Abstract
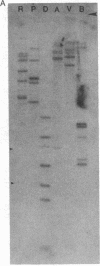
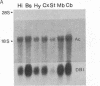
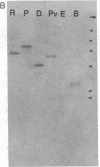
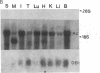
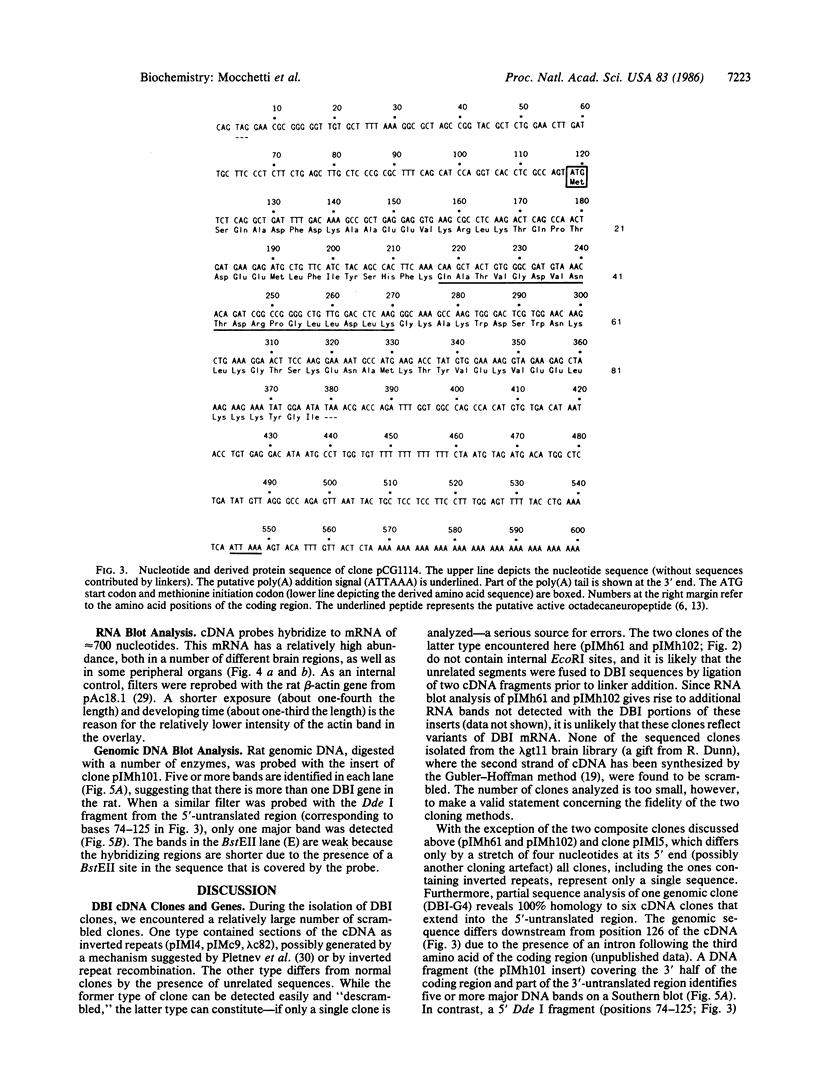
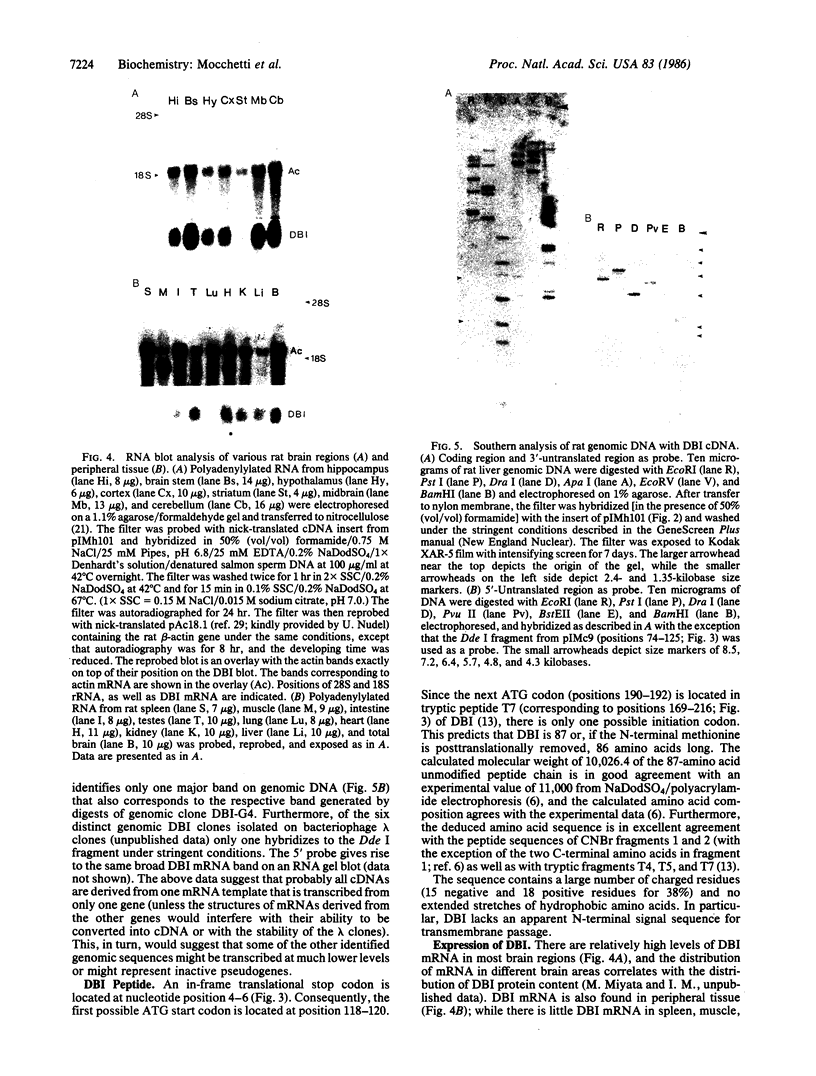
cDNA clones corresponding to the polypeptide that has been shown to be an endogenous diazepam binding inhibitor and may act as a physiological ligand for the benzodiazepine/beta-carboline receptor have been isolated from bacteriophage lambda recombinant libraries from rat hypothalamus, total brain, and liver. The clones contain an open reading frame corresponding to 87 amino acids. A signal sequence is not present. In addition to high levels of mRNA in various brain regions, RNA blot analysis reveals an abundance of diazepam binding inhibitor mRNA in many peripheral organs (e.g., testes, kidney, liver, and heart) that are known to be rich in peripheral benzodiazepine recognition sites. The size of the mRNA in all tissue examined is approximately 0.7 kilobase. Southern blot analysis of genomic DNA suggests the presence of about six genes in the rat, some of which may be pseudogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Costa E., Ferrero P., Fujimoto M., Cosenza-Murphy D., Guidotti A. Diazepam-binding inhibitor: a neuropeptide located in selected neuronal populations of rat brain. Science. 1985 Jul 12;229(4709):179–182. doi: 10.1126/science.3892688. [DOI] [PubMed] [Google Scholar]
- Anholt R. R., De Souza E. B., Kuhar M. J., Snyder S. H. Depletion of peripheral-type benzodiazepine receptors after hypophysectomy in rat adrenal gland and testis. Eur J Pharmacol. 1985 Mar 26;110(1):41–46. doi: 10.1016/0014-2999(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Anholt R. R., De Souza E. B., Oster-Granite M. L., Snyder S. H. Peripheral-type benzodiazepine receptors: autoradiographic localization in whole-body sections of neonatal rats. J Pharmacol Exp Ther. 1985 May;233(2):517–526. [PubMed] [Google Scholar]
