Abstract
This study evaluated the difference in retinoid receptor expression between non-neoplastic lymph nodes and nodal lymphoma in dogs. Retinoid receptor expression was evaluated by immunohistochemistry in 32 canine lymph nodes. The lymph nodes had been previously diagnosed as non-neoplastic (6 normal and 7 hyperplastic lymph nodes) and B- and T-cell lymphoma (19 cases). Immunohistochemistry for retinoic acid receptors and retinoid-X receptors (and their subtypes α, β, and γ) was performed in all cases. In addition, immunohistochemistry for CD3 and CD79a was performed in all lymphoma cases. Non-neoplastic lymphocytes were negative for all retinoid receptors. Retinoic acid receptor-γ was detected in 100% of B-cell lymphoma and 78% of T-cell lymphoma, while retinoid X receptor-γ was positive in 78% of T-cell lymphoma cases. When normal lymph node architecture was still present, a contrast between retinoid-negative benign cells and retinoid-positive malignant cells was clear. Retinoid receptors were expressed in neoplastic, but not in benign lymphocytes, suggesting their value for both diagnosis and treatment of canine lymphoma.
Résumé
Détection des récepteurs aux rétinoïdes dans les ganglions lymphatiques canins non néoplasiques et dans les lymphomes. Cette étude a évalué la différence dans l’expression des récepteurs de l’acide rétinoïque entre les ganglions lymphatiques non néoplasiques et les lymphomes ganglionnaires chez les chiens. L’expression des récepteurs de l’acide rétinoïde a été évaluée par immunohistochimie dans 32 ganglions lymphatiques canins. Les ganglions lymphatiques avaient été antérieurement diagnostiqués comme étant non néoplasiques (6 ganglions lymphatiques normaux et 7 hyperplasiques) et les lymphomes B et T (19 cas). L’immunohistochimie pour les récepteurs de l’acide rétinoïque et les récepteurs X de rétinoïde (et leurs sous-types α, β et γ) a été réalisée dans tous les cas. De plus, l’immunohistochimie pour CD3 et CD79a a été réalisée dans tous les cas de lymphomes. Les lymphocytes non néoplasiques étaient négatifs pour tous les récepteurs de rétinoïde. Le récepteur-γ d’acide rétinoïque a été détecté dans 100 % des lymphomes B et dans 78 % des lymphomes T, tandis que le récepteur-γ X de rétinoïde était positif dans 78 % des cas de lymphome T. Lorsqu’une architecture normale des ganglions lymphatiques était présente, le contraste entre les cellules bénignes négatives pour la rétinoïde et les cellules malignes positives pour la rétinoïde était clair. Les récepteurs de rétinoïde étaient exprimés dans les lymphocytes néoplasiques, mais non dans les lymphocytes bénins, suggérant leur valeur pour le diagnostic et le traitement des lymphomes canins.
(Traduit par Isabelle Vallières)
Introduction
Lymphoma (LSA) is the most common hematopoietic malignancy in dogs and the disease most commonly treated by chemotherapy (1). Treatment for LSA has been well-documented and usually consists of multi-drug chemotherapy regimens. Most dogs with multicentric LSA respond to doxorubicin-based chemotherapy protocols. Remission rates of > 90% and median survival times ranging from 6 to 17 mo have been reported (2,3). Survival for dogs treated with doxorubicin-based protocols varies from 25% to 50% (1-year survival), and 13% to 27% (2-year survival) (3–5). The common induction of multi-drug resistance means that most dogs will still die or be euthanized after progression of disease. Long-term chemotherapy does not prolong survival in humans compared with short-term aggressive protocols. The same seems to be true for canine patients with 1 major difference being that the induction protocol is usually much more aggressive in humans than it is in dogs (2,3). Chemotherapy treatment intensity may be one of the reasons for the much higher cure rate in humans than dogs (3–7). Clearly, additional methods of therapy are needed to prolong remission times in dogs. One of these emerging approaches is the use of retinoids (8).
