essential for endogenous CCK-induced modifications of neurochemical phenotype in cell bodies of the nodose ganglia (De Lartigue et al. 2012): In rats that were fasted for 24h before being fed for 1 h (a model of meal-induced endogenous CCK release), fastinginduced elevation of CB1 receptor expression in nodose ganglia of DIO animals was not downregulated by feeding and it was suggested that this was due to the leptin resistant status of the rats (De Lartigue et al. 2012). It has been reported that after 8 weeks on a high fat diet vagal afferent neurons of DIO but not DR exhibited leptin resistance (De Lartigue et al. 2011). In the present study, animals were fed a high fat diet for only 4 weeks and the DR vs DIO phenotype had not presented; thus it is unlikely that those rats were leptin resistant compared to the chow-fed controls. Our findings suggest that changes in CCK signaling in rats fed a high fat diet, which were heavier than chow-fed controls, may result in a loss of CCK-induced inhibition of CB1 receptor immunoreactivity, and thus maintain the higher levels of basal CB1 activity we observed in fed ad libitum rats. The data suggest that the ingestion of a high fat diet or a heavier body weight alters the ability of CCK to negatively influence endocannabinoid signaling and this may play a role in the maintenance of a positive energy balance.
The CB1 immunoreactivity changes in the nodose ganglia observed in response to feeding status, high fat feeding and body weight were not similarly seen in OX-1R immunoreactivity in the current study. In the ad libitum fed state immunoreactivity to OX-1R was present and a 24 h fast did not modify this in the nodose ganglia of chow fed rats. OX-1R gene expression in lean rat nodose ganglia was not altered by fasting (Burdyga et al. 2004) and the present study suggests that protein expression is similarly not altered by food deprivation in this structure. Furthermore we present data suggesting that the proportion of OX-1R immunoreactive cells in DIO rats and heavier chow-fed rats was comparable to leaner chow-fed animals and this was not modified by feeding status. A high fat diet had no effect on hypothalamic OX-1R mRNA levels (White et al. 2005) and the current study suggests that high fat feeding does not alter OX-1R protein in the nodose ganglia. However, it would appear that in animals resistant to the obesogenic effects of high fat feeding, food deprivation induces a down-regulation in the number of OX-1R receptor immunoreactive cells. This was not observed in weight-matched rats that were fed a standard chow diet and thus suggests this occurs in diet resistant animals only when exposed to a high fat diet. This reduction in orexin-1 receptor may play a role in the generation of the leaner phenotype observed in these rats.
Conclusion
These results suggest that a heavier body weight may contribute to an elevated proportion of neurons expressing CB1-NT immunoreactivity in the nodose ganglion of rats fed ad libitum and that a high-fat diet blunts the increase of CB1-NT immunoreactivity in response to fasting, and also suppresses the inhibitory action of CCK on CB1 immunoreactivity in nodose ganglia. This indicates that the two parameters of a greater body weight and high fat feeding may act in differing ways but with the same outcome: that CB1 activation in the nodose ganglia is elevated which may contribute to a physiologically-inappropriate stimulation of food intake, leading to the maintenance of obesity.
Acknowledgments
The authors thank Winnie Ho for technical assistance. These studies were supported by grants from the Canadian Institutes of Health Research (Team grant in the Neurobiology of Obesity: no. OTG-183738) (K.A.S. and D.R.), the Biotechnology and Biological Sciences Research Council (G.J.D and G.B.) and the NIH (DA011322 and DA021696) (K.M.). K.A.S. is an AHFMR Medical Scientist and the Crohn's and Colitis Foundation of Canada Chair in Inflammatory Bowel Disease Research. N.L.C. is an Alberta Innovates - Health Solutions (AI-HS) fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors disclose no conflicts of interest.