- Anholt R. R., Murphy K. M., Mack G. E., Snyder S. H. Peripheral-type benzodiazepine receptors in the central nervous system: localization to olfactory nerves. J Neurosci. 1984 Feb;4(2):593–603. doi: 10.1523/JNEUROSCI.04-02-00593.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt R. R., Pedersen P. L., De Souza E. B., Snyder S. H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986 Jan 15;261(2):576–583. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J., Malgouris C., Imbault F., Begassat F., Uzan A., Renault C., Dubroeucq M. C., Gueremy C., Le Fur G. "Peripheral type" benzodiazepine binding sites in rat adrenals: binding studies with [3H]PK 11195 and autoradiographic localization. Arch Int Pharmacodyn Ther. 1983 Nov;266(1):38–49. [PubMed] [Google Scholar]
- Braestrup C., Schmiechen R., Neef G., Nielsen M., Petersen E. N. Interaction of convulsive ligands with benzodiazepine receptors. Science. 1982 Jun 11;216(4551):1241–1243. doi: 10.1126/science.6281892. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Corda M. G., Ferrari M., Guidotti A., Konkel D., Costa E. Isolation, purification and partial sequence of a neuropeptide (diazepam-binding inhibitor) precursor of an anxiogenic putative ligand for benzodiazepine recognition site. Neurosci Lett. 1984 Jun 29;47(3):319–324. doi: 10.1016/0304-3940(84)90533-0. [DOI] [PubMed] [Google Scholar]
- Costa E., Corda M. G., Guidotti A. On a brain polypeptide functioning as a putative effector for the recognition sites of benzodiazepine and beta-carboline derivatives. Neuropharmacology. 1983 Dec;22(12B):1481–1492. doi: 10.1016/0028-3908(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Mao C. C., Suria A. New concepts on the mechanism of action of benzodiazepines. Life Sci. 1975 Jul 15;17(2):167–185. doi: 10.1016/0024-3205(75)90501-9. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Huston V. Peripheral benzodiazepine binding sites in heart and their interaction with dipyridamole. Eur J Pharmacol. 1981 Jul 17;73(2-3):209–211. doi: 10.1016/0014-2999(81)90092-3. [DOI] [PubMed] [Google Scholar]
- De Souza E. B., Anholt R. R., Murphy K. M., Snyder S. H., Kuhar M. J. Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinology. 1985 Feb;116(2):567–573. doi: 10.1210/endo-116-2-567. [DOI] [PubMed] [Google Scholar]
