Abstract
Diesel exhaust particulate matter contains many semivolatile organic compounds (SVOCs) of environmental and health significance. This study investigates the composition, emission rates, and integrity of 25 SVOCs, including polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs (NPAHs), and diesel biomarkers hopanes and steranes. Diesel engine particulate matter (PM), generated using an engine test bench, three engine conditions, and ultra-low sulfur diesel (ULSD), was collected on borosilicate glass fiber filters. Under high engine load, the PM emission rate was 0.102 g/kWh, and emission rate of ΣPAHs (10 compounds), ΣNPAHs (6 compounds), Σhopanes (2 compounds), and Σsteranes (2 compounds) were 2.52, 0.351, 0.02 ~ 2 and 1μg/kWh, respectively. Storage losses were evaluated for three cases: conditioning filters in clean air at 25 °C and 33% relative humidity (RH) for 24 h; storing filter samples (without extraction) wrapped in aluminum foil at 4 °C for up to one month; and storing filter extracts in glass vials capped with Teflon crimp seals at 4 °C for up to six months. After conditioning filters for 24 h, 30% of the more volatile PAHs were lost, but lower volatility NPAHs, hopanes and steranes showed negligible changes. Storing wrapped filters and extracts at 4 °C for up to one month did not lead to significant losses, but storing extracts for five months led to significant losses of PAHs and NPAHs; hopanes and steranes demonstrated greater integrity. These results suggest that even relatively brief filter conditioning periods, needed for gravimetric measurements of PM mass, and extended storage of filter extracts can lead to underestimates of SVOC concentrations. Thus, SVOC sampling and analysis protocols should utilize stringent criteria and performance checks to identify and limit possible biases occurring during filter and extract processing.
Keywords: Polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs (NPAHs), diesel biomarkers, filter conditioning, sample storage
Introduction
Many semivolatile organic compounds (SVOCs) are environmentally significant due to their persistence, potential for bioaccumulation, and toxicity (ATSDR 1995). SVOC monitoring has focused on polycyclic aromatic hydrocarbons (PAHs), especially the 16 compounds identified by US EPA many years ago, which range from volatile naphthalene to semivolatile indeno[1,2,3-cd]pyrene (OFR 1982). Other classes of SVOCs are also of interest. Nitrogen-substituted PAHs (NPAHs) can have stronger carcinogenic and mutagenic activity than their parent PAHs, e.g., 1,8-dinitropyrene's mutagenic activity in Salmonella typhimurzum TA98 is three orders of magnitudes higher than benzo[a]pyrene's, which is often considered one of the most toxic PAHs (Schantz et al. 2000). Both PAHs and NPAHs can be formed through incomplete combustion, and important urban sources include vehicle emissions, especially diesel engine exhaust (Chen et al. 2004; Neff 1979; Logan 2007). Two additional classes of SVOCs, hopanes and steranes, are also emitted by vehicles, and have been used as “markers” or “tracers” of diesel exhaust given their resistance to environmental degradation (Prince et al. 2007) and specificity to the engine lubricating oil used in diesel and gasoline engines (Schauer et al. 1999; Kleeman et al. 2007; Schauer et al. 2002). Monitoring of these and other SVOCs is needed to trend and apportion emission sources, to estimate risk, and for other purposes.
Air sampling involves, in brief, collection of a sufficient sample on an appropriate matrix, e.g., 10 to 150 m3 of air on a Teflon or quartz filter for the particulate fraction, and a polyurethane foam cartridge for the vapor fraction, followed by storage, extraction, purification and analysis.
Because PAHs and NPAHs can volatize, decompose or transform during sample handling and storage, an important consideration in measuring SVOCs is the integrity of collected samples, especially for air samples, which involve multiple, sequential and complex steps. Sample integrity must be characterized and maintained, otherwise the measurements cannot be used quantitatively, and even some qualitative uses may be limited. Several sampling and analysis protocols specify sample storage times. EPA Method SW-846 for solid samples (e.g., soil, sediment, sludge, ash, etc.) requires extraction within 14 days of sample collection, and analysis within 40 days following extraction (EPA 2008). EPA method 3542 for air sampling specifies the same extraction and analysis times, and also specifies storage of filters at 4 °C(EPA 1996).
A few studies have investigated the storage losses of SVOC samples. Mussel tissues stored at −80 °C and −120 °C showed PAH concentrations that were stable for up to 10 years (Schantz et al. 2000). Soils stored at 4 °C for two weeks showed significant losses of 2- to 5-ring PAHs due to biodegradation; storage at −20 °C or the addition of a biocide reduced losses (Rost et al. 2002). Particulate matter (PM) collected on Teflon filters wrapped in foil, packed in glass bottles, and stored at room temperature in a darkened room for up to 118 days gave consistent readings for PAHs ranging in volatility from fluoranthene/pyrene to coronene (Oda et al. 1998). PM collected on quartz fiber filters stored for one week at 4 °C were stable for 50 of 61 PAHs, although 11 or the more volatile PAHs showed losses of 50 to 80% (Oda et al. 1998). Tests using artificially generated particles and quartz filters indicated that PAHs were stable for up to 120 days when stored at −79 °C (Sverdrup et al. 1990). Only one NPAH study was identified, which tested the storage losses of a single NPAH compound (2-nitrofluorene) on quartz filters. Filter samples stored at both −79 °C and 20 °C showed losses of about 40% after 30 days of storage (Sverdrup et al. 1990).
The integrity of PAH samples following extraction, typically dissolved in organic solvents, has received some attention. Ampouled PAH solutions in acetonitrile and toluene were stable after 1-year storage at both −20 and 20 °C (Vaessen et al. 1988). 61 PAHs tested stored in acetonitrile were stable after 4 weeks of storage at 4 °C (Oda et al. 1998). Three PAHs (fluorene, anthracene and benzo[a]pyrene) stored in six separate organic solvents (methanol, acetonitrile, DMSO, dichloromethane, hexane and cyclohexane) were stable for 20 days, with the exceptions of anthracene and benzo[a]pyrene in DMSO (Dabrowska et al. 2008). Post-extraction integrity of NPAH, hopane and sterane samples has not been demonstrated. Air sampling can involve storage and conditioning of PM filters if the gravimetric mass is determined from the same filter. No information was identified pertaining to losses of hopanes and steranes during filter storage and conditioning, or during extract storage. Similarly, no information was located regarding integrity of PAH and NPAH compounds during filter conditioning.
This study investigates the composition and integrity of particulate phase PAHs, NPAHs, hopanes and steranes in diesel exhaust sampled on PTFE-bonded glass fiber filters. Samples obtained from well-controlled engine dynamometer laboratory tests are used to investigate effects of filter conditioning, storage of filters before extraction, and storage of extracts. We believe that this is the first study examining losses of NPAHs and diesel biomarkers in both filters and extracts. This study is also unique in its use of PM from a real engine, which is significant because sample integrity may be affected by the PM composition (or matrix). The study's recommendations have implications for the design, methods, and quality assurance activities of future studies.
Materials and methods
Experimental design
Several series of laboratory experiments were used to investigate the integrity of SVOCs collected as PM samples of diesel engine exhaust. As target compounds, 14 PAHs, 7 NPAHs, 2 hopanes and 2 steranes were selected since they are frequently detected in airborne PM. Table 1 lists these compounds, CAS numbers, selected chemical properties, abbreviations used in this paper, and instrumental detection limits (IDLs). Table 2 summarizes the test conditions for PM sampling. Diesel exhaust was generated by a 6.4 L 2008 Ford Power Stroke diesel engine using conventional ultra low sulfur diesel (ULSD). The engine was operated under three engine conditions: idle (650 rpm, 0 bar Brake Mean Effective Pressure or BMEP); low load (1500 rpm, 6 bar BMEP); and high load (2500 rpm, 9 bar BMEP). The engine configuration represented a 2004 engine calibration without exhaust gas after-treatment. This configuration and operating conditions were selected because they produce relatively high PM concentrations, which facilitated sample collection, and because they are representative of many current in-use diesel engines.
