Abstract
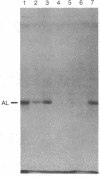
Using antibodies specific for argininosuccinate lyase (EC 4.3.2.1), we isolated two cDNA clones by screening a human liver cDNA library constructed in the lambda gt11 expression vector. The identity of these isolates was confirmed by in vitro translation of plasmid-selected mRNA. One of these isolates was used to rescreen the cDNA library and a 1565-base-pair (bp) clone was identified. The entire nucleotide sequence of this clone was determined. An open reading frame was identified which encoded a protein of 463 amino acids with a predicted molecular weight of 51,663. The clone included 115 bp of 5' untranslated sequence and 46 bp of 3' untranslated sequence. A canonical poly(A) addition site was present in the 3' end, 16 bp from the beginning of the poly(A) tract. Comparison of the deduced amino acid sequence of the human enzyme with that of the yeast enzyme revealed a 56% homology, when conservative amino acid changes were taken into consideration. The yeast protein is also 463 amino acids long, with a molecular weight of 51,944. By use of a genomic DNA panel from human-Chinese hamster somatic cell hybrids, the human gene was mapped to chromosome 7. Another hybridizing region, corresponding to a portion of the 5' end of the cDNA, was found on chromosome 22.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adcock M. W., O'Brien W. E. Molecular cloning of cDNA for rat and human carbamyl phosphate synthetase I. J Biol Chem. 1984 Nov 10;259(21):13471–13476. [PubMed] [Google Scholar]
- Beacham I. R., Schweitzer B. W., Warrick H. M., Carbon J. The nucleotide sequence of the yeast ARG4 gene. Gene. 1984 Sep;29(3):271–279. doi: 10.1016/0378-1119(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce F. M., Anderson G. M., Rusk C. D., Freytag S. O. Human argininosuccinate synthetase minigenes are subject to arginine-mediated repression but not to trans induction. Mol Cell Biol. 1986 Apr;6(4):1244–1252. doi: 10.1128/mcb.6.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Ratner S. Argininosuccinase from bovine kidney: comparison of catalytic, physical, and chemical properties with the enzyme from bovine liver. Arch Biochem Biophys. 1971 Oct;146(2):531–541. doi: 10.1016/0003-9861(71)90158-5. [DOI] [PubMed] [Google Scholar]
- Carritt B., Povey S. Regional asssignments of the loci AK3, ACONS, and ASS on human chromosome 9. Cytogenet Cell Genet. 1979;23(3):171–181. doi: 10.1159/000131323. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Beaudet A. L., Bock H. G., O'Brien W. E. Molecular structure of the human argininosuccinate synthetase gene: occurrence of alternative mRNA splicing. Mol Cell Biol. 1984 Oct;4(10):1978–1984. doi: 10.1128/mcb.4.10.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard L. J., Bui Q. T., Nygaard R., Raushel F. M. Acid-base catalysis in the argininosuccinate lyase reaction. J Biol Chem. 1985 May 10;260(9):5548–5553. [PubMed] [Google Scholar]
- HAVIR E. A., TAMIR H., RATNER S., WARNER R. C. BIOSYNTHESIS OF UREA. XI. PREPARATION AND PROPERTIES OF CRYSTALLINE ARGININOSUCCINASE. J Biol Chem. 1965 Jul;240:3079–3088. [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., O'Brien W. E., Beaudet A. L. Arginine-mediated regulation of an argininosuccinate synthetase minigene in normal and canavanine-resistant human cells. Mol Cell Biol. 1986 Jun;6(6):2257–2261. doi: 10.1128/mcb.6.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby L. B. Adaptation of cultured human lymphoblasts to growth in citrulline. Exp Cell Res. 1974 Mar 15;84(1):167–174. doi: 10.1016/0014-4827(74)90393-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Simard L. R., Ray P. N., McInnes R. R. Molecular cloning of cDNA for rat argininosuccinate lyase and its expression in rat hepatoma cell lines. Mol Cell Biol. 1986 May;6(5):1722–1728. doi: 10.1128/mcb.6.5.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P., Graf L., Mercer J. F., Wake S. A., Hudson P., Hoogenraad N. The primary structure of the imported mitochondrial protein, ornithine transcarbamylase from rat liver: mRNA levels during ontogeny. DNA. 1985 Apr;4(2):147–156. doi: 10.1089/dna.1985.4.147. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Klebe R. J., Shows T. B. Argininosuccinic aciduria: assignment of the argininosuccinate lyase gene to the pter to q22 region of human chromosome 7 by bioautography. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6159–6162. doi: 10.1073/pnas.75.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Nuzum C. T., Snodgrass P. J. Urea cycle enzyme adaptation to dietary protein in primates. Science. 1971 Jun 4;172(3987):1042–1043. doi: 10.1126/science.172.3987.1042. [DOI] [PubMed] [Google Scholar]
- Nyunoya H., Broglie K. E., Widgren E. E., Lusty C. J. Characterization and derivation of the gene coding for mitochondrial carbamyl phosphate synthetase I of rat. J Biol Chem. 1985 Aug 5;260(16):9346–9356. [PubMed] [Google Scholar]
- O'Brien W. E., Barr R. H. Argininosuccinate lyase: purification and characterization from human liver. Biochemistry. 1981 Mar 31;20(7):2056–2060. doi: 10.1021/bi00510a049. [DOI] [PubMed] [Google Scholar]
- Palekar A. G., Mantagos S. Human liver arginiosuccinase purification and partial characterization. J Biol Chem. 1981 Sep 10;256(17):9192–9194. [PubMed] [Google Scholar]
- Patel P. I., Nussbaum R. L., gramson P. E., Ledbetter D. H., Caskey C. T., Chinault A. C. Organization of the HPRT gene and related sequences in the human genome. Somat Cell Mol Genet. 1984 Sep;10(5):483–493. doi: 10.1007/BF01534853. [DOI] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- SCHIMKE R. T. ENZYMES OF ARGININE METABOLISM IN MAMMALIAN CELL CULTURE. I. REPRESSION OF ARGININOSUCCINATE SYNTHETASE AND ARGININOSUCCINASE. J Biol Chem. 1964 Jan;239:136–145. [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Snodgrass P. J., Lin R. C., Müller W. A., Aoki T. T. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem. 1978 Apr 25;253(8):2748–2753. [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Increased translatable messenger ribonucleic acid for argininosuccinate synthetase in canavanine-resistant human cells. Biochemistry. 1981 May 12;20(10):2956–2960. doi: 10.1021/bi00513a037. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Su T. S., Nussbaum R. L., Airhart S., Ledbetter D. H., Mohandas T., O'Brien W. E., Beaudet A. L. Human chromosomal assignments for 14 argininosuccinate synthetase pseudogenes: cloned DNAs as reagents for cytogenetic analysis. Am J Hum Genet. 1984 Sep;36(5):954–964. [PMC free article] [PubMed] [Google Scholar]
- Su T. S., O'Brien W. E., Beaudet A. L. Genomic DNA-mediated gene transfer for argininosuccinate synthetase. Somat Cell Mol Genet. 1984 Nov;10(6):601–606. doi: 10.1007/BF01535225. [DOI] [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]