Abstract
A 1-year-old, intact female mixed-breed dog was presented to St. George’s University Small Animal Clinic in Grenada for a third eyelid mass. The dog was diagnosed with a rare ocular transmissible venereal tumor (TVT) and concurrent anaplasmosis, ehrlichiosis and dirofilariasis. Treatment with vincristine sulfate resulted in complete resolution of the TVT.
Résumé
Cas de tumeur vénérienne canine oculaire transmissible. Une chienne de race croisée intacte âgée de 1 an a été présentée à la clinique pour petits animaux de l’Université St. George de la Grenade pour une masse de la troisième paupière. La chienne a été diagnostiquée avec une rare tumeur vénérienne oculaire transmissible (TVT) et l’anaplasmose, l’ehrlichiose et la dirofilariose concomitantes. Le traitement au sulfate de vincristine a produit une résolution complète de la TVT.
(Traduit par Isabelle Vallières)
Case description
A 1-year-old, 15-kg, intact, mixed breed, female dog was presented to St. George’s University Small Animal Clinic (SGU SAC) for evaluation of a suspected prolapse of her right third eyelid and unilateral ocular discharge. The dog had been adopted by a veterinary student 3 days earlier after it was found wandering the neighborhood. On presentation the right third eyelid was prominent, light pink in color with diffuse punctate, raised lesions. There was moderate, yellowish-brown crusting around the eye. A neuro-ophthalmic examination showed the eyes to be normal bilaterally. Tear production, intraocular pressures, and a fundoscopic examination were normal in both eyes. There was no corneal uptake of fluorescein stain bilaterally. Clavamox (Pfizer Animal Health, New York, New York, USA) 16 mg/kg body weight (BW) PO, q12h for 14 d, Rimadyl (Caplets and Chewables; Pfizer Animal Health) 2.5 mg/kg BW PO, q12h for 5 d, and chloramphenicol ophthalmic ointment (Vetachloracin Ophthalmic Ointment 1%; Dechra Veterinary Products, Overland Park, Kansas, USA) OD ¼ inch strip applied topically q12h for 7 d were prescribed. Despite these therapies chemosis of the right third eyelid worsened and the ocular discharge increased.
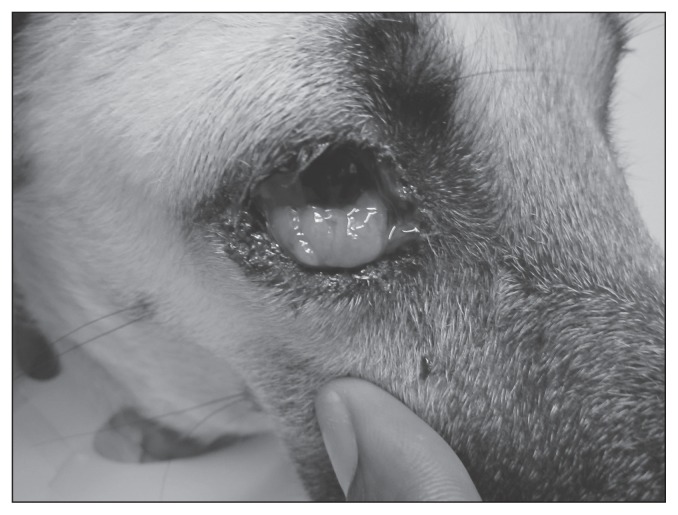
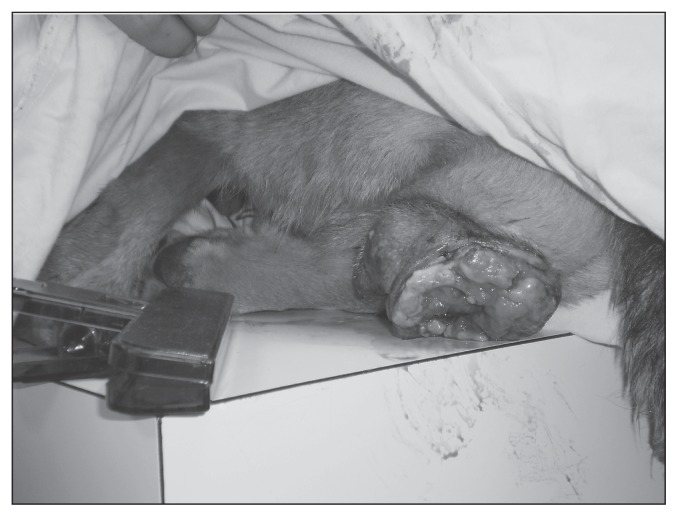
Three days later the dog was re-examined at the SGU SAC. The right third eyelid was noticeably more swollen with punctate hemorrhages, scalloped edges, and several lobulated masses evident. Blood-tinged epiphora with moderate brown-black crusting around the eye was seen (Figure 1). The third eyelid mass was friable and bled when manipulated with a cotton tip applicator. The left eye was normal.