Table 1.
List of target compounds, showing abbreviations in this paper, CAS number, number of rings, vapor pressure, instrument detection levels (IDLs) and concentration in the spiking solution.
| Group | Compound | Abbrev. | CAS # | # of rings | Vapor pressure (mmHg at 25 °C) | IDL (ng/mL) | Concentration in spiking solution (ng/μL) |
|---|---|---|---|---|---|---|---|
| Naphthalene | NAP | 91-20-3 | 2 | 8.50E-02 | 0.1 | 2.5 | |
| Acenaphthylene | ACY | 208-96-8 | 3 | 6.68E-03 | 0.2 | 2 | |
| Acenaphthene | ACT | 83-32-9 | 3 | 2.15E-03 | 0.1 | 5 | |
| Phenanthrene | PHE | 85-01-8 | 3 | 1.21E-04 | 0.1 | 10 | |
| Anthracene | ANT | 120-12-7 | 3 | 6.53E-06 | 0.1 | 1 | |
| Fluoranthene | FLA | 206-44-0 | 4 | 9.22E-06 | 0.2 | 7.5 | |
| PAHs | Pyrene | PYR | 129-00-0 | 4 | 4.50E-06 | 0.1 | 2 |
| Benzo[a]anthracene | BAA | 56-55-3 | 4 | 2.10E-07 | 0.1 | 10 | |
| Chrysene | CHR | 218-01-9 | 4 | 6.23E-09 | 0.2 | 10 | |
| Benzo[b]fluoranthene | BBF | 205-99-2 | 5 | 5.00E-07 | 0.3 | 10 | |
| Benzo[k]fluoranthene | BKF | 207-08-9 | 5 | 9.65E-10 | 0.3 | 0 | |
| Benzo[a]pyrene | BAP | 50-32-8 | 5 | 5.49E-09 | 0.1 | 1 | |
| Indeno[1,2,3-c,d]pyrene | IND | 193-39-5 | 6 | 1.25E-10* | 0.8 | 10 | |
| Dibenzo[a,h]anthracene | DBA | 53-70-3 | 5 | 9.55E-10 | 1.1 | 10 | |
| 1-Nitronaphthalene | 1-NNAP | 86-57-7 | 2 | 4.80E-04 | 0.2 | 0 | |
| 2-Nitronaphthalene | 2-NNAP | 581-89-5 | 2 | 2.83E-04* | 0.2 | 1 | |
| NPAHs | 2-Nitrobiphenyl | 2-NBPH | 86-00-0 | 2 | 5.21E-04* | 0.2 | 0 |
| 3-Nitrobiphenyl | 3-NBPH | 2113-58-8 | 2 | 1.01E-04* | 0.1 | 0.5 | |
| 4-Nitrobiphenyl | 4-NBPH | 92-93-3 | 2 | 3.01E-05* | 0.6 | 0 | |
| 2-Nitrofluorene | 2-NFL | 607-57-8 | 3 | 4.43E-06* | 0.2 | 0 | |
| 6-Nitrochrysene | 6-NCHR | 7496-02-8 | 4 | 7.61E-09* | 0.1 | 0.5 | |
| Hopanes | 17α(h),21β(h)-Hopane | Hop19 | 471-62-5 | 5 | 3.91E-07* | 0.2 | 0 |
| 17α(h)-22,29,30-Trisnorhopane | Hop15 | 53584-59-1 | 5 | 2.09E-06* | 0.6 | 0 | |
| Steranes | 20s-5α(h),14α(h),17α(h)-Cholestane | Ster42 | 481-21-0 | 4 | 8.79E-06* | 0.3 | 0 |
| 20r-5α(h),14β(h),17β(h)-Cholestane | Ster43 | 69483-47-2 | 4 | n/a | 0.3 | 0 |
Predicted by US EPA. [2011]. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.10.
Values without an asterisk were taken from the PhysProp Database, Syracuse Research Corporation (SRC), USA.
Table 2.
Engine and PM sampling conditions for each filter.
| Filter ID | Type | Speed (rpm) | BMEPa (bar) | Start of injection (degree ATDC) | EGRb (%) | Sampling time (s) | Sampling volume (L) | Cut into quarters |
|---|---|---|---|---|---|---|---|---|
| S1, S2, S3, S4, S5, S6 | Sample | 1500 | 6 | 3.5 | 14 | 330 | 55 | Yes |
| B1, B2, B3 | Field blank | n/a | n/a | n/a | n/a | n/a | n/a | Yes |
| S7, S8, S9 | Sample | 1500 | 6 | 3.5 | 14 | 330 | 55 | No |
| S10, S11, S12 | Sample | 2500 | 9 | 3.5 | 17 | 90 | 15 | No |
| S13, S14, S15 | Sample | 650 | 0 | 3.5 | 8 | 480 | 80 | No |
| S16 | Exhaust blank | n/a | n/a | n/a | n/a | 330 | 55 | No |
| S17, S18 | Field blank | n/a | n/a | n/a | n/a | n/a | n/a | No |
BMEP: Brake Mean Effective Pressure.
EGR: Exhaust Gas Recirculation.
A total of 24 filters were collected, including 1 exhaust blank and 5 field blanks, using the exhaust gas dilution sampling system (described below). Since samples were obtained consecutively, side-by-side replicate samples could not be collected. To obtain replicates and increase the number of samples available for the tests, nine filters were cut into quarters using a cleaned razor blade, and designated as sub-samples a, b, c and d. While these quarter filters had only one-fourth the mass of a full filter, loadings were designed to obtain sufficient mass for analytical purposes.
The first set of experiments tested the effect of filter conditioning, a standard practice to equilibrate air and exhaust sampling filters to a constant temperature and humidity needed to obtain repeatable weight measurements down to 1 or 2 μg (or better). These experiments used three loaded filters that had been sectioned into quarters. (Online Resource Table S1 shows details pertaining to each set of experiments). Selected quarter filters were spiked with 1 μL spiking solution (described in Table 1) that contained known concentrations of the target compounds. These spiked filters served as a performance check to guarantee that the target SVOCs had large enough masses and can be measured quantitatively (i.e., well above MDLs). In addition, with the spiked and unspiked quarter filters, the possible difference in SVOC losses between high and low concentrations could be examined. For spiking, each quarter filter was supported by glass tubes placed on aluminum foil, and 1 μL of the spiking solution was slowly transferred from a 2 μL syringe throughout the filter. The spiking solution was not allowed to penetrate to the aluminum foil. Selected quarter filters were conditioned by placing them on clean aluminum foil in a glove box maintained at 25°C and 33% RH for 24 h. During conditioning, filters were unwrapped and exposed to air in the glove box.
A second set of experiments examined the effect of filter storage. These experiments used six loaded filters that were sectioned into quarters, and selected quarter filters were spiked as described above (Online Resource Table S1). In this case, quarter filters were folded in half, individually wrapped in aluminum foil, packed in a zip-lock bag, and placed in a clean refrigerator at 4 °C for 0, 7 or 30 days.
The third experiment examined the integrity of extracts for extended storage periods (up to six months). A total of 12 filters were used. Full (uncut) and unspiked loaded filters were conditioned for 24 h after sampling, extracted, fractionated into three portions, and immediately analyzed, as described below. These extracts were then stored in 2 mL glass vials (Fisherbrand, Cat. No. 03-391-5) capped with Teflon crimp seals (National Scientific Company, Part No. C4011-1A), placed in a refrigerator at 4 °C, and reanalyzed after 1, 5 and 6 months.
