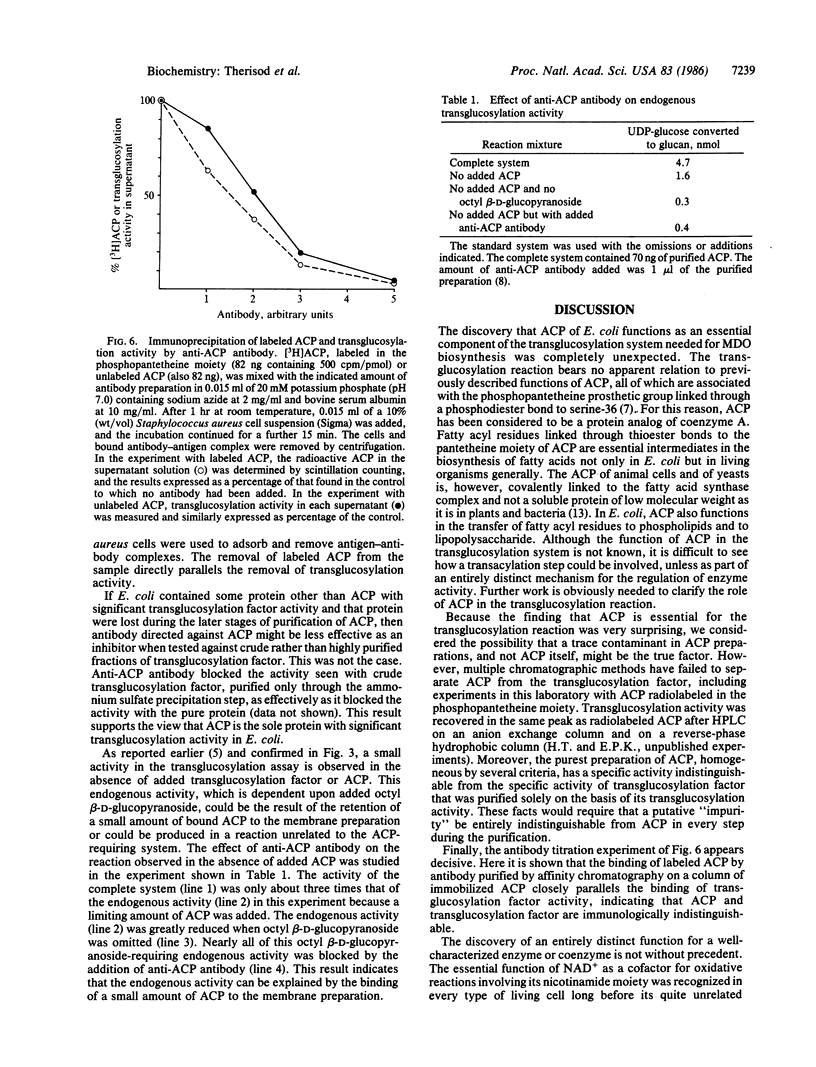
Abstract
Membrane-derived oligosaccharides are branched, substituted beta-glucans localized in the periplasmic space of Escherichia coli and other Gram-negative bacteria. The biosynthesis of membrane-derived oligosaccharides and of analogous periplasmic oligosaccharides found in plant bacteria is of particular interest because it is subject to strict osmotic regulation [Miller, K.J., Kennedy, E.P., and Reinhold, V.N. (1986) Science 231, 48-51]. An enzyme system catalyzing the synthesis of the (beta 1-2)-linked glucan backbone of E. coli membrane-derived oligosaccharides from UDP-glucose requires both a membrane component and a cytosolic protein termed transglucosylation factor. The factor has now been purified to apparent homogeneity and has been found to be identical to acyl carrier protein (ACP), the phosphopantetheine-containing protein of low molecular weight that has long been known to be essential for fatty acid synthesis in E. coli and other organisms. Both are small, heat-stable, highly anionic proteins with identical chromatographic and electrophoretic behavior. ACP of the highest purity has an activity in the transglucosylation system indistinguishable from that of the protein independently purified as transglucosylation factor. Antibody raised against pure ACP completely inhibits transglucosylation activity; this inhibition is overcome by titration of the antibody with either ACP or transglucosylation factor. These findings provide evidence for an essential function of ACP unrelated to the biosynthesis of lipid.