Figure 1.
Ocular transmissible venereal tumor (TVT) of the right third eyelid and bulbar conjunctival tissue in a dog.
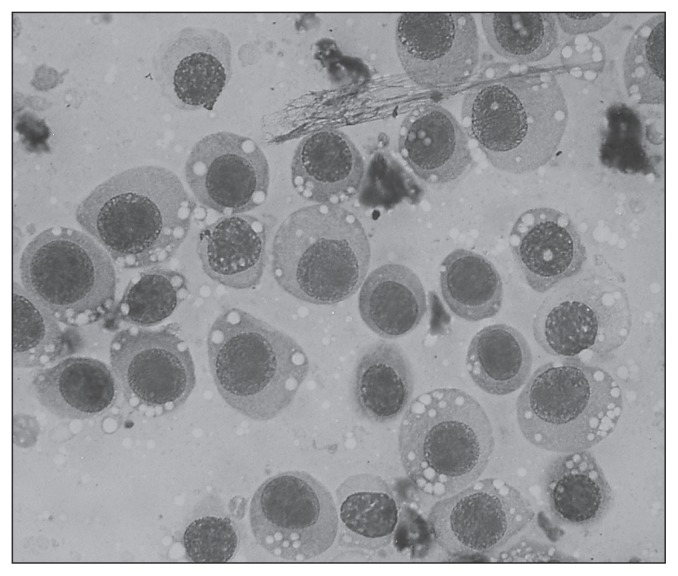
A complete blood (cell) count (CBC), serum biochemistry profile, and analysis of urine collected by cystocentesis were performed along with a Snap 4Dx test (IDEXX Laboratories, Westbrook, Maine, USA) to screen for vectorborne disease agents Dirofilaria immitis (heartworm disease), Ehrlichia canis (ehrlichiosis), Anaplasma phagocytophilum (anaplasmosis), and Borrelia burgdorferi (Lyme disease). In addition, impression smear cytology of the third eyelid mass was done. The CBC revealed a mild non-regenerative anemia [hematocrit (HCT) 34%; reference interval (RI): 37% to 55%, reticulocyte count 1.5%)] and marked thrombocytopenia (36 × 109/L; RI: 175 to 500 × 109/L). The serum biochemistry profile revealed mild hyperphosphatemia (2.45 mmol/L; RI: 0.81 to 2.2 mmol/L), and moderate hyperproteinemia (93 g/L; RI: 52 to 82 g/L) characterized by a hyperglobulinemia (63 g/L; RI: 25 to 45 g/L). The urine specific gravity was 1.045 and the urine sediment examination was unremarkable. The dog was positive for D. immitis, E. canis, and A. phagocytophilum and negative for B. burgdorferi on the IDEXX 4Dx Snap test. Impression smear cytology of the right third eyelid mass using Giemsa stain revealed a neoplastic round cell population with some inflammatory cells (75% neutrophils and 25% macrophages). Features of malignancy in the round cell population included mild to moderate anisocytosis, anisokaryosis, numerous mitotic figures, and abundant faint basophilic cytoplasm. The cells had an increased nuclear to cytoplasmic ratio and the cytoplasm contained 3 to 4 distinct vacuoles characteristic for transmissible venereal tumor (TVT) (Figure 2). Given the findings consistent with TVT a vaginal examination was performed. No vaginal mass was visualized or palpated.