Materials
PM samples were collected on 47 mm diameter Polytetrafluoroethylene (PTFE)-bonded glass fiber filters (Emfab™ TX40-HI20WW, borosilicate glass microfibers, reinforced with woven glass cloth and bonded with PTFE; Pall Corporation, Port Washington, NY, USA). PTFE-bonded glass fiber filters have been the filter media of choice for sampling diesel and gasoline engine particulate emissions to characterize PAHs and/or mutagenicity (Alsberg et al. 1985; Salmeen et al. 1984; Zinbo et al. 1995), and these filters are also used to sample PM emissions in engine compliance tests (CFR 2013).These filters can be more heavily loaded than PTFE membrane filters and the pressure drop across them rises more slowly. The mutagenicity and composition of diesel exhaust PMs collected on these and two other types of filters (PTFE membrane, quartz) are indistinguishable(Gorse Jr et al. 1982). Thus, PTFE-bonded glass fiber filters should be a suitable filter media to collect particulate phase SVOCs in diesel exhaust and then study their composition and integrity during filter conditioning and storage.
Solvents were HPLC grade and obtained from Fisher Scientific Inc. (Pittsburgh, PA, USA). Florisil (60-100 mesh) and sodium sulfate (anhydrous, certified ACS granular, 10-60 mesh) for column chromatography were supplied by the same vendor. The spiking solution, which included 14 PAHs and 3 NPAHs at the concentrations listed in Table 1, was prepared from standard solutions of individual compounds (Cambridge Isotope Laboratories Inc., Andover, MA, USA).
Filter conditioning and weighing
Filters were conditioned and weighed before and after being PM loading, following engine testing procedures (CFR 2013). Filters were conditioned in a glove box (Series 100 twin plastic glove box isolator, Terra Universal, Inc.) at 25°C and 33% RH for 24 h. Electrostatic charges on filters and instruments were neutralized using an ionizer (Terra Universal, Inc) placed in the filter weighing chamber for 30 min right before weighing. Then the filters were weighed twice to 1 μg precision using a microbalance (ME 5, Sartorius Inc., Edgewood, NY, USA). If the weights agreed within 5 μg, the results were averaged; otherwise filters were reweighed. After loading, selected filters were carefully cut into four equal sections.
Sample collection
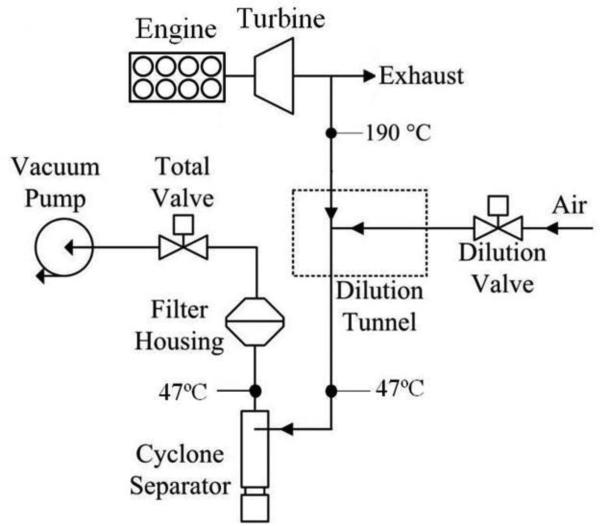
Diesel exhaust was sampled using a partial flow dilution tunnel (BG-2, Sierra Instruments Inc., Monterey, CA, USA) at a flow rate of 10 L/min through a 0.95 cm diameter stainless steel sample probe inserted into the center of a straight section of exhaust pipe, facing upstream, 2 m downstream of the engine's turbine (Fig. 1). The sample probe was 40 cm long and heated to191°C. The dilution tunnel, at the end of the sample probe, mixed raw exhaust with filtered air using a dilution ratio of 6:1. The mixture then passed through a transfer tube (40 cm length × 16 mm i.d.) to a 2.5 μm cyclone separator (Sierra Instruments Inc., Monterey, CA, USA), a second transfer tube (20 cm × 16 mm i.d.), and to a Teflon filter cassette holder (Sierra Instruments Inc., Monterey, CA, USA) that supported a filter on a perforated stainless steel backing plate. In the cassette, the exposed area of the filter was 39 mm in diameter. The transfer tubes, cyclone separator and filter were maintained at 47 ± 5°C. The filter flow rate was 60 L/min (face velocity of 91 cm/s).
Fig. 1.
PM sampling flow diagram
Before sampling, dilution and total flows were stabilized at set points for 60 s, and additional dilution air was back-flushed into the exhaust pipe. Sampling times were adjusted to sufficiently load filters (>280 μm of PM), at which point the pressure drop across the filter reached approximately 30 kPa. After sampling, the filter and cassette were immediately wrapped with Parafilm, placed in a sealed metal container, and transported to the filter conditioning and weighing laboratory. A 30-second high-flow purge cycle (100 L/min) was used to minimize PM accumulation in the dilution tunnel and transfer line prior to the next sample.
As much as possible, the PM sampling and analysis protocols were consistent with US EPA engine testing procedures (CFR 2013), ISO-DIS 16183 (ISO 2009), verbal recommendations by Sierra Instruments, and internal standard operating protocols (SOPs). To quantify the overall reproducibility (including engine, dilution tunnel, and filter conditioning, and filter weighing), nine PM samples were collected at specified conditions over a 2 day period. These tests showed good repeatability, i.e., the 95% confidence interval was ± 5.1% of the collected PM mass.
Extraction, fractionation and analysis
Filters were extracted by placing each in a 50 mL centrifuge tube, adding 25 mL of dichloromethane/hexane (4:1, v/v) to immerse the entire filter, and sonicating for 30 min (1510R-MTH, Branson Ultrasonics Corporation, Danbury, CT). The filter was then removed using a cotton stick and discarded. Extracts were passed through an activated Florisil column and fractionated into 3 portions using different solvents: fraction A was eluted with 15 mL of hexane; fraction B with 15 mL of hexane/acetone (1:1, v/v); and fraction C with 30 mL of methanol. Each fraction was then evaporated under nitrogen gas to 0.25 mL. Fractions A, B and C were analyzed for hopanes and steranes, PAHs, and NPAHs, respectively. No additional cleaning of each fraction was necessary.
SVOC were quantified using gas chromatography-mass spectrometry (GC-MS; HP 6890/5973, Agilent Industries, Palo Alto, CA, USA), an autosampler, and splitless 2 μL injections. Injector and detector temperatures were 275 and 280 °C, respectively. Separations used a capillary column (DB-5, 30 m × 0.25 mm id; film thickness 0.25 μm; J&W Scientific, Folsom, CA, USA). The carrier gas was helium (1.5 mL/min, pressure of 37.4 kPa, average velocity of 31 cm/s). The MS reagent gas was ultra high purity methane. The PAH analyses used a temperature program that started at 80 °C; then increased at 15 °C /min to 150 °C, then at 5 °C/min to 200 °C, and finally increased by 10 °C /min to 300 °C, which was held for 20 min, giving a total run time of 44.7 min. The MS detector was operated in electron impact (MSD-EI) mode. Scan mode was used to evaluate chromatography, and selective ion monitoring (SIM) mode was used for quantitative analysis with seven time windows (4, 5.8, 10, 14.25, 19, 22.2 and 25 min) and multiple ions. NPAH analyses used a temperature program that started at 40 °C held for 1.7 min; then increased by 25 °C/min to 150 °C and held for 10 min; increased by 10 °C/min to 220 °C and held for 10 min; and increased by 10 °C/min to 310 °C and maintained for 15 min giving a total run time of 57.1 min. The MSD was operated in negative chemical ionization (NCI) mode, again in scan mode to evaluate chromatography and in SIM mode for quantitative analysis (using ions 223, 247 and 297). Hopanes and steranes analyses used a third program: an initial oven temperature of 50 °C and no hold, ramping at 6 °C /min to 300 °C and holding for 10 min, giving a total run time of 41.7 min. Analyses used MSD-EI, again in scan mode to evaluate chromatography, and in selective ion monitoring (SIM) mode for quantitative analysis (using ion 191 for hopanes and ions 217 and 218 for steranes).