Retinoids are natural or synthetic derivatives of vitamin A shown to modulate cell growth, differentiation, and apoptosis in vivo and in vitro. Retinoids induce growth inhibition in tumor cells by induction of terminal differentiation, cell cycle arrest, and apoptosis (8,9). These effects occur through interaction with retinoid-specific nuclear receptors which function as ligand-dependent transcription factors; retinoid binding and activation of the receptor is followed by transcription of responsive genes. Retinoid receptors are divided into 2 classes: the retinoic acid receptors (RARs); and the retinoid X receptors (RXRs) and their 3 subtypes α, β, and γ (8,9). In humans, retinoids are used successfully for the treatment of promyelocytic leukemia, cutaneous lymphoma, lung and thyroid carcinomas, and glioblastomas (8–14).
Variation in retinoid receptor expression occurs in cancer and is associated with aggressiveness, response to treatment, and overall survival (8,10,11). In dogs, retinoids have been used in dermatology as differentiation agents in actinic keratosis, sebaceous adenitis, and benign pilomatrixomas (15–16). Few studies have demonstrated the effects of retinoids in canine cancer. In a study of 14 dogs with cutaneous lymphoma treated with retinoids as single agents, a clinical response rate of 42% was achieved (17). In addition, research in canine mast cell tumor (MCT) cell lines revealed that these cells expressed retinoid receptors and the level of expression of RARα mRNA correlated well with growth inhibition caused by all-trans retinoic acid (ATRA) (18,19). Furthermore, concentration-dependent cell death was achieved at micromolar levels of retinoid-treated MCT cell lines from grade II and III tumors (20). With the development of synthetic retinoids, specific receptor targeting has led to superior results in both cancer prevention and treatment in human medicine (8,11). The understanding of the pattern of retinoid receptor expression in canine lymph nodes may also lead to the use of specific receptor interacting agents to enhance responses in dogs with LSA. This study was conducted to evaluate the expression of retinoid receptors in lymph nodes of dogs. We hypothesized that the expression of retinoid receptors varies from non-neoplastic lymph nodes to lymphoma and between B- and T-cell lymphoma.
Materials and methods
Formalin-fixed paraffin embedded biopsy specimens from cases of lymphoma and lymphoid hyperplasia used in this study were consecutive cases retrieved from the archives of the University of Illinois, College of Veterinary Medicine. Lymph nodes from cases diagnosed with lymphoid hyperplasia had follow-up of 12 mo. Dr. David M. Vail from the University of Wisconsin-Madison College of Veterinary Medicine graciously provided the normal lymph nodes. Anti-human antibodies were used for retinoid receptor detection in our study. All antibodies are indicated by the manufacturer to react against human and mice retinoid receptors. Some of them, RARβ, RXRβ, and RXRγ are described to also react against canine retinoid receptors [Santa Cruz Biotechnology, Santa Cruz, California, USA, RARα: sc-551 and blocking peptide (BP) sc-551P; RARβ: sc-552 and BP sc-552P; RARγ: sc-550 and BP sc-550P; RXRα: sc-553 and BP sc-553P; RXRβ: sc-831 and BP sc-831; RXRγ: sc-555 and BP sc-555P]. These antibodies were chosen based on a study by Mori et al (21) and a previous study from our laboratory, in which we confirmed that rabbit antibodies against retinoid receptors cross react with canine tissue (22). In addition, we performed an amino acid homology search [BLAST program, National Center for Biotechnology Information (NCBI), Bethesda, Maryland]. This search revealed a high degree of homology between all human and canine retinoid receptor proteins (RARα 99%, GenBank Accession No. NP-001012663.1; RARβ 92%, XP-862280.1; RARγ 94%, XP-849260.1; RXRα 89%, XP-548399.2; RXRβ 96%, XP-862727.1; and RXRγ 97%, XP-536146.2).