Figure 2.
Impression smear cytology of the third eyelid transmissible venereal tumor (TVT) revealing characteristic features of a TVT showing a round cell population with mild to moderate anisocytosis, anisokaryosis, a few mitotic figures and abundant faint basophilic cytoplasm with characteristic cytoplasmic vacuoles. Image courtesy of Dr. Kumthekar.
Treatment with doxycycline (Westward Pharmaceutical Corp, Edmonton, New Jersey, USA), 10 mg/kg BW, PO q12h for 30 d, for ehrlichiosis and anaplasmosis was initiated. In addition, Iverhart Max Chewable tablet containing ivermectin 136 μg, PO, q30d, pyrantel pamoate 114 mg, and praziquantel 114 mg (Virbac Animal Health, Fort Worth, Texas, USA) were prescribed for heartworm and intestinal parasites. For the TVT, treatment with vincristine sulfate (Hospira, Lake Forest, Illinois, USA), 0.025 mg/kg BW, IV, q7d for a total of 6 treatments was initiated. Due to financial limitations of the owner the dog was monitored with a CBC every other week instead of every week.
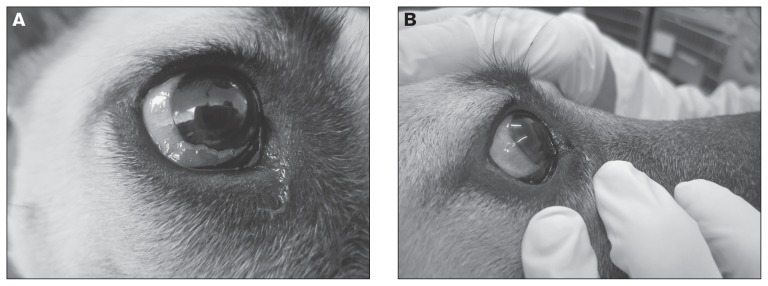
Photographs of the right nictitating membrane lesion were taken at each follow-up visit to monitor progress (Figures 3a, b). After the first vincristine treatment only minimal improvement was noted. A CBC performed prior to the second vincristine treatment revealed no improvement in the anemia (HCT 34%), a persistent but improved thrombocytopenia (162 × 109/L; RI: 175 to 500 × 109/L), and a mild eosinophilia (1.7 × 109/L; RI: 0.5 to 1.5 × 109/L).
Figure 3.
A — Partial resolution of the third eyelid transmissible venereal tumor (TVT) in a dog after 3 weekly vincristine sulphate treatments (left), and B — Complete resolution of the third eyelid TVT in a dog after 6 weekly treatments with vincristine sulfate (right).
By the third week the right third eyelid mass had decreased dramatically in size. By the fourth week only a small remnant of the mass was visible (Figure 3a) and by week 6, the mass was no longer evident without exteriorization of the third eyelid. However, marked thrombocytopenia (25 × 109/L) persisted, along with a now regenerative anemia (HCT 35.9%). A manual platelet count with only 1 platelet seen per high power field (hpf ) confirmed the thrombocytopenia. Because the thrombocytopenia was attributed to bone marrow suppression from the vincristine the sixth, and final vincristine treatment, was postponed by a week. Prior to the sixth treatment a recheck CBC revealed normalization of the platelet count (200 × 109/L; RI: 175 to 500 × 109/L) and resolution of the anemia (HCT 37.5%). At this time the dog was sedated with Propofol (PropoFlo; Abbot Laboratories, Abbott Park, Illinois, USA), 0.2 mg/kg BW, IV and her right third eyelid was everted to permit examination. Only a very small remnant of the mass was still visible. Complete resolution was evident 1 week later after the final treatment (Figure 3b). With resolution of the ocular TVT, heartworm adulticide treatment was initiated and was well-tolerated with no complications. Eighteen months after initial presentation, there was no visible recurrence of the dog’s TVT.