Data analysis
Spiking reproducibility was quantified by calculating the absolute percent difference between the two quarters cut from the same filter that used the same conditioning and storage conditions. These statistics were calculated for each PAH and NPAH, as well as sum of the target PAHs and NPAHs (denoted as ΣPAHs and ΣNPAHs).
The effect of filter conditioning was evaluated as the relative change for each compound (using the difference between the averages of conditioned and unconditioned quarter filters, divided by the average of unconditioned filters). The uncertainty of the change was evaluated as its standard deviation, estimated by propagating the standard deviations of the average mass in conditioned and unconditioned quarter filters.
The effect of filter storage was evaluated as the relative change between quarter filters obtained from the same filter stored for different lengths of time. This helps to avoid variation due to engine emissions, spiking retention, and other factors. Specifically, unstored filter subsamples (unspiked: S5a and S5b; spiked: S2a and S2b) were compared to 7 day subsamples (unspiked: S5c and S5d; spiked: S2c and S2d). Similarly, subsamples stored for 7 days (unspiked: S6a and S6b; spiked: S3a and S3b) were compared to subsamples stored for 30 days (unspiked: S6c and S6d; spiked: S3c and S3d). Unspiked and spiked filters were compared separately.
The effect of extract storage was calculated as the relative change between stored and directly analyzed (unstored) extracts. Because losses for individual compounds across the three engine conditions were generally similar, results are averaged. Significant differences are discussed in the results.
Calibration and quality assurance
Calibration standards were prepared using three mixtures: 16 PAHs; 8 NPAHs (both Sigma-Aldrich, St. Louis, MO, USA); SRM 2266 and individual standards for one hopane (Hop19) and one sterane (Ster42) (Chiron Laboratories, Trondheim, Norway). Five concentrations were used: 0.01, 0.05, 0.10, 0.50, and 1.00ng/μL. Instrumental detection limits (IDLs) for each compound are reported in Table 1. All analytes were individually quantified against authentic standards when present as mixtures. Fluoranthene-d10 (Cambridge Isotope Laboratories Inc, Andover, MA, USA) and an internal standard (IS) PAH mixture (Wellington Laboratories, Guelph, ON, Canada) were used as ISs for PAH analyses. Nitrofluoanthene-d9 (Cambridge Isotope Laboratories Inc, Andover, MA, USA) was used as an IS for NPAH analyses. Lastly, n-tetracosane-d50 (Chiron Laboratories, Trondheim, Norway) was used as an IS for hopanes and steranes. Using a 25 μL syringe, 15 μL of the internal standard was added to each sample prior to GC-MS analysis.
Quality assurance (QA) measures included the regular use of blanks, replicates, spike recovery tests, and standard reference materials (SRM 2585, NIST, USA). To check for possible contamination, solvent blanks, lab blanks and field blanks were collected and analyzed using the procedures described above. No contamination of target compounds was found in the three types of blanks.
Spike recovery was 90-98% during the study, and the shift (abundance of target compounds in standard solutions before and after running a batch of samples) was within 20%. The reproducibility of spiked quarter filters for ΣPAH and ΣNPAH measurements, shown in Online Resource Table S2, was within 10% for most filters. Reproducibility for individual compounds was similar (data not shown), indicating that spiking was reproducible. However, large differences were shown by one sample (S1, including subsamples S1a, S1b, S1c, and S1d). This was the first filter spiked, and the variability may be due to different spiking volumes among the quarter filters, penetration and loss of the spiking solution through the filter, or some other reason. (The reproducibilty of spiking for hopanes and steranes was not calculated as these compounds were not contained in the spiking solution.)
Results and discussion
SVOCs in diesel exhaust PM
Table 3 shows the concentrations and emission rates of target SVOCs measured in diesel exhaust PM (from samples S7 through S15, Table 2). The PM emission rates under low-load and high-load conditions were 0.033 and 0.102 g/kW-hr, respectively, which is comparable to previous studies (Sharp et al. 2000a, 2000b; Hori and Narusawa 2001; Lea-Langton et al. 2008; Tanaka et al. 1998; Ratcliff et al. 2010; Gerald Liu et al. 2010; Gambino et al. 2001) (Table 4). During idling, the PM emission rate was 0.11 mg/s.
Table 3.
Concentrations and emission rates of target SVOCs in diesel exhaust PM. Results show average and standard deviation (in parentheses).
| Emission rate |
Mass per PM |
||||||
|---|---|---|---|---|---|---|---|
| Group | Compound | Idle (ng/s) | Low-load (μg/kWh) | High-load (μg/kWh) | Idle ng/mg PM | Low-load ng/mg PM | High-load ng/mg PM |
| Naphthalene | 1.80 (0.52) | 0.256 (0.058) | 1.617 (0.185) | 16.2 (4.3) | 7.6 (1.5) | 15.8 (2.2) | |
| Acenaphthylene | 0.13 (0.04) | 0.030 (0.008) | 0.159 (0.060) | 1.2 (0.5) | 0.9 (0.2) | 1.6 (0.6) | |
| Acenaphthene | 0.16 (0.02) | 0.030 (0.011) | 0.311 (0.202) | 1.5 (0.3) | 0.9 (0.3) | 3.0 (1.9) | |
| Phenanthrene | n/aa | n/aa | 0.003 (0.004) | n/aa | n/aa | 0.0 (0.0) | |
| Anthracene | n/aa | n/aa | 0.007 (0.001) | n/aa | n/aa | 0.1 (0.0) | |
| Fluoranthene | n/aa | n/aa | n/aa | n/aa | n/aa | n/aa | |
| Pyrene | 0.23 (0.03) | 0.042 (0.004) | 0.350 (0.012) | 2.1 (0.6) | 1.2 (0.1) | 3.4 (0.2) | |
| PAHs | Benzo[a] anthracene | n/aa | n/aa | 0.017 (0.009) | n/aa | n/aa | 0.2 (0.1) |
| Chrysene | n/aa | n/aa | 0.016 (0.005) | n/aa | n/aa | 0.2 (0.0) | |
| Benzo[b]fluoranthene | n/aa | n/aa | 0.003 (0.005) | n/aa | n/aa | 0.0 (0.0) | |
| Benzo[k]fluoranthene | n/aa | n/aa | n/aa | n/aa | n/aa | n/aa | |
| Benzo[a]pyrene | n/aa | n/aa | 0.040 (0.002) | n/aa | n/aa | 0.4 (0.0) | |
| Indeno[1,2,3-c,d]pyrene | n/aa | n/aa | n/aa | n/aa | n/aa | n/aa | |
| Dibenzo[a,h]anthracene | n/aa | n/aa | n/aa | n/aa | n/aa | n/aa | |
| ΣPAHs | 2.32 (0.50) | 0.359 (0.083) | 2.522 (0.242) | 21.0 (4.5) | 10.7 (2.1) | 24.6 (2.7) | |
| 1-Nitronaphthalene | n/aa | n/aa | n/aa | n/aa | n/aa | n/aa | |
| 2-Nitronaphthalene | 0.0013 (0.0013) | 0.001 (0.001) | 0.012 (0.004) | 0.01 (0.01) | 0.02 (0.02) | 0.12 (0.04) | |
| 2-Nitrobiphenyl | 0.0201 (0.0134) | 0.014 (0.008) | 0.017 (0.012) | 0.19 (0.15) | 0.41 (0.25) | 0.17 (0.12) | |
| NPAHs | 3-Nitrobiphenyl | 0.0185 (0.0126) | 0.004 (0.001) | 0.065 (0.024) | 0.18 (0.12) | 0.14 (0.04) | 0.64 (0.23) |
| 4-Nitrobiphenyl | n/aa | n/aa | 0.014 (0.002) | n/aa | n/aa | 0.13 (0.02) | |
| 2-Nitrofluorene | n/aa | 0.019 (0.003) | 0.071 (0.011) | n/aa | 0.57 (0.09) | 0.69 (0.11) | |
| 6-Nitrochrysene | n/aa | 0.019 (0.002) | 0.171 (0.015) | n/aa | 0.57 (0.07) | 1.67 (0.12) | |
| ΣNPAHs | 0.0398 (0.0163) | 0.056 (0.009) | 0.351 (0.041) | 0.38 (0.21) | 1.17 (0.29) | 3.42 (0.33) | |
Compound was not detected.