Deparaffinization and antigen retrieval were performed at room temperature as follows. Slides were placed in 3 changes of xylene for at least 3 min for each change. This was followed by 2 changes of 100% ethanol, 2 changes of 95% ethanol, and 1 change of 70% ethanol for at least 2 min per change. Slides were placed in running water for at least 1 min and then in 3% hydrogen peroxide in methanol for 15 min prior to rinsing in Optimax wash buffer (500M Optim; Biogenex, San Ramon, California, USA) for 5 min. Slides were placed in a container of citrated buffer, pH 6, to be microwaved on high power until the buffer boiled. Power was reduced to a level that produced intermittent boiling for 10 min. Slides were cooled for 20 min and rinsed again in Optimax buffer.
Primary antibodies used in the study were: RARα C-20, RARβ C-19, and RARγ C-19 (rabbit polyclonal biotinylated); RXRα D-20, RXRβ C-20, and RXRγ Y-20 (rabbit polyclonal biotinylated) (Santa Cruz Biotechnology). For the negative controls, specific blocking peptides (100 μg/0.5mL) were added to each antibody (25 μL of blocking peptide to 5 μL of antibody) and incubated for 2 h before dilution. The Supersensitive kit (streptavidin-biotin system) was used for all antibodies (Biogenex, San Ramon, California, USA). Immunoreaction was visualized with 3,39-diaminobenzidine substrate. Final dilutions were RARα (1:100), RARβ (1:100), RARγ (1:75), RXRα (1:100), RXRβ (1:100), and RXRγ (1:100). Finally, the slides were counterstained with hematoxylin for 1 min, rinsed in water, dehydrated, and mounted. Hematoxylin-eosin stained slides from a total of 32 cases previously diagnosed as normal lymph node (6 cases), lymphoid hyperplasia (7 cases), B-cell LSA (10 cases), and T-cell LSA (9 cases) were reviewed by 1 pathologist (VEOV). For all the lymphoma cases, slides immunophenotyped for CD3 and CD 79a expression by IHC were also re-evaluated (Dako-Cytomation, Carpinteria, California, USA). Formalin-fixed paraffin embedded tissues were used to create 15 sections of 3-μm thickness from each case. Slides were prepared from all blocks for immunostaining using all the markers described. Immunohistochemistry analysis was performed on all lymph nodes at the same time and by using the same biotin-streptavidin-immunoperoxidase amplified detection system as recently described (22). For retinoid receptors, mouse pup eyes were used as positive controls, and blocking peptides were used as negative controls. This decision was based on our previous study that compared the retinoid receptor retinal staining patterns of mice and dogs. That study showed that staining of the same retinal structures was similar in mice and dogs. We decided to use mice eyes due to their greater availability (Figure 1) (22). The immunostaining for retinoid receptors had to be strong in the nucleus of the cell in order for the sample to be considered positive. One pathologist (VEOV) evaluated all the IHC slides. Patterns of staining were recorded for further analysis.
Figure 1.
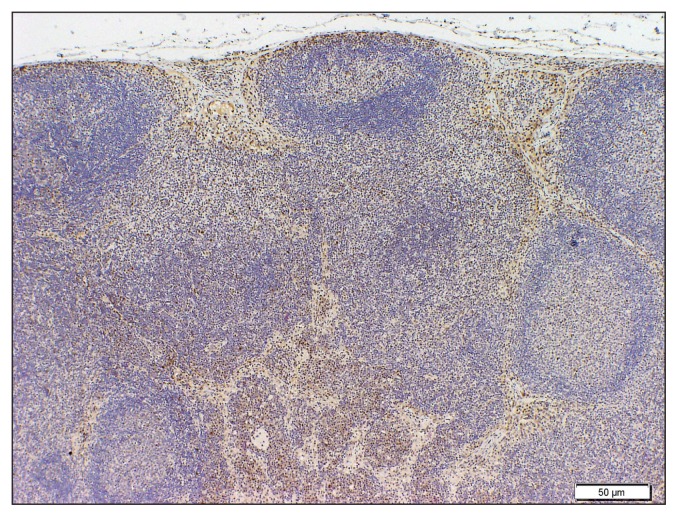
RXRβ staining in a case of lymphoid hyperplasia showing numerous RXRβ positive macrophages concentrated mostly at the nodal sinusoids.