- Ferrero P., Guidotti A., Conti-Tronconi B., Costa E. A brain octadecaneuropeptide generated by tryptic digestion of DBI (diazepam binding inhibitor) functions as a proconflict ligand of benzodiazepine recognition sites. Neuropharmacology. 1984 Nov;23(11):1359–1362. doi: 10.1016/0028-3908(84)90061-3. [DOI] [PubMed] [Google Scholar]
- Ferrero P., Santi M. R., Conti-Tronconi B., Costa E., Guidotti A. Study of an octadecaneuropeptide derived from diazepam binding inhibitor (DBI): biological activity and presence in rat brain. Proc Natl Acad Sci U S A. 1986 Feb;83(3):827–831. doi: 10.1073/pnas.83.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish M., Fares F. Solubilization of peripheral benzodiazepine-binding sites from rat kidney. J Neurosci. 1985 Nov;5(11):2889–2893. doi: 10.1523/JNEUROSCI.05-11-02889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I., Schwartz J. P., Costa E. Use of mRNA hybridization and radioimmunoassay to study mechanisms of drug-induced accumulation of enkephalins in rat brain structures. Mol Pharmacol. 1985 Jul;28(1):86–91. [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnev A. G., Scobeleva N. A., Frolova LYu, Kisselev L. L. Nucleotide sequence of the anemic pigeon alpha-globin gene. Structural rearrangements in the cloned cDNA. Gene. 1983 Nov;25(1):93–100. doi: 10.1016/0378-1119(83)90171-3. [DOI] [PubMed] [Google Scholar]
- Polc P., Bonetti E. P., Schaffner R., Haefely W. A three-state model of the benzodiazepine receptor explains the interactions between the benzodiazepine antagonist Ro 15-1788, benzodiazepine tranquilizers, beta-carbolines, and phenobarbitone. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(4):260–264. doi: 10.1007/BF00498510. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Wang J. K., Spector S. Properties of [3H] diazepam binding to rat peritoneal mast cells. Life Sci. 1980 Jul 14;27(2):171–178. doi: 10.1016/0024-3205(80)90460-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Wang J. K., Spector S. [3H]Diazepam binding sites on rat heart and kidney. Biochem Pharmacol. 1982 Feb 15;31(4):589–590. doi: 10.1016/0006-2952(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Taniguchi T., Spector S. Properties of [3H]diazepam binding sites on rat blood platelets. Life Sci. 1980 Nov 17;27(20):1881–1888. doi: 10.1016/0024-3205(80)90434-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]