Discussion
Transmissible venereal tumor (TVT), also referred to as Sticker’s tumor, venereal granuloma, transmissible venereal sarcoma, infective venereal tumor, and transplantable lymphosarcoma is a tumor that primarily affects the genitalia of sexually mature dogs. It is a common tumor in tropical and subtropical regions where there is a high population of stray, malnourished dogs (1–4). A TVT is unique in its pathogenesis compared with other neoplasms since it does not arise spontaneously but is transmitted from one animal to another (2). The transmissible nature is suggestive of an infectious etiology; however, no infectious particles have ever been detected within the tumor cells (2–3). These tumor cells are not the patient’s own cells transformed into cancer cells. The TVT is a tumor that grafts itself from one dog’s body onto another dog’s body (5).
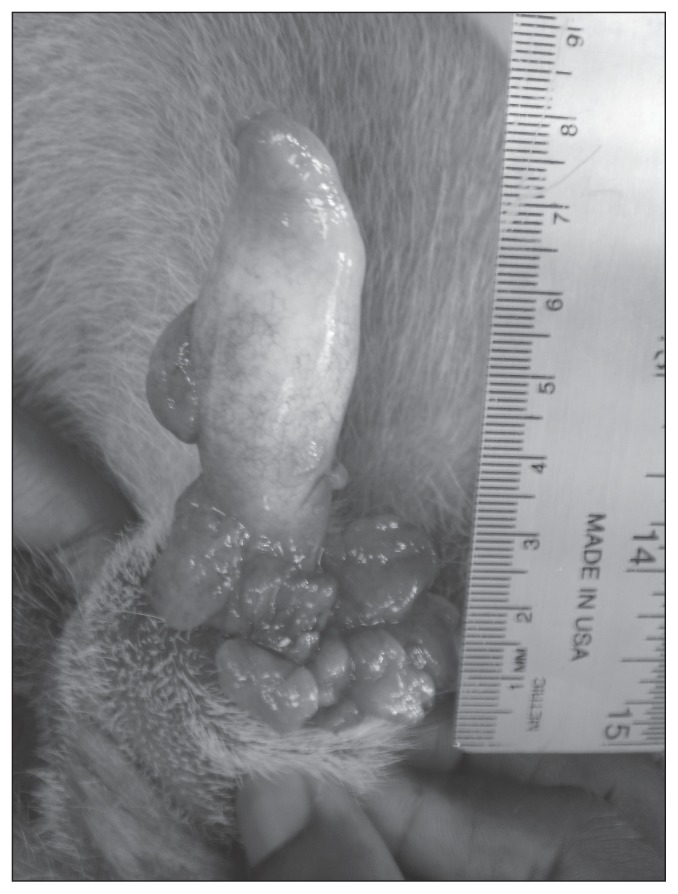
In male dogs the tumor typically affects the caudal aspect of the penis (Figure 4) and occasionally the prepuce. Phimosis or paraphimosis may occur as a complication (2). In female dogs lesions are most common at the posterior part of the vagina, particularly at the vestibulovaginal junction (2). Due to this deeper location a visible genital growth may not be evident; however, in some cases the mass may be large enough to protrude from the vulva (Figure 5) (2,4). A digital vaginal examination and possibly vaginoscopy should be performed in a female dog with a confirmed extragenital TVT to look for a primary vaginal tumor. Unlike other genital tumors in dogs that tend to occur in older dogs (> 10 y of age), TVT tends to occur in young dogs, typically 2- to 5-years old (2).
Figure 4.
Multilobulated, ulcerated transmissible venereal tumor (TVT) on the base of the penis in a dog. Courtesy of Dr. Emma Hage.
Figure 5.
Large cauliflower-like, ulcerated, hemorrhagic vaginal transmissible venereal tumor (TVT) in a dog. Courtesy of Dr. Emma Hage.