Results
Retinoid receptor expression was not detected in any of the normal lymph node specimens. In cases of lymphoid hyperplasia, expression of RAR and RXR (α, β, and γ) was only present in sinus macrophages (Figure 2). Sinusoidal macrophages are the primary cell on nodal sinus and were identified by cell morphology, nuclear size and morphology, and by the common presence of hemosiderin granules in the cytoplasm. All LSA cases expressed at least 2 of the receptor subtypes. In such cases, > 95% of the cells were positive. The most commonly expressed receptor was RARγ, positive in 17 cases (89% overall; 100% in the cases of B-cell LSA), followed by RXRγ, positive in 9 cases (47% overall; 78% in T-cell LSA specimens) (Figure 3). The other subtypes were positive in small percentages of cases (Table 1). In the LSA cases evaluated, where neoplastic cells had not effaced the lymph node, normal structures such as mantle cell cuff and germinal centers, when benign, were uniformly negative for retinoid receptor expression (Figures 3 and 4).
Figure 2.
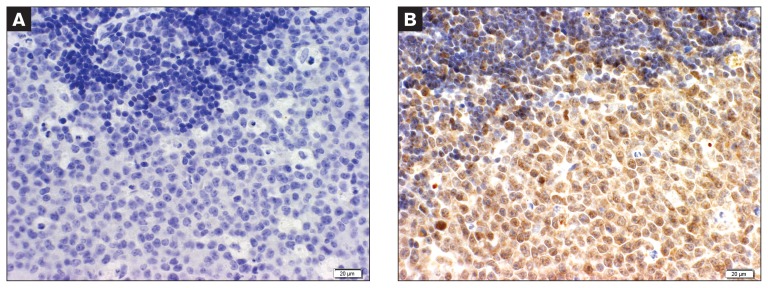
B-cell Marginal zone lymphoma case showing a clear distinction between neoplastic cells (bottom) and benign mantle cuff cells (darker cells on top). Blocking peptide was added (A); same case showing strong staining for RARγ that spares the benign mantle cell cuff (B).
Figure 3.
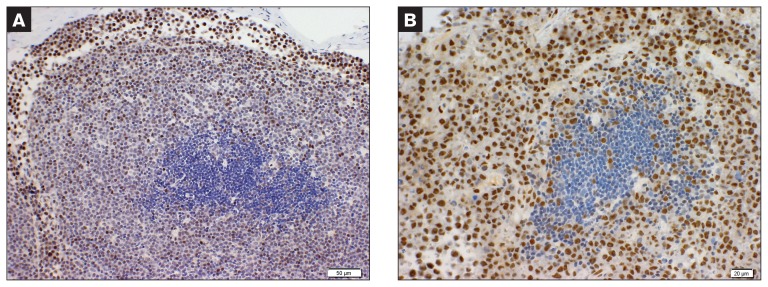
Two cases of T-cell lymphoma positive for RARα. The strong staining of neoplastic cells contrasts with the negative benign germinal center.
Table 1.
Distribution of retinoid receptor expression among 19 cases of canine nodal lymphoma
| Retinoid Receptor Subtype | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| LSA phenotype | RARα | RARβ | RARγ | RXRα | RXRβ | RXRγ |
| B-cell (10 cases) | 4 (40%) | 1 (10%) | 10 (100%) | 0 (0%) | 1 (10%) | 7 (70%) |
| T-cell (9 cases) | 4 (44%) | 1 (11%) | 7 (78%) | 3 (33%) | 2 (22%) | 2 (22%) |
LSA — lymphoma; RAR — retinoic acid receptor; RXR — retinoid X receptor.