Table 4.
Comparison of PM, ΣPAHs, ΣNPAHs, Σhopanes, and Σsteranes measurements in the present study and previous studies.
| Engine | Displacement (L) | Power (kW) | Condition | PM (g/kWh) | ΣPAHs (μg/kWh) |
ΣNPAHs (μg/kWh) |
ΣHopanes (μg/kWh) |
ΣSteranes (μg/kWh) |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2008 Ford Power Stroke | 6.4 | 261 | 1500 rpm, 6 bar | 0.033 | 0.36 | 0.06 | 0.003 ~ 0.3 | 0.2 | This study |
| 2500 rpm, 9 bar | 0.10 | 2.52 | 0.35 | 0.02 ~ 2 | 1.0 | ||||
| 1997 Cummins N14 | 276 (rated power) | EPA transient cycle | 0.142 | C.A. Sharp, 2000a | |||||
| 1997 DDC Series 50 | 205 (rated power) | EPA transient cycle, no catalyst | 0.137 | ||||||
| 1997 DDC Series 50 | 205 (rated power) | EPA transient cycle, with catalyst | 0.101 | ||||||
| 1997 Cummins N14 | 276 (rated power) | EPA transient cycle | 14.6 | 0.210 | C.A. Sharp, 2000b | ||||
| 1997 DDC Series 50 | 205 (rated power) | EPA transient cycle, no catalyst | 6.36 | 0.184 | |||||
| 1997 DDC Series 50 | 205 (rated power) | EPA transient cycle, with catalyst | 2.21 | 0.177 | |||||
| 6-cylinder DI | 8.0 | 147 (maximum power, at 2900 rpm) | 1000 rpm, 25% load | 0.1-0.2 | S. Hori, 2001 | ||||
| 1000 rpm, 75% load | 0.3-0.5 | ||||||||
| Perkins Phaser 180Ti 6 cylinder DI | 6.0 | 134 (maximum power, at 2600 rpm) | 23 kW power output | 0.060 | 544 | A. Lea-Langton. 2008 | |||
| 47 kW power output | 0.030 | 80 | |||||||
| 6-cylinder DI | 7.961 | 191 (rated power, at 2700 rpm) | Japanese 13 mode test cycle | 0.181-0.241 | 9.6-20.9 | S. Tanaka, 1998 | |||
| 2002 Cummins ISB | 5.9 | 224 | AVL 8-mode test cycle | 0.063 | 0.006 | M.A. Ratcliff, 2010 | |||
| 2004 Heavy-duty | 15 | EPA transient cycle | 1382 | 1.57 | 15.1 | 9.67 | Z. Gerald Liu, 2010 | ||
| 2007 Heavy-duty | Average 324 | 16-hr transient cycle | 0.0014 | 992 | 0.94 | 0.80 | 0.54 | I.A. Khalek, 2011 | |
| 6-cylinder EURO 2 DI | 7.8 | 159 (maxium power, at 2100 rpm) | EURO 2 13 mode test cycle | 0.26 | 9.661 | 0.351 | M.Gambino, 2001 |
Emission rates and the number of target compounds detected (PAHs, NPAHs, hopanes and steranes) were highest under high-load conditions. For the PAHs, the larger molecular weight compounds, e.g., benzo[b]fluoranthene and benzo[a]pyrene, along with phenanthrene, anthracene, benzo[a]anthracene and chrysene, were detected only under high-load condition. Emission rates of ΣPAHs under loaded conditions were slightly lower than previous studies (Table 4), but emission rates under high-load (2.52 μg/kW-hr) was similar to that in a study (2.21 μg/kW-hr, DDC 50 engine with catalyst) (Sharp et al. 2000b).ΣNPAH emission rates were similar to those in previous studies (Sharp et al. 2000b; Gambino et al. 2001).
Hopane and sterane emission rates in the literature vary by a large amount, e.g., 10 to 100 times (Gerald Liu et al. 2010; Khalek et al. 2011) probably due to true variation in emissions, but also due to analytical issues including a lack of standards for most compounds. In the present study, two hopanes and two steranes were selected (Table 1) based on authentic standards and SRMs. Due to co-elution of target and unidentified hopanes, they were quantified as a range that represents the minimum and maximum expected concentration, thus results for Σhopanes are presented as a range (Table 4). This uncertainty does not affect the sample integrity tests (discussed below) since the same analytical approach is used throughout and all chromatograms in these tests were very similar.
SVOC concentrations were low in the extracts of unspiked whole filters (S7 – S15, Table 2), thus, results for some compounds may be affected by detection limit issues. This is not an issue for PAHs such as naphthalene, pyrene and acenaphthylene, which had concentrations 100, 10 and 2 times higher than their IDLs, respectively. However, concentrations of acenaphthene, phenanthrene, benzo[b]fluoranthene, benzo[a]anthracene, chrysene and benzo[a]pyrene were close to IDLs, and other PAHs were not detected. For the NPAHs, concentrations of six of the seven target compounds exceeded IDLs by at least several times, but 1-nitronaphthalene was not detected. Diesel biomarkers hopanes and steranes were detected at levels close to IDLs with the exception of 20s-5α(h)14α(h)17α(h)-cholestrane, which exceeded the IDL by 10-fold.Thus, the absence of several target SVOCs in our results is likely due to levels below the detection limit. Additionally, measurement variation will increase at low concentrations.
Filter conditioning
Unconditioned and conditioned filters (24 h at 25 °C and 33% RH) are compared in Table 5. PAH and NPAH results represent only unspiked filters collected at the medium load condition (thus avoiding possible errors from spiking); hopane and sterane results include both spiked and unspiked filters (since spiking did not contain these compounds). Under the test conditions, five PAHs were detected on the quarter filters with masses from 0.02 ng for phenanthrene to 1.16 ng for naphthalene (Online Resource Table S3). The average (± standard deviation) loss from conditioning was 27 ± 20% across the five PAHs. Acenaphthylene and phenanthrene had the highest losses, 39 ± 22% and 35 ± 36%, respectively. These PAHs had four or fewer rings and relatively high vapor pressure (Table 1), which may explain the losses. Considering that significant PAH levels have been detected downstream of PM filters in other studies (Schauer et al. 1999), it is not surprising that filter conditioning at 25°C for 24 h can reduce PAHs in PM samples. Greater losses would be expected with longer conditioning periods, e.g., a 60% loss of fluorene has been reported for surrogate filter samples stored for 30 days at 20 °C (Sverdrup et al. 1990).
Table 5.