The TVT is typically transmitted during coitus (2–4). Transmission can also occur through dogs’ normal social behavior when they lick, sniff, scratch, or bite at their own genitalia (autoimplantation) or if they lick the genitalia of an affected dog (heteroimplantation) (2,4,5). Early on, the genital tumors appear as small hyperemic papules but with time progress to become nodular, papillary multilobulated, friable masses with cauliflower-like or pedunculated proliferations that can measure up to 15 cm in diameter (2). Ulceration of the surface of the tumor with secondary inflammation is common (2,5). Transmissible venereal tumors also bleed easily and often have a malodorous serosanguinous discharge especially if they become secondarily infected (2,4,5–7). The bloody discharge can be confused with signs of estrus or cystitis in females or prostatitis in male dogs (2,4).
Transmissible venereal tumor can also occur in extragenital sites, even in the absence of pre-existing genital lesions as in the case described (2,4,8,9–11). This is typically the result of primary auto- or hetero-implantation. Much less commonly this can result from metastatic spread from a primary genital tumor via hematogenous or lymphatic routes (2,5,7,12). Metastases occur in less than 5% of cases and most commonly affect regional lymph nodes. However, metastasis to other internal organs such as the skin and subcutaneous tissue, pharynx, tonsils, liver, spleen, kidneys, brain, eyes, and lymph nodes has been reported (2,4,6,8). Diagnosis of a TVT at an extragential site such as the eye, when there is no concurrent genital tumor, as in the case described, can be challenging. The clinical signs seen with TVT in the eye can range from chemosis to episcleritis, severe uveitis, corneal edema, and glaucoma (13,14). There are only a few reported cases of ocular TVT in the literature. Most cases have involved other structures of the eye (sclera, cornea) and have been clearly the result of metastasis from genital lesions (5,6,12,13). In this case, the third eyelid TVT tumor was suspected to be a primary tumor and not to have resulted from metastasis since no external lesions were visible or evident on digital vaginal examination. Given the conjunctival location it is speculated that the dog developed this lesion after being licked on the face by an affected dog.
Tumors of the third eyelid are rare, seen in older dogs, and are typically malignant (15). They usually arise from the conjunctival or glandular tissue. Types of tumors include adenocarcinoma (most common), hemangiosarcoma, mastocytoma, squamous cell carcinoma, hemangioma, fibrosarcoma, and malignant melanoma (15). Transmissible venereal tumors should also be considered, especially in a young to middle-aged sexually intact dog which resides in or has visited a region where TVT is endemic. Importantly, with the exception of TVT, the treatment for other forms of neoplasia of the third eyelid is amputation of the third eyelid and associated gland (15).
A presumptive diagnosis of canine TVT is often made based on the combination of epidemiological factors (young- to middle-aged sexually active dog living in an endemic region) and the location of the tumor (genitalia involvement). Definitive diagnosis of TVT, with differentiation from other round cell tumors, can usually be made from impression smear cytology or fine-needle aspirate cytology (1,2,16). Differential diagnoses include other round-cell tumors such as histiocytomas, lymphoma, poorly differentiated mast cell tumors and carcinomas, and amelanotic melanomas (2,7,12,13). Impression smear cytology is preferred over biopsy for establishing the diagnosis of TVT because it is minimally invasive, inexpensive, and highly sensitive (1,2,16). There is also less cellular distortion with cytology than with formalin-fixed biopsy samples. Histopathology, immunohistochemistry, and electron-microscopy can also be used to establish a diagnosis of TVT (2) and differentiate TVT from other round-cell tumors (3,13) if required. For example, canine TVT cells possess 57 to 64 chromosomes (average of 59), in contrast with the normal 78 in canine somatic cells (2,17). This finding on electron microscopy can be used to help support the diagnosis of TVT when the diagnosis is questioned.