Discussion
Important findings in our study were the absence of retinoid receptor binding in lymphocytes and plasma cells of benign canine nodal tissue and a positive pattern present only in macrophages of hyperplastic lymph nodes. In contrast, we detected strong retinoid receptor expression in canine lymphomas. In addition, the most common receptor sub-type expressed varied with phenotype. This dramatic difference between retinoid receptor expression in non-neoplastic (normal lymph nodes and lymphoid hyperplasia) and lymphoma cases indicates that retinoid receptor expression is associated with the malignant phenotype of nodal lymphoma in dogs. A striking finding of our study was the dramatic retinoid receptor staining contrast of benign versus neoplastic cells. In cases where normal lymph node architecture was still present, the interface between the strong positive neoplastic cells and the negative benign cells was very clear after IHC. To our knowledge this fact has not been reported in the literature. The strong retinoid receptor-staining pattern displayed by macrophages within lymph nodes demonstrating lymphoid hyperplasia was an expected finding. It has been previously shown in humans that dendritic cells and macrophages express retinoid receptors during inflammation and that this expression is associated with cytokine production (23–25).
The retinoid receptor expression of lymphocytes has been evaluated in neonatal mice and young children. In mice, lymphocyte retinoid receptor expression varies throughout the embryonic development of the thymus (25). Both RARα and RARγ continue to be present in lymphocytes after birth and expression of RARγ is particularly strong in both CD4+ and CD8+ mature cells (25,26). Based on studies in mice, retinoid receptor activation is important during B- and T-cell activation, where it is thought to modulate homing of cells to specific lymphoid organs and to alter cytokine production (26–29). In children, retinoid receptor expression is highest between the ages of 1 and 3 years, decreasing after that. Terminally differentiated lymphoid cells do not express or have low-expression of retinoid receptors (30). Despite the fact that retinoid receptor expression was evaluated in a small number of cases of non-neoplastic lymph nodes in this study, this lack of expression seems to be similar to that in lymph nodes of older children, when the immune system is fully developed. An alternative possibility to this negative finding is that retinoid receptor expression was present but at levels too low for detection. In contrast, the common expression of some subtypes (RARγ and RXRγ) in LSA cases suggests that retinoid receptor expression plays a role in this disease, which merits further investigation. Due to unavailability of information regarding any treatments, response rates, or survival times of the cases from which lymph node material was gathered for the study, we were not able to assess the value of retinoid receptor expression as a prognostic factor. The importance of specific receptor subtype has been underscored by various studies in humans.
The expression, or sometimes loss of expression, of specific retinoid receptor subtypes is associated with biologic behavior and overall prognosis in a variety of carcinomas and also in melanomas in humans (31–35) Retinoic acid receptor and RXR expression in human thyroid carcinomas can predict their response to retinoids and related drugs (35–37). Also in humans, the expression of RARβ correlates with less aggressive behavior in a variety of malignancies (31,35). In contrast, loss of RARβ has been associated with more aggressive behavior and this receptor is now considered to serve a tumor suppressive function, although precise mechanisms for this effect have yet to be elucidated (38). Loss of RARα, RARγ, and RXRβ expression also occurs in lung and prostatic cancer in humans (38,39). In addition, recent studies in breast cancer cells showed that natural and synthetic retinoids can induce expression of RARβ and down-regulate levels of Bcl-2 and survivin, which in turn leads to an increase in cellular apoptosis (40).