Full text
PDF
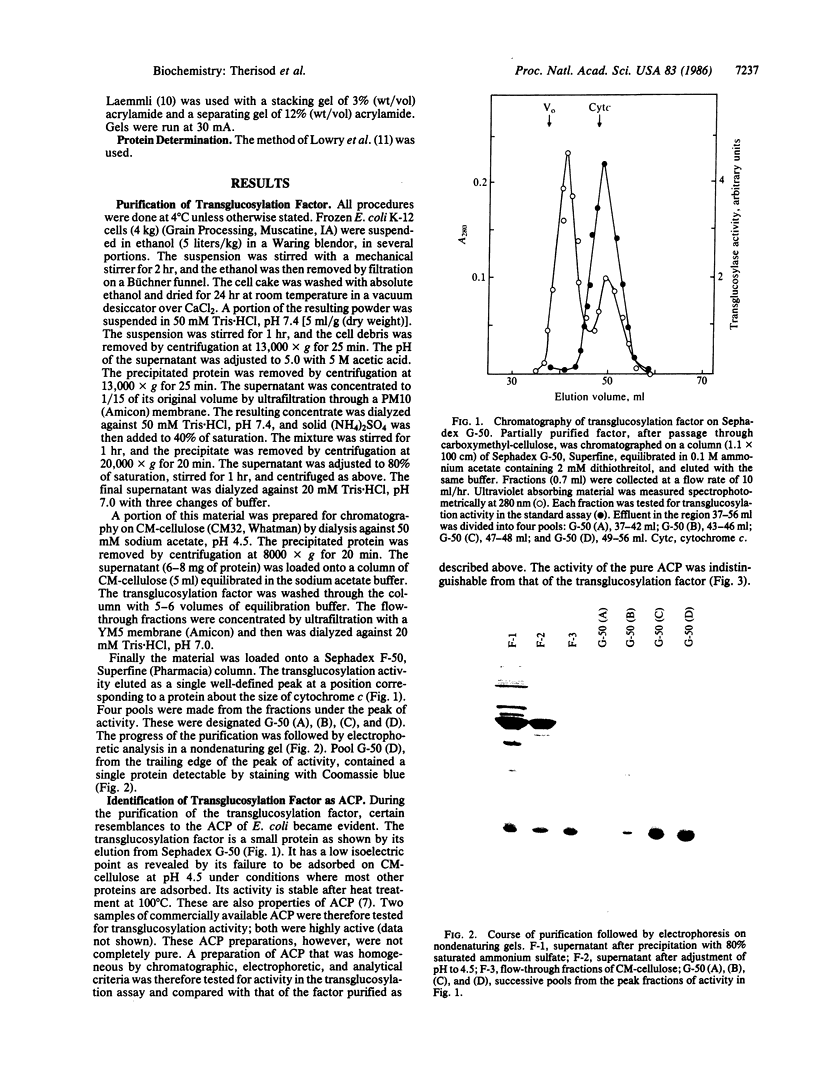
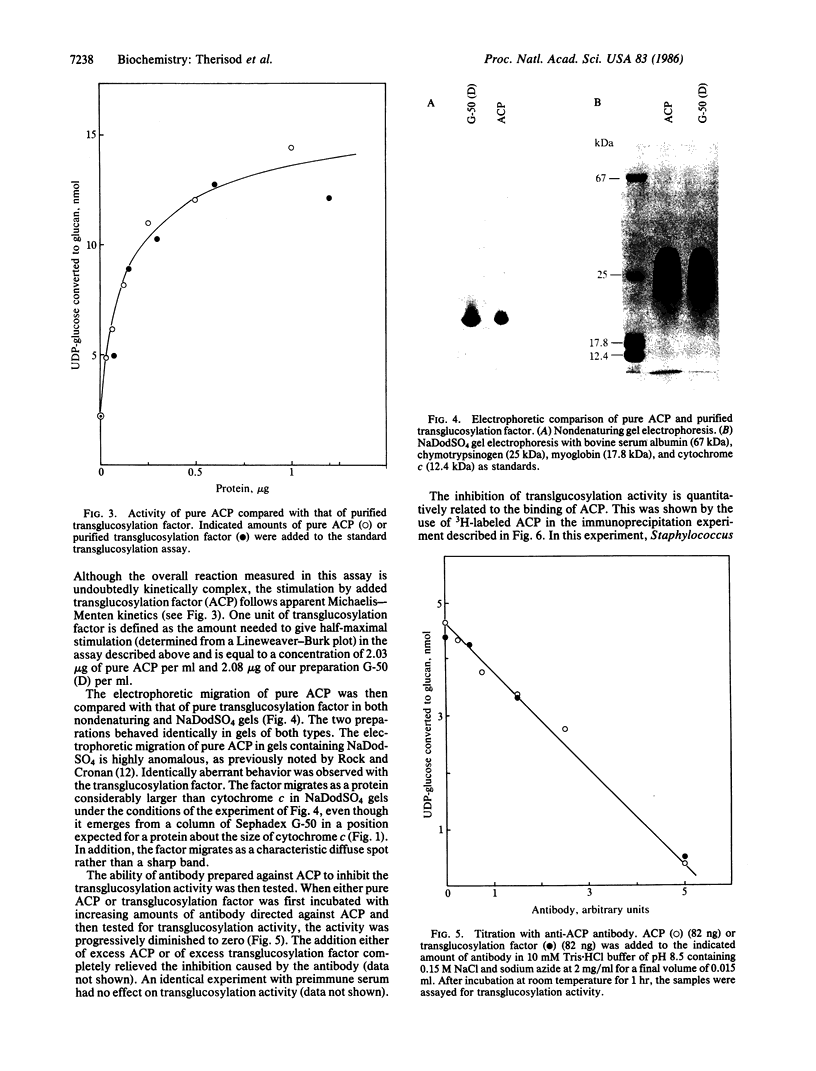

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Kennedy E. P. Mapping of a locus (mdoA) that affects the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Bacteriol. 1984 Mar;157(3):956–957. doi: 10.1128/jb.157.3.956-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):15186–15191. [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Diphosphopyridine nucleotide: a cofactor for the polynucleotide-joining enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1700–1704. doi: 10.1073/pnas.57.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Re-evaluation of the solution structure of acyl carrier protein. J Biol Chem. 1979 Oct 10;254(19):9778–9785. [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Weissborn A. C., Kennedy E. P. Biosynthesis of membrane-derived oligosaccharides. Novel glucosyltransferase system from Escherichia coli for the elongation of beta 1----2-linked polyglucose chains. J Biol Chem. 1984 Oct 25;259(20):12644–12651. [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]