Vincristine sulfate, as a single agent given IV once weekly is the most common treatment protocol for TVT (2,16–19). Four to 8 doses are usually necessary to effect a cure (17). Complete regression of the right third eyelid TVT in this dog was seen after 6 treatments with vincristine sulfate, with no recurrence or metastasis. The most common side effects with vincristine are gastrointestinal upset and myelosuppression evident by leukopenia (8,17). In the case described, the dog was already severely thrombocytopenic and slightly anemic when vincristine therapy was initiated. The initial thrombocytopenia was attributed to ehrlichiosis and anaplasmosis. Treatment for these diseases with doxycycline resulted in marked improvement in the thrombocyte count; however, there was no improvement in the anemia for several weeks. This was likely because the pathophysiology of the anemia was complex and at least in part explained by chronic inflammatory disease. After the fifth treatment with vincristine, the dog became markedly thrombocytopenic again. This was attributed to myelosuppression from vincristine because the thrombocytopenia resolved with a short delay of further vincristine administration. Transmissible venereal tumors have been reported to be immunogenic tumors; therefore, occurrence, metastasis, and recurrence can depend on the strength of the dog’s immune system (17). It is possible that being a stray and being malnourished, in addition to having 3 concurrent infectious diseases may have predisposed this dog to the development of TVT. The lack of recurrence and metastasis may have also been attributable to improved nutrition and successful ongoing treatment of the concurrent infections.
Medical treatment options for TVT include other chemotherapy drugs such as monotherapy with doxorubicin or vinblastine or combination chemotherapy protocols (2,17). Surgical excision is also an option, but due to the locally invasive nature of TVTs and the risk of tumor transplantation into surgical wounds from contaminated instruments and gloves, the results are often unrewarding with recurrence being common (2,8,17). Radiation therapy has also resulted in complete remission of TVTs but this requires special equipment and personnel, which would have been prohibitively expensive in this case (2,17).
The dog herein was also diagnosed with heartworm disease and there was some concern with immunosuppression from chemotherapy. However, the recommendations of the American Heartworm Society (AHS) were followed (20). This dog was diagnosed with stage 1 (asymptomatic) heartworm disease; the initial recommended treatment is to give a heartworm preventative monthly for 3 mo along with doxycycline at 10 mg/kg BW, PO, q12h for 28 d with adulticide treatment being delayed until days 60, 90, and 91 following diagnosis (20). Doxycycline is recommended to reduce the numbers of the rickettsial bacterium, Wolbachia spp. that works symbiotically with D. immitis in all stages of heartworm disease (20). Killing the Wolbachia spp. disrupts heartworm transmission and reduces pathology associated with adult heartworms (20). Cage rest was also recommended with only short, slow leash walks as needed. This treatment was well-tolerated by the dog.
The typical presentation for TVT often involves the genitalia; however, as the present case illustrates primary ocular TVT may occur. The dog was also diagnosed with 3 concurrent infectious diseases which may have contributed to the dog’s risk of developing a TVT tumor. Treatment of all the diseases was initiated simultaneously and there was complete resolution of the TVT.
Acknowledgments
The authors thank Dr. Sachin Kumthekar, Veterinary Pathology, for his expertise in cytological interpretation of the ocular tumor and for providing 1 of the photographs; Dr. Tara Paterson and Dr. Emma Hage for assistance in management of the case. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Santos do Amaral A, Bassani-Silva S, Ferreira I, et al. Cytomorphological characterization of transmissible canine venereal tumor. RPCV. 2007;102:253–260. [Google Scholar]