The pattern of retinoid receptors in adult humans with lymphoma and leukemias, other than promyelocytic leukemia, is unknown. Only 2 studies correlated the presence of retinoid receptors with response to retinoid/rexinoid therapy in lymphomas and leukemia. In 1 of these studies, response to the RXR selective agent, bexarotene, was associated with clinical remission. When relapse occurred despite treatment, evaluation of the resistant cells revealed the RXR-α had been down-regulated. As in any immunohistochemistry study, ours has limitations such as the inability to detect functionality of the detected proteins. Despite that and a small number of cases, our findings demonstrate that retinoid receptor expression varies markedly between non-neoplastic and neoplastic lymphoid tissue. In addition, the high amino acid homology shared by dogs and humans increases the strength of our results. Correlation of retinoid receptor and tumor grade, to extend our findings into the realm of low-grade tumors, might be useful in determining the impact of IHC as a diagnostic modality to differentiate reactive from neoplastic lymphocyte proliferation. Since the presence or absence of specific retinoid receptors has been associated with response to treatment, a study correlating specific retinoid receptor isotype to response to treatment, remission time, and survival is warranted. Furthermore, our results suggest that retinoids such as isotretinoin and etretinate (RAR binding drugs) and bexarotene (RXR binding drug), may prove to be of therapeutic benefit in the treatment of nodal LSA in dogs.
Acknowledgments
We thank Dr. David M. Vail for providing the slides of cases of normal lymph nodes. The authors also thank Mrs. Jane Chladny for preparing the slides for the IHC procedures. CVJ
Footnotes
This project was conducted at The College of Veterinary Medicine, University of Illinois at Urbana-Champaign, 1008 West Hazelwood Drive, Urbana, Illinois, USA.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Work was supported by funding provided by Dr. Victor E.O. Valli.
References
- 1.Dorn CR, Taylor DON, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318. [PubMed] [Google Scholar]
- 2.Fan TM. Lymphoma updates. Vet Clin of North Am Small Animal Pract. 2003;33:455–471. doi: 10.1016/s0195-5616(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 3.Vail DM, Young KM. Withrow and MacEwen’s Small Animal Clinical Oncology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2007. pp. 699–733. [Google Scholar]
- 4.Rebhun RB, Lana SE, Ehrhart EJ, Charles JB, Thamm DH. Comparative analysis of survivin expression in untreated and relapsed canine lymphoma. J Vet Intern Med. 2008;22:989–995. doi: 10.1111/j.1939-1676.2008.0143.x. [DOI] [PubMed] [Google Scholar]
- 5.Hosoya K, Kisseberth WC, Lord LK, Alvarez FJ, et al. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1355–1363. doi: 10.1892/06-284.1. [DOI] [PubMed] [Google Scholar]
- 6.Rassnick KM, McEntee WC, Erb HN, et al. Comparison of 3 protocols for treatment after induction of remission in dogs with lymphoma. J Vet Intern Med. 2007;21:1364–1373. doi: 10.1892/07-057.1. [DOI] [PubMed] [Google Scholar]
- 7.Simon D, Nolte I, Eberle N, Abbrederis N, Killich M, Hirchberger J. Treatment of dogs with lymphoma using a 12-week, maintenance-free combination chemotherapy protocol. J Vet Intern Med. 2006;20:948–954. doi: 10.1892/0891-6640(2006)20[948:todwlu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronmeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 9.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 10.Fontana JA, Rishi AK. Classic and novel retinoids: Their targets in cancer therapy. Leukemia. 2002;16:463–472. doi: 10.1038/sj.leu.2402414. [DOI] [PubMed] [Google Scholar]