References
- Andersson H, D'Antona AM, Kendall DA, Von HG, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol.Pharmacol. 2003;64:570–577. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- Bello NT, Coughlin JW, Redgrave GW, Ladenheim EE, Moran TH, Guarda AS. Dietary conditions and highly palatable food access alter rat cannabinoid receptor expression and binding density. Physiol Behav. 2012;105:720–726. doi: 10.1016/j.physbeh.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am.J.Physiol Regul.Integr.Comp Physiol. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–140. doi: 10.1016/s0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am.J.Physiol Endocrinol.Metab. 2000;278:E563–E569. doi: 10.1152/ajpendo.2000.278.3.E563. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J.Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics, and role in influencing neurochemical phenotype. Am.J.Physiol Gastrointest.Liver Physiol. 2010 doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br.J.Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Barbier De la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS.One. 2012;7:e32967. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lartigue G, Barbier de la SC, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am.J.Physiol Endocrinol.Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, Romano B, Orlando P, Capasso F, Izzo AA. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br.J.Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol.Biochem.Behav. 1986;25:577–582. doi: 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J.Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, Devi LA. Conformation state-sensitive antibodies to G-protein-coupled receptors. J.Biol.Chem. 2007;282:5116–5124. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur.J.Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hanus L, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003;983:144–151. doi: 10.1016/s0006-8993(03)03046-4. [DOI] [PubMed] [Google Scholar]
- Hu H, Ho W, Mackie K, Pittman QJ, Sharkey KA. Brain CB(1) receptor expression following lipopolysaccharide-induced inflammation. Neuroscience. 2012;227:211–222. doi: 10.1016/j.neuroscience.2012.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br.J.Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, DiPatrizio NV. Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav.Pharmacol. 2005;16:373–380. doi: 10.1097/00008877-200509000-00009. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Larsen PJ, Vrang N. The effect of leptin receptor deficiency and fasting on cannabinoid receptor 1 mRNA expression in the rat hypothalamus, brainstem and nodose ganglion. Neurosci.Lett. 2009;463:125–129. doi: 10.1016/j.neulet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br.J.Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Triscari J, Sullivan AC. Relationship between sympathetic activity and diet-induced obesity in two rat strains. Am J Physiol. 1983;245:R364–R371. doi: 10.1152/ajpregu.1983.245.3.R364. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Stella N, Xu C, Tarzia G, Piomelli D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg.Med.Chem.Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J.Clin.Endocrinol.Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol.Cell Endocrinol. 2008;286:S66–S78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. CB1 receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur.J.Biochem. 2001;268:499–505. doi: 10.1046/j.1432-1327.2001.01810.x. [DOI] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am.J.Physiol Regul.Integr.Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Nie J, Lewis DL. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience. 2001;107:161–167. doi: 10.1016/s0306-4522(01)00335-9. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Depetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J.Clin.Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino G, Barbier De la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am.J.Physiol Endocrinol.Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, Salamone JD. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol.Biochem.Behav. 2010 doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int.J.Obes.Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Richard D, Guesdon B, Timofeeva E. The brain endocannabinoid system in the regulation of energy balance. Best.Pract.Res.Clin.Endocrinol.Metab. 2009;23:17–32. doi: 10.1016/j.beem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA, Pittman QJ. Central and peripheral signaling mechanisms involved in endocannabinoid regulation of feeding: a perspective on the munchies. Sci.STKE. 2005;2005:e15. doi: 10.1126/stke.2772005pe15. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A Complete Protocol for Insitu Hybridization of Messenger-Rnas in Brain and Other Tissues with Radiolabeled Single-Stranded Rna Probes. Journal of Histotechnology. 1989;12:169–181. [Google Scholar]
- Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di Marzo V. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a highfat diet. Obesity.(Silver.Spring) 2008;16:553–565. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- Swartz TD, Duca FA, Covasa M. Differential feeding behavior and neuronal responses to CCK in obesity-prone and -resistant rats. Brain Res. 2010;1308:79–86. doi: 10.1016/j.brainres.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Baraboi ED, Poulin AM, Richard D. Palatable high-energy diet decreases the expression of cannabinoid type 1 receptor messenger RNA in specific brain regions in the rat. J.Neuroendocrinol. 2009;21:982–992. doi: 10.1111/j.1365-2826.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamamoto S. Anandamide amidohydrolase (fatty acid amide hydrolase) Prostaglandins Other Lipid Mediat. 2000;61:19–28. doi: 10.1016/s0090-6980(00)00052-6. [DOI] [PubMed] [Google Scholar]
- White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–2338. doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]