- 2.Das U, Das AK. Review of canine transmissible venereal sarcoma. Vet Res Commun. 2000;24:545–556. doi: 10.1023/a:1006491918910. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim AM, Porter BF. Pathology in practice. Transmissible venereal tumor located on the bulbus glandis and body of the penis. J Am Vet Med Assoc. 2012;241:707–7099. doi: 10.2460/javma.241.6.707. [DOI] [PubMed] [Google Scholar]
- 4.Kabuusu RM, Stroup DF, Fernandez C. Risk factors and characteristics of canine transmissible venereal tumors in Grenada, West Indies. Vet Comp Oncol. 2010;8:50–55. doi: 10.1111/j.1476-5829.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues GN, Alessi AC, Laus JL. Intraocular transmissible venereal tumor in a dog. Cienc Rural. 2001;31:141–143. [Google Scholar]
- 6.Pigatoo JAT, Hunning PS, Bercht BS, de Albuquerque L. Transmissible venereal tumor in the palpebral conjunctiva of a dog: Case report. Semina: Ciencias Agraias. 2011;32:1139–1144. [Google Scholar]
- 7.Rogers KS, Walker MA, Dillon HB. Transmissible venereal tumor: A retrospective study of 29 cases. J Am Anim Hosp Assoc. 1998;34:463–470. doi: 10.5326/15473317-34-6-463. [DOI] [PubMed] [Google Scholar]
- 8.Oduye OO, Ikede BO, Esuruoso GO, Akpokodje JU. Metastatic transmissible venereal tumour in dogs. J Small Anim Pract. 1973;14:625–637. doi: 10.1111/j.1748-5827.1973.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Kim Y, Kang MS, et al. Disseminated transmissible venereal tumor in a dog. J Vet Diagn Invest. 2006;18:130–133. doi: 10.1177/104063870601800123. [DOI] [PubMed] [Google Scholar]
- 10.Yang TJ. Metastatic transmissible venereal sarcoma in a dog. J Am Vet Med Assoc. 1987;190:555–556. [PubMed] [Google Scholar]
- 11.Chikweto A, Kumthekar SM, Bhaiyat MI, Tiwari KP, Larkin H. Genital and extragenital canine transmissible venereal tumor (TVT) in Grenada, West Indies. Vet Path J Abstracts ACVP. 2011;66:146–148. [Google Scholar]
- 12.Ferreira AJA, Jaggy A, Varejäo AP, et al. Brain and ocular metastases from a transmissible venereal tumor in a dog. J Small Anim Pract. 2000;41:165–168. doi: 10.1111/j.1748-5827.2000.tb03187.x. [DOI] [PubMed] [Google Scholar]
- 13.Pereira JS, Silva AB, Martins AL, Ferreira AM, Brooks DE. Immunohistochemical characterization of intraocular metastasis of a canine transmissible venereal tumor. Vet Ophthalmol. 2000;3:43–47. doi: 10.1046/j.1463-5224.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Abbott PK. Venereal transmissible tumour on eyelid of dog. Aust Vet J. 1966;42:29. doi: 10.1111/j.1751-0813.1966.tb04609.x. [DOI] [PubMed] [Google Scholar]
- 15.Aquino SM. Management of eyelid neoplasms in the dog and cat. Clin Tech Small Anim Pract. 2007;22:46–54. doi: 10.1053/j.ctsap.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Varughese E, Singla V, Ratnakaran U, Gandotra V. Successful management of metastatic transmissible venereal tumour to skin of mammary region. Reprod Domest Anim. 2012;47:366–369. doi: 10.1111/rda.12105. [DOI] [PubMed] [Google Scholar]
- 17.Mello Martins MI, Ferreira de Souza F, Gobello C. The canine transmissible venereal tumor: etiology, pathology, diagnosis and treatment. In: Concannon PW, England G, Verstegen J III, Linde-Forsberg C, editors. Recent Advances in Small Animal Reproduction. Ithaca, New York: International Veterinary Information Service; 2005. [Last accessed September 16, 2013]. p. A1233.0405. Available from: http://www.biologia.studies.uj.edu.pl/~joachimiak/WWW/Joachimiak/ciekawostki/Canine%20tumor.pdf. [Google Scholar]
- 18.Nak D, Nak Y, Cangul IT, Tuna B. A Clinico-pathological study on the effect of vincristine on transmissible venereal tumour in dogs. J Vet Med Series A. 2005;52:366–370. doi: 10.1111/j.1439-0442.2005.00743.x. [DOI] [PubMed] [Google Scholar]
- 19.Scarpelli KC, Valladão ML, Metze K. Predictive factors for the regression of canine transmissible venereal tumor during vincristine therapy. Vet J. 2010;183:362–363. doi: 10.1016/j.tvjl.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 20.American Heartworm Society. Current canine guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs (revised 2012 January) [Last accessed September 16, 2013]. Available from: http://www.heartwormsociety.org/veterinary-resources/canine-guidelines.html.