Effect of filter conditioning and storage, showing average percentage change and standard deviation (in parentheses).
| Filter conditioning |
Filter storage |
|||||
|---|---|---|---|---|---|---|
| Changea (conditioned vs. unconditioned) (%) | Change (Day7-1 vs. Day0) |
Change (Day30 vs. Day7-2) |
||||
| Group | Compound | Unspiked (%) | Spiked (%) | Unspiked (%) | Spiked (%) | |
| Naphthalene | − 27 (3) | n/a | − 4 (8) | − 11 (12) | 0 (3) | |
| Acenaphthylene | − 39 (22) | 0 (31) | 0 (86) | − 4 (5) | − 6 (46) | |
| Acenaphthene | − 3 (5) | − 6 (23) | 123 (257) | − 1 (31) | 0 (3) | |
| Phenanthrene | − 35 (36) | n/a | 12 (48) | 0 (1) | − 4 (6) | |
| Anthracene | n/a | n/a | 2 (82) | n/a | − 3 (0) | |
| Fluoranthene | n/a | n/a | 0 (21) | n/a | − 2 (12) | |
| Pyrene | − 29 (15) | − 2 (21) | − 10 (16) | − 7 (6) | − 9 (3) | |
| PAHs | Benzo[a]anthracene | n/a | n/a | n/a | n/a | − 2 (7) |
| Chrysene | n/a | n/a | n/a | n/a | − 2 (10) | |
| Benzo[b]fluoranthene | n/a | 17 (44) | 57 (13) | n/a | − 3 (8) | |
| Benzo[k]fluoranthene | n/a | n/a | n/a | n/a | 7 (24) | |
| Benzo[a]pyrene | n/a | − 1 (32) | 12 (43) | n/a | 2 (6) | |
| Indeno[1,2,3-c,d]pyrene | n/a | n/a | 26 (16) | n/a | − 5 (21) | |
| Dibenzo[a,h]anthracene | n/a | n/a | n/a | n/a | − 4 (6) | |
| Average | − 27 (20) | 2 (32) | 11 (46)b | − 4 (15) | − 2 (16) | |
| 1-Nitronaphthalene | n/a | − 8 (8) | − 30 (9) | − 11 (19) | − 25 (8) | |
| 2-Nitronaphthalene | − 14 (4) | 34 (8) | − 9 (37) | − 5 (40) | − 2 (29) | |
| 2-Nitrobiphenyl | − 10 (61) | 1 (9) | 8 (8) | − 15 (7) | − 1 (2) | |
| NPAHs | 3-Nitrobiphenyl | − 9 (45) | − 32 (15) | − 23 (4) | − 9 (8) | − 2 (7) |
| 4-Nitrobiphenyl | − 13 (31) | − 29 (8) | 10 (49) | n/a | − 4 (4) | |
| 6-Nitrochrysene | 2 (21) | − 21 (11) | − 14 (46) | 3 (18) | 0 (2) | |
| Average | − 8 (38) | − 9 (10) | − 10 (32) | − 7 (20) | − 6 (13) | |
| 17α(h),21β(h)-Hopane | 7 (27) | − 2(14) | − 13 (16) | |||
| Hopanesc | 17α(h)-22,29,30-Trisnorhopane | 39 (40) | − 2 (21) | 25 (27) | ||
| Average | 23 (34) | − 2 (18) | 6 (22) | |||
| 20s- 5α(h),14α(h),17α(h)-Cholestane | 2 (14) | − 8 (11) | 5 (11) | |||
| Steranesc | 20r- 5α(h),14β(h),17β(h)-Cholestane | 13 (26) | − 9 (13) | 1 (18) | ||
| Average | 8 (21) | − 8 (12) | 3 (15) | |||
Results for PAHs and NPAHs include only unspiked filters to avoid possible errors due to spiking (see text).
Excluded ACT.
Results for hopanes and steranes combine both spiked and unspiked filters since the spiking solution did not contain these compounds.
n/a: Compound was not detected.
Six NPAHs were detected on the quarter filters (masses from 0.04 ng for 4-nitrobiphenyl to 0.72 ng for 6-nitrochrysene; Online Resource Table S3). Conditioning produced an average loss of only 8 ± 38%. The replicates showed relatively large variation, probably because concentrations were low and sometimes near detection limits. The NPAHs have lower volatility than their parent PAHs(Dusek et al. 2002), and thus lower losses.
Hopane and sterane levels on the filters after conditioning increased by 23 ± 34% and 8 ± 21%, respectively, but these changes were not statistically significant (Online Resource Table S3). Reproducibility was only fair for 17α(H)-22,29,30 trisnorhopane, probably because concentrations were low and close to detection limits. These compounds are known to be stable and non-volatile (Prince et al. 2007), thus filter conditioning was not expected to produce significant losses.
In summary, filter conditioning produced significant losses of the most volatile PAHs, but other SVOCs were not affected. Experimental variation somewhat exceeded the normal criterion of 25%, a result of several factors. First, although samples S1 and S4 were collected under the same engine operating condition, they were obtained sequentially (over a 1 h period), and thus reflect any variation in engine emissions and sampling conditions. Second, experimental variation reflects two sets of measurements (pre- and post-conditioning). Third, sectioning of filters can yield different amounts of PM and SVOCs. Fourth, GC-MS analyses on different days can vary due to instrumental fluctuations (Kloster et al. 1992; Grimmer 1988). Finally, as mentioned, concentrations of some compounds were low and near IDLs. Given these factors, the variation is reasonable.
Filter storage
Filter storage tests showed mostly small or negligible changes for PAHs, e.g., on average, PAHs gained 2 ± 32% after 7 days of storage, and lost 4 ± 15% after 30 days, as compared to 7 days (Table 5). These results are based on the seven PAHs found on the unspiked quarter filters, which were mostly four or fewer ring compounds as seen in the filter conditioning tests. Spiked filters also showed small changes for PAHs, with the exception of acenaphthene (ACT) due to an anomalously high concentration in sample S2d, a possible instrumental error. Excluding this compound, spiked PAHs gained 11 ± 46% after 7 days of storage, and lost 2 ± 16% after 30 days. Overall, PAHs in the stored filters were stable for 30 days, including the relatively volatile PAHs such as naphthalene, which had 30% losses after one day of conditioning.
NPAH changes due to filter storage were also small, e.g., average losses combining spiked and unspiked quarter filters) were 9 ±24% and 6 ± 17% after 7 and 30 days of storage, respectively, for combined spiked and unspiked quarter filters (Table 5). Several spiked and unspiked comparisons differed, e.g., 1-nitronaphthalene in spiked and unspiked quarter filters lost 30 ±9% and 8 ± 8%, respectively, after 7 days of storage, these results likely reflect measurement variation at the low concentrations seen (the spiking solution did not include 1-nitronaphthalene).
Changes in hopane and sterane levels during filter storage fell within the range expected for experimental variation, e.g., average changes below 12% (Table 5). Thus, filter storage for 30 days had little effect on these compounds.
Overall, SVOC changes over the 30 day period were modest, indicating many SVOCs will be retained for at least 30 days in filters that are wrapped in aluminum foil and stored at 4 °C. The variability in the experimental results is due to the same factors discussed in the section on filter conditioning, e.g., low concentration samples, changes in the engine exhaust concentrations, and instrument fluctuations.
Extract storage
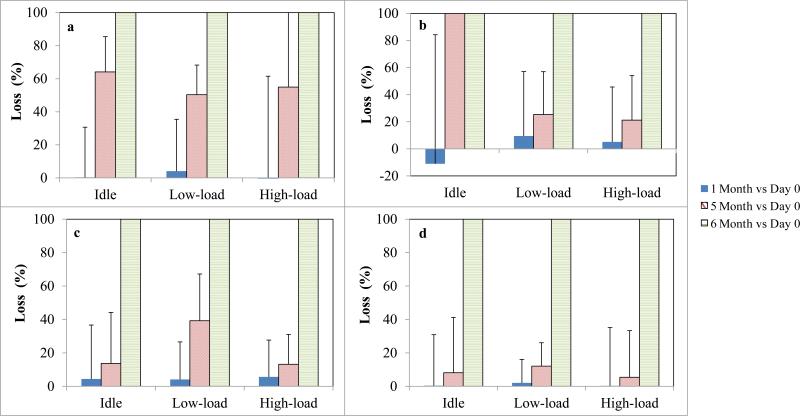
Extract storage tests are summarized in Fig. 2. For PAHs dissolved in hexane/acetone, storage for one month had no effect, e.g., losses averaged 1 ± 44% across the three engine conditions. The high-load condition (2500 rpm - 9 bar) showed greater variation, however, individual filters were fairly consistent with those measured in the directly analyzed extract (data not shown). After five months, PAH losses became appreciable (56 ± 31%) and several compounds were not detected, e.g., anthracene and benzo[a]pyrene that were initially found at low concentrations, 0.08 and 0.45 ng/mL, respectively)(Online Resource Fig. S1C). After six months, no PAHs were detected. These results indicate that storage of PAH extracts in hexane/acetone solvents in glass vials with Teflon crimped seals at 4 °C should be limited to one month.
