Abstract
Background
Alcohol use, abuse and dependence remain a pressing public health problem. Based on its mechanism of action, varenicline seemed to be a likely candidate for treating alcohol dependence.
Methods
Alcohol dependent subjects (n = 40) were enrolled in a 13-week double-blind placebo controlled clinical trial. Subject visits were once per week. At each visit, subjects were tested for breath alcohol levels, provided self-report data on alcohol and nicotine use, and on mood and craving. In addition, subjects received once a week medical management (MM).
Results
There was no difference between varenicline and placebo treated groups on any of the drinking outcomes. Compared to placebo-treated subjects, varenicline treated subjects had decreased rates of alcohol craving and cigarette smoking, as well as greater mood improvements during the later part of the study (weeks 6-13). In addition, among subjects who were cigarette smokers, those treated with varenicline were significantly less likely to report heavy drinking during the trial.
Conclusions
Although varenicline was not significantly more effective than placebo at reducing drinking during the trial, its effects on alcohol craving and mood suggest that future investigation of the mechanism of action of varenicline, as well as additional clinical studies may be warranted. In particular, the findings regarding the influence of smoking status on heavy drinking among varenicline-treated subjects should be investigated in future studies.
Keywords: treatment, pharmacotherapy, alcohol dependence, nicotine dependence, varenicline
1. INTRODUCTION
Varenicline is a partial agonist for the α4β2 nicotinic acetylcholine receptor subtypes. It has demonstrated efficacy as a treatment for smoking cessation (Oncken et al., 2006; Nides et al., 2006) and relapse prevention (Tonstad et al., 2006). In addition to its partial agonist activity at heteromeric α4β2 nicotinic acetylcholine receptors, varenicline has also been shown to be a full agonist at homomeric α7 nicotinic acetylcholine receptors, which may be key in reducing alcohol withdrawal and craving during early alcohol abstinence, as α7 receptors are implicated in the neural reward circuitry activated by alcohol use (Mihalak et al., 2006; Bowers et al., 2005). Varenicline’s ability to occupy the α7 and α4β2 nicotinic receptors, blocking alcohol’s effects on those receptors, should reduce the euphoric and reinforcing effects of alcohol ingested during varenicline treatment. Support for this comes from the non-specific nAChR antagonist mecamylamine, which attenuates the reinforcing effects of alcohol, reducing alcohol consumption in animal models (Larsson and Engel, 2004; Le et al., 2000). Mecamylamine studies in humans have shown that social drinkers treated with mecamylamine experience less euphoria and stimulating effects from alcohol than normal (Chi and de Wit, 2003) as well as decreases in the reinforcing effects of alcohol, and BAL decreases (Blomqvist et al., 2002). However, as mecamylamine causes autonomic side effects it is an impractical treatment for alcohol dependence.
A promising study examining the impact of varenicline on alcohol self-administration in rats showed a decrease in ethanol self-administration with acute administration of varenicline at doses of both 1 mg/kg and 2 mg/kg (Steensland et al., 2007). As testing of sucrose self-administration showed no decrease with varenicline, varenicline appears to be a highly specific target for alcohol-derived reinforcement. A human laboratory study examining the effects of varenicline on drinking behavior among heavy drinking smokers also showed reduced alcohol self-administration during varenicline treatment, as well as reduced alcohol craving (McKee et al., 2009). Taken together, the findings from these seminal studies make a strong case for testing varenicline clinically for the treatment of alcohol dependence in humans.
2. METHODS
2.1 Subjects
We randomized 40 treatment-seeking participants from the greater metropolitan Philadelphia area to participate in this trial. The University of Pennsylvania Human Investigations Committee (IRB) approved the protocol as well as all print advertisements that were used for recruitment. Subjects provided written informed consent to participate in the trial. Subjects met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for alcohol dependence and reported drinking on at least 12 of the past 30 days. Individuals were excluded from the study if they were dependent on any other substance (except nicotine) or had active and serious medical or psychiatric illness, were taking psychotropic medications or agents that could interact with varenicline, or had abnormal baseline laboratory findings. Pregnant and breastfeeding women were excluded and women of childbearing potential were only randomized if they agreed to use acceptable birth control methods.
2.2 Study Design
The primary study objective was to evaluate the efficacy of varenicline treatment for alcohol dependence based on self-reported use, gathered using the Time-Line Follow Back (TLFB). A screening period (3-4 visits) included a comprehensive medical history, physical examination, clinical laboratory studies, vital signs and a 12-lead electrocardiogram (ECG), and was repeated at the end of the study after discontinuation of study medications. Current alcohol dependence was established with a Structured Clinical Interview for DSM IV (SCID; First et al., 1996), and other psychiatric disorders were ruled out with the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). After screening, eligible patients were randomized to varenicline 2mg/day (n=19), or matching placebo (n=21) for the 12-week treatment course. A research pharmacist generated the allocation sequence, assigned group participation, and was solely aware of the medication assignment code that was only available for emergency access. Research personnel who enrolled, treated, and assessed the patients were unaware of patient assignments. Urn randomization was used to stratify patients across the experimental conditions based on gender, race and cigarette smoking status. The study physician dispensed study medications (varenicline and matched placebo provided by Pfizer for use in this study) on a weekly basis in blister packs that contained a 9-day supply to cover missed visits. Patients were paid $5 for each returned blister pack to facilitate accurate pill counts. Study medications were initiated at .5 mg/day and titrated (0.5mg o.d. for days 1-3, 0.5mg b.i.d. for days 4-7), up to the full dose (1mg b.i.d.) by the end of the first week. Subjects were reduced to 1 mg/day for the final week of medication during the study.