- 11.Sun SY, Yue P, Mao L, et al. Identification of receptor-selective retinoids that are potent inhibitors of the growth of human head and neck squamous cell carcinoma cells. Clin Can Res. 2000;6:1563–1573. [PubMed] [Google Scholar]
- 12.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2009;11:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 13.Schmutzler C, Kohrle J. Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid. 2000;10:393–406. doi: 10.1089/thy.2000.10.393. [DOI] [PubMed] [Google Scholar]
- 14.Coelho SM, Vaisman M, Carvalho DP. Tumor re-differentiation effect of retinoic acid: A novel therapeutic approach for advanced thyroid cancer. Curr Pharm Des. 2005;11:2525–2531. doi: 10.2174/1381612054367490. [DOI] [PubMed] [Google Scholar]
- 15.White SD, Rosychuck RA, Scott KV, Hargis AM, Jonas L, Trettien A. Sebaceous adenitis in dogs and results of treatment with isotretinoin and etretinate: 30 cases (1990–1994) J Am Vet Med Assoc. 1995;207:197–200. [PubMed] [Google Scholar]
- 16.Toma S, Noli C. Isotretinoin in the treatment of multiple benign pilomatrixomas in a mixed-breed dog. Vet Dermatol. 2005;16:346–350. doi: 10.1111/j.1365-3164.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 17.White SD, Rosychuck RAW, Scott KV, et al. Use of isotretinoin and etretinate for the treatment of benign cutaneous neoplasia and cutaneous lymphoma in dogs. J Am Vet Med Assoc. 1993;202:387–391. [PubMed] [Google Scholar]
- 18.Ohashi M, Miyajima N, Nakagawa T, et al. Retinoids induce growth inhibition and apoptosis in mast cell tumor cell lines. J Vet Med Sci. 2006;68:797–802. doi: 10.1292/jvms.68.797. [DOI] [PubMed] [Google Scholar]
- 19.Miyajima N, Watanabe M, Ohashi E, et al. Relationship between retinoic acid receptor α gene expression and growth-inhibitory effect of all-trans retinoic acid on canine tumor cells. J Vet Intern Med. 2006;20:348–354. doi: 10.1892/0891-6640(2006)20[348:rbrarg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Pinello KC, Nagamine M, Silva TC, et al. In vitro chemosensitivity of canine mast cell tumors grades II and III to all-trans-retinoic acid (ATRA) Vet Res Comm. 2009;33:581–588. doi: 10.1007/s11259-008-9202-3. [DOI] [PubMed] [Google Scholar]
- 21.Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–1318. [PubMed] [Google Scholar]
- 22.de Mello Souza CH, Valli VEO, Selting KA, Kiupel M, Kitchell BE. Immunohistochemical detection of retinoid receptors in tumors from 30 dogs diagnosed with cutaneous lymphoma. J Vet Intern Med. 2010;24:1112–1117. doi: 10.1111/j.1939-1676.2010.0571.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhelyaznik N, Mey J. Regulation of retinoic acid receptors α, β and retinoid X receptor α after sciatic nerve injury. Neuroscience. 2006;141:1761–1744. doi: 10.1016/j.neuroscience.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Dzhagalov I, Chambon P, You-Wen He. Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma. J Immunol. 2007;178:2113–2121. doi: 10.4049/jimmunol.178.4.2113. [DOI] [PubMed] [Google Scholar]
- 25.Santhakumar M, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol. 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson HD, Collins G, Pyle R, Key M, Taub DD. The retinoic acid receptor-α mediates human T-cell activation and Th2 cytokine and chemokine production. BMC Immunol. 2008;9:16. doi: 10.1186/1471-2172-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordy C, Dzhagalov I, He Y-W. Regulation of CD8 + T cell functions by RARγ. Semin Immunol. 2009;21:2–7. doi: 10.1016/j.smim.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J Immunol. 2008;180:138–145. doi: 10.4049/jimmunol.180.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang SS, Kim YU, Lee W, Lee GR. Differential expression of nuclear receptors in T helper cells. J Microbiol Biotechnol. 2009;19:208–214. doi: 10.4014/jmb.0811.618. [DOI] [PubMed] [Google Scholar]