Fig. 2.
Effect of storage time for SVOCs in extracts. Panels a through d show average percentage loss for sum of the detected PAHs, NPAHs, hopanes and steranes, respectively. Low-load is 1500 rpm – 6 bar. High-load is 2500 rpm – 9 bar. Error bars show one standard deviation.
NPAHs dissolved in methanol and stored at 4 °C were stable for one month. Losses for the NPAHs detected (3, 5 and 6 compounds were detected under idle, low-load and high-load conditions, respectively) averaged 1 ± 66%. The large variation is due to the low concentrations of NPAHs in the extracts. After five months, losses averaged 23 ± 32% for the detected NPAHs (5 and 6 compounds in samples collected under low and high load, respectively) (Online Resource Fig. S2B& S2C). (The 5 month idle samples were considered invalid due to broken vial inserts. We attempted to restore these samples by re-dissolving the partially dried extract with additional solvent and transferring the mixture to new inserts and vials, but the NPAHs were not successfully recovered.) No NPAHs were detected in the extracts after six months of storage.
For the hopanes and steranes dissolved in hexane, storage for one month also showed negligible change (average loss of 2 ± 27%). After five months, losses for hopanes under the low-load condition (Online Resource Fig. S3B) were larger and variable (39 ± 28%), probably due to the low concentrations (0.04 – 0.07 ng/mL) of the extracts. Excluding the low-load condition, losses for hopanes and steranes averaged 10 ± 28% at five months. After six months, hopanes and steranes were not detected. These results suggest that hopane and sterane extracts dissolved in hexane and stored at 4 °C are stable for at least one month, and five months may be acceptable with small (10%) losses.
After 6-month storage of extracts, most of the samples needed solvent additions (due to evaporation), which could introduce additional errors, e.g., incomplete mixing and transfer losses if vial inserts were replaced. This may explain the fact that no target SVOCs were detected in the extracts after six month.
In summary, storage of PAH, NPAH, hopane and sterane extracts in glass vials with Teflon crimped seals at 4 °C should be limited to about one month, although longer storage may not be detrimental for hopanes and steranes. Losses and variability will increase if solvents evaporate and solvent additions are used to re-dissolve extracts prior to analysis.
Strengths and limitations
This study was designed to investigate effects of processing and storage of SVOC samples collected as air samples. The study's strengths include the use of PM samples collected from diesel engine exhaust under controlled conditions, which allowed characterization using reproducible, real world and relevant samples. This is important since storage integrity may be affected by the matrix, i.e., the physical and chemical properties of the sample. Thus, results in the present paper should be more representative than those obtained using artificially generated PM. Other strengths include the use of environmentally relevant concentrations, consideration of a wide range of SVOCs, and the use of real extracts. On the last point, extracts may be affected by co-contaminants, impurities and other factors, and previous studies used known solutions of pure PAHs in solvents (Oda et al. 1998; Sverdrup et al. 1990; Vaessen et al. 1988). In addition, the use of sectioned filters provided replicates with filter-specific controls that helped to eliminate differences between filters, although it has the disadvantages of reducing sample mass and sensitivity, potentially increasing variability, and restricting PM mass measurements.
The study has several limitations. First, replicate filters collected under the same engine test condition were not ‘true’ replicates since samples were taken sequentially, not simultaneously. Although engine conditions were kept constant, variation in emissions and sampling conditions could alter the mass and composition of collected PM, as suggested by our results (Online Resource Tables S4-S6). The temporal variation could be minimized using parallel sampling trains. The number of samples in each filter conditioning and storage test was small, and thus statistical hypothesis testing was not feasible. While the overall trends were clear, larger sample sizes would help to confirm the findings of the present study. Finally, we recognize that ambient sampling networks, such as IMPROVE and Speciation Trends networks (Kleiman et al. 2003), use Teflon filters to collect PM2.5, not the borosilicate glass fiber filters used in the engine tests. We did not evaluate other filter types, but anticipate that our estimates of volatilization losses during filter conditioning will apply to other types of filters.
Conclusions
In this study, well controlled tests investigated the emission rates and integrity of SVOCs samples collected from diesel engine exhaust. Under high engine load conditions, the emission rate of PM was 0.102 g/kWh; emission rates of ΣPAHs, ΣNPAHs, Σhopanes and Σsteranes were 2.52, 0.351, 0.02 ~ 2 and 1 μg/kWh, respectively. These emission rates are generally comparable to previous studies. Conditioning filters for 24 h at 25 °C and 33% RH for weighing purposes did not significantly change concentrations of NPAHs, hopanes and steranes, however, approximately 30% of the more volatile PAHs were lost. Filters loaded with PM can be held for at least one month without appreciable losses of these four classes of compounds if the filter is wrapped in aluminum foil and held at 4 °C. Filter extracts (PAHs in hexane/acetone, NPAHs in methanol, hopanes and steranes in hexane) can be stored at 4 °C for at least one month without significant losses. Hopane and sterane extracts may be stored for five months or more with acceptable results.
Our findings show that even the relatively brief periods used to condition filters, which are needed for gravimetric measurements of PM mass, can lead to underestimates of PAH concentrations. Ideally, filter conditioning would not be used for SVOC measurements, and a separate parallel sampler would be used to determine gravimetric concentrations. Often, this is not feasible. To reduce potential biases, conditioning protocols might be altered by lowering temperatures (e.g., from 25 to 10 °C) and/or reducing conditioning times (e.g., from 24 to 12 h). Such temperatures will require refrigeration, and shorter times may not work if the filter or the collected PM is hydroscopic, e.g., PM containing a large fraction of sulfate aerosols. Additionally, SVOC sampling and analysis protocols might utilize performance checks and criteria aimed at identifying and limiting potential biases occurring during filter and extract processing, e.g., PM samples on glass fiber filters should be sealed appropriately, extracted within 30 days of collection, and analyzed within one month.
Supplementary Material
Acknowledgements
The authors thank the Walter E. Lay Auto Laboratory at the University of Michigan, and Mehdi Abarham, Tejas Chafekar, Ashwin Salvi, Feng-Chiao Su, and Dongyan Sun for laboratory assistance. This study was supported in part by US Environmental Protection Agency grant GL00E00690-0 entitled “PAHs, Nitro-PAHs & Diesel Exhaust Toxics in the Great Lakes: Apportionments, Impacts and Risks.” Additional support was provided by the Support for this research was provided by grant P30ES017885 from the National Institute of Environmental Health Sciences, National Institutes of Health entitled “Lifestage Exposure and Adult Disease.”