Subjects attended one clinic visit per week and provided breath samples during each visit, which assessed recent drinking. TLFB data on all drinking and other drug use were collected at each visit, as were data on mood, adverse events, concomitant medications, and global improvement. Individual, manual-guided MM was provided once weekly (total of 12 sessions). Safety data was also collected at each visit, including blood pressure, pulse, temperature, body weight, urine testing for other substances and adverse events.
2.3 Outcome Measures, Schedule of Assessments, and Sample Size
The primary measure of efficacy was alcohol use based on self-reported alcohol use collected using the TLFB (Sobell and Sobell, 1995). Our secondary efficacy measures included self-reported smoking behavior; mood as measured by the Hamilton Anxiety Scale (Ham A; Hamilton, 1959) and Hamilton Depression Scale (Ham D; Hamilton, 1976), global improvement as measured by the nurse-rated Clinical Global Impression-Objective Scale (CGI-O; Guy, 1976), and the Clinical Global Impression Scale-Subjective Scale (CGI-S; Guy, 1976), as well as alcohol craving s measured by the Penn Alcohol Craving Scale (PACS; Flannery et al., 1999). Additional clinical and psychosocial characteristics were assessed at baseline and at end of study with the Addiction Severity Index (ASI; McLellan et al., 1992).
2.4 Attendance Contingencies
Subjects were encouraged to attend all visits through use of attendance contingencies. Subjects earned payments on an escalating scale for attendance and completion of all visit requirements.
2.5 Statistical analysis
Baseline measures between the Varenicline and placebo groups were compared using t- tests for continuous variables and χ2-tests for dichotomized variables. The number of sessions attended for each group during the trial was compared by using a t-test. Self-reported drinking results, as gathered by the TLFB, were compared by the generalized estimating equations (GEE; Diggle et al., 1994), using Poisson models for counts of drinking and heavy drinking days, and logistic regression models for absence/presence binary indicators of drinking. In the GEE model, the pre-treatment of the response was included as a covariate, together with the treatment group indicator, and a linear time effect. The two-way interactions between these covariates were considered for inclusion by examining the p-values of regression coefficients for the GEE model. For the GEE model for the drinking outcomes, a compound symmetry structure was used for the working correlation matrix.
3. RESULTS
3.1 Baseline demographics
On the whole, the two study groups, varenicline and placebo, were very similar in demographics and baseline use characteristics (see Table 1). There were more African American subjects in the varenicline group as compared to the placebo group (p = 0.06).
Table 1.
Baseline Demographics
| Varenicline | Placebo | |
|---|---|---|
| Male(%) | 78.9 | 90.5 |
| African American (%) | 57.9 | 28.6 |
| Age (years) | 44.8(12.3) | 48.1(10.5) |
| Days of alcohol use in past 30 days | 18.4(8.8) | 17.6(9.1) |
| $ spent for alcohol in past 30 days | 197(152) | 165(137) |
| Years of alcohol use, lifetime | 18.7(10.7) | 21.2(12.0) |
| ASI Composite Alcohol Score | 0.61(0.16) | 0.60(0.15) |
| ASI Composite Employment Score | 0.52(0.32) | 0.46(0.27) |
| ASI Composite Legal Score | 0.04(0.11) | 0.02(0.08) |
| ASI Composite Family/Social Score | 0.18(0.23) | 0.14(0.20) |
| ASI Composite Psychiatric Score | 0.06(0.10) | 0.08(0.14) |
| ASI Composite Medical Score | 0.16(0.30) | 0.14(0.24) |
3.2 Alcohol Use Results (TLFB)
There were no significant group effects for weekly days of alcohol use (beta=log(rate)=-0.14, exp(beta)=rate=0.87, χ2(1) = 0.18, p=0.67) or for presence/absence of alcohol use (beta=logodds ratio=-0.16, exp(beta)=odds ratio=0.86, χ2(1) = 0.07, p=0.80). The varenicline group had slightly lower numbers of heavy drinking days (Figure 1a; beta=-0.67, exp(beta)=0.51, χ2(1) = 0.2.71, p=0.10), corresponding to the placebo group having an average of 1.95 times more heavy drinking days per week. There was no significant effect for presence/absence of heavy drinking (beta=-0.86, OR=0.42, χ2(1) = 1.98, p=0.16), although the varenicline group was less than half as likely as the placebo group to have heavy drinking in a given week.
Figure 1.