- 30.Wei D, Yang Y, Wang W. The expression of retinoic acid receptors in lymph nodes of young children and the effect of all-trans-retinoic acid on the B cells from lymph nodes. J Clin Immunol. 2007;27:88–94. doi: 10.1007/s10875-006-9059-6. [DOI] [PubMed] [Google Scholar]
- 31.Khuri FR, Latan R, Kemp BL, et al. Retinoic acid receptor-beta as a prognostic indicator in stage I non-small-cell lung cancer. J Clin Oncol. 2000;18:2798–2804. doi: 10.1200/JCO.2000.18.15.2798. [DOI] [PubMed] [Google Scholar]
- 32.Hatoum A, El-Sabban ME, Khoury J, Yuspa SH, Darwiche N. Overexpression of retinoic acid receptors alpha and gamma into neoplastic epidermal cells causes retinoic acid-induced growth arrest and apoptosis. Carcinogenesis. 2001;22:1955–1963. doi: 10.1093/carcin/22.12.1955. [DOI] [PubMed] [Google Scholar]
- 33.Gorgun G, Foss F. Immunomodulatory effects of RXR retinoids: Modulation of high-affinity IL-2R expression enhances susceptibility to denileukin diftox. Blood. 2002;100:1399–1403. doi: 10.1182/blood-2002-01-0300. [DOI] [PubMed] [Google Scholar]
- 34.Chakravarti N, Mathur M, Bahadur S, Shukla NK, Ralhan R. Retinoic acid receptor-alpha as a prognostic indicator in oral squamous cell carcinoma. Intl J Can. 2003;103:544–549. doi: 10.1002/ijc.10819. [DOI] [PubMed] [Google Scholar]
- 35.Cheung B, Hocker JE, Smith SA, Norris MD, Haber M, Marshall GM. Favorable prognostic significance of high-level retinoic acid receptor β expression in neuroblastoma mediated by effects on cell cycle regulation. Oncogene. 1998;17:751–759. doi: 10.1038/sj.onc.1201982. [DOI] [PubMed] [Google Scholar]
- 36.Haugen BR, Larson LL, Pugazenthi U, et al. Retinoic acid and retinoid-X receptors are differentially expressed in thyroid cancer cells and predict response to treatment with retinoids. J Clin Endocrinol Metabol. 2004;89:272–280. doi: 10.1210/jc.2003-030770. [DOI] [PubMed] [Google Scholar]
- 37.Klopper JP, Hays WR, Sharma V, Baumbusch MA, Hershman JM, Haugen BR. Retinoid X receptor-γ and peroxisome proliferators-activated receptor-γ expression predicts thyroid carcinoma cell response to retinoid and thiazolinedione treatment. Mol Cancer Ther. 2004;3:1011–1020. [PubMed] [Google Scholar]
- 38.Xu XC. Tumor suppressive activity of retinoic acid receptor-β in cancer. Can Lett. 2007;253:14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brabender J, Metzger R, Salonga D, et al. Comprehensive expression analysis of retinoic acid receptors and retinoid X receptors in non-small cell lung cancer: Implications for tumor development and prognosis. Carcinogenesis. 2005;26:525–530. doi: 10.1093/carcin/bgi006. [DOI] [PubMed] [Google Scholar]
- 40.Pratt MAC, Niu M, White D. Differential regulation of protein expression, growth, and apoptosis by natural and synthetic retinoids. J Cell Biochem. 2003;90:692–708. doi: 10.1002/jcb.10682. [DOI] [PubMed] [Google Scholar]
- 41.Cheng AL, Chuang SE, Su IJ. Factors associated with the therapeutic efficacy of retinoic acids on malignant lymphoma. Formos Med Assoc. 1997;96:525–534. [PubMed] [Google Scholar]
- 42.Lin JH, Kim EJ, Bansal A, et al. Clinical and in vitro resistance to bexarotene in adult T-cell leukemia: Loss of RXR-α receptor. Blood. 2008;112:2484–2488. doi: 10.1182/blood-2008-03-141424. [DOI] [PMC free article] [PubMed] [Google Scholar]