References
- Alsberg T, Stenberg U, Westerholm R, Strandell M, Rannug U, Sundvall A, et al. Chemical and biological characterization of organic material from gasoline exhaust particles. Environmental Science & Technology. 1985;19(1):43–50. [Google Scholar]
- ATSDR . Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) (Update) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 1995. [PubMed] [Google Scholar]
- CFR . Code of Federal Regulations Title 40 Part 1065: Engine Testing Procedures. U.S. National Archives and Records Administration; 2013. [Google Scholar]
- Chen B, Xuan X, Zhu L, Wang J, Gao Y, Yang K, et al. Distributions of Polycyclic Aromatic Hydrocarbons in Surface Waters, Sediments and Soils of Hangzhou City, China. Water Research. 2004;38(16):3558–3568. doi: 10.1016/j.watres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Dabrowska D, Kot-Wasik A, Namiesnik J. Stability studies of selected polycyclic aromatic hydrocarbons in different organic solvents and identification of their transformation products. Polish Journal of Environmental Studies. 2008;17(1):17–24. [Article] [Google Scholar]
- Dusek B, Hajskova J, Kocourek V. Determination of nitrated polycyclic aromatic hydrocarbons and their precursors in biotic matrices. Journal of Chromatography A. 2002;982(1):127–143. doi: 10.1016/s0021-9673(02)01340-7. [DOI] [PubMed] [Google Scholar]
- EPA . Method 3542: Extraction of Semivolatile Analytes Collected Using Method 0010 (Modified Method 5 Sampling Train) U.S. Environmental Protection Agency; Washington, D.C.: 1996. [Google Scholar]
- EPA . SW-846: Test Methods for Evaluating Solid Waste, Physical/Chemical Methods. Third edition U.S. Environmental Protection Agency; Washington, D.C.: 2008. [Google Scholar]
- Gambino M, Iannaccone S, Battistelli CL, Crebelli R, Iamiceli AL, Turrio Baldassarri L. Exhaust emission toxicity evaluation for heavy duty diesel and natural gas engines. Part I: regulated and unregulated emissions with diesel fuel and a blend of diesel fuel and biodiesel. International Combustion Engines, SAE_NA Technical Paper Series, 2001-01-044. 2001.
- Gerald Liu Z, Berg DR, Vasys VN, Dettmann ME, Zielinska B, Schauer JJ. Analysis of C1, C2, and C10 through C33 particle-phase and semi-volatile organic compound emissions from heavy-duty diesel engines. Atmospheric Environment. 2010;44(8):1108–1115. [Google Scholar]
- Gorse R, Jr, Salmeen I, Clark C. Effects of filter loading and filter type on the mutagenicity and composition of diesel exhaust particulate extracts. Atmospheric Environment (1967) 1982;16(6):1523–1528. [Google Scholar]
- Grimmer G. QUALITY GUARANTEE OF SAMPLING PROCEDURE FOR POLYCYCLIC AROMATIC-HYDROCARBONS. Staub Reinhaltung Der Luft. 1988;48(11):401–404. [Article] [Google Scholar]
- Hori S, Narusawa K. The Influence of Fuel Components on PM and PAH Exhaust Emissions from a DI Diesel Engine-Effects of Pyrene and Sulfur Contents. SAE TRANSACTIONS. 2001;110(4):2386–2392. [Google Scholar]
- ISO . ISO-DIS 16183: Heavy duty engines -- Measurement of gaseous emissions from raw exhaust gas and of particulate emissions using partial flow dilution systems under transient test conditions. International Organization for Standardization; 2009. [Google Scholar]
- Khalek IA, Bougher TL, Merritt PM, Zielinska B. Regulated and unregulated emissions from highway heavy-duty diesel engines complying with US Environmental Protection Agency 2007 emissions standards. Journal of the Air & Waste Management Association (1995) 2011;61(4):427. doi: 10.3155/1047-3289.61.4.427. [DOI] [PubMed] [Google Scholar]
- Kleeman MJ, Riddle SG, Robert MA, Jakober CA. Lubricating oil and fuel contributions to particulate matter emissions from light-duty gasoline and heavy-duty diesel vehicles. Environmental Science & Technology. 2007;42(1):235–242. doi: 10.1021/es071054c. [DOI] [PubMed] [Google Scholar]
- Kleiman G, Graham J, Savelli E, Bowen RS. Review of Speciation Trends Network and IMPROVE Chemically Speciated Data. Northeast States for Coordinated Air Use Management; Boston, MA: 2003. [Google Scholar]
- Kloster G, Niehaus R, Stania H. Storage stability of polycyclic aromatic hydrocarbons collected from ambient air using solid supports. Fresenius’ journal of analytical chemistry. 1992;342(4):405–408. [Google Scholar]
- Lea-Langton A, Li H, Andrews G. Comparison of particulate PAH emissions for diesel, biodiesel and cooking oil using a heavy duty DI diesel engine. SAE Technical Paper. 2008:01–1811. [Google Scholar]
- Logan DT. Perspective on Ecotoxicology of PAHs to Fish. Human and Ecological Risk Assessment: An International Journal. 2007;13(2):302–316. [Google Scholar]
- Neff JM. Polycyclic Aromatic Hydrocarbons in The Aquatic Environment. Sources, Fates and Biological Effects. Applied Science Publishers Ltd.; London, UK: 1979. [Google Scholar]
- Oda J, Yasuhara A, Matsunaga K, Saito Y. Stability of polycyclic aromatic hydrocarbons and their oxygenated derivatives during various storage. Japanese Journal of Toxicology and Environmental Health. 1998;44(5):352–363. [Article] [Google Scholar]
- OFR . Appendix A: priority pollutants. Vol. 47. Office of the Federal Registration; Federal Register: 1982. p. 52309. [Google Scholar]
- Prince R, Walters C, Wang Z, Stout S. Biodegradation of oil hydrocarbons and its implications for source identification. Oil Spill Environmental Forensics. 2007:349–379. [Google Scholar]
- Ratcliff MA, Dane AJ, Williams A, Ireland J, Luecke J, McCormick RL, et al. Diesel Particle Filter and Fuel Effects on Heavy-Duty Diesel Engine Emissions. Environmental Science & Technology. 2010 doi: 10.1021/es1008032. [DOI] [PubMed] [Google Scholar]
- Rost H, Loibner AP, Hasinger M, Braun R, Szolar OHJ. Behavior of PAHs during cold storage of historically contaminated soil samples. Chemosphere. 2002;49(10):1239–1246. doi: 10.1016/s0045-6535(02)00497-6. [DOI] [PubMed] [Google Scholar]
- Salmeen IT, Pero AM, Zator R, Schuetzle D, Riley TL. Ames assay chromatograms and the identification of mutagens in diesel particle extracts. Environmental Science & Technology. 1984;18(5):375–382. doi: 10.1021/es00123a017. [DOI] [PubMed] [Google Scholar]
- Schantz MM, Porter BJ, Wise SA. Stability of Polycyclic Aromatic Hydrocarbons in Frozen Mussel Tissue. Polycyclic Aromatic Compounds. 2000;19(1):253–262. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Environmental Science & Technology. 1999;33(10):1578–1587. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 5. C1-C32 organic compounds from gasoline-powered motor vehicles. Environmental Science & Technology. 2002;36(6):1169–1180. doi: 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- Sharp CA, Howell S, Jobe J. The Effect of Biodiesel Fuels on Transient Emissions From Modern Diesel Engines-Part I: Regulated Emissions and Performance. SAE Technical Paper 2000-01-1967. 2000a.
- Sharp CA, Howell S, Jobe J. The Effect of Biodiesel Fuels on Transient Emissions from Modern Diesel Engines, Part II Unregulated Emissions and Chemical Characterization. SAE Technical Paper 2000-01-1968. 2000b.
- Sverdrup GM, Buxton BE, Chuang JC, Casuccio GS. Determination of optimal storage conditions for particle samples. Environmental Science & Technology. 1990;24(8):1186–1195. [Google Scholar]
- Tanaka S, Takizawa H, Shimizu T, Sanse K. Effect of fuel compositions on PAH in particulate matter from DI diesel engine. SAE TRANSACTIONS. 1998;107:1941–1951. [Google Scholar]
- Vaessen HAMG, van de Kamp CG, Jekel AA. Preparation and stability of ampouled polycyclic aromatic hydrocarbon solutions. Zeitschrift für Lebensmitteluntersuchung und-Forschung A. 1988;186(4):308–310. doi: 10.1007/BF01027032. [DOI] [PubMed] [Google Scholar]
- Zinbo M, Korniski TJ, Weir JE. Relationship between the composition of engine particulate emissions and emission control system performance. Industrial & engineering chemistry research. 1995;34(2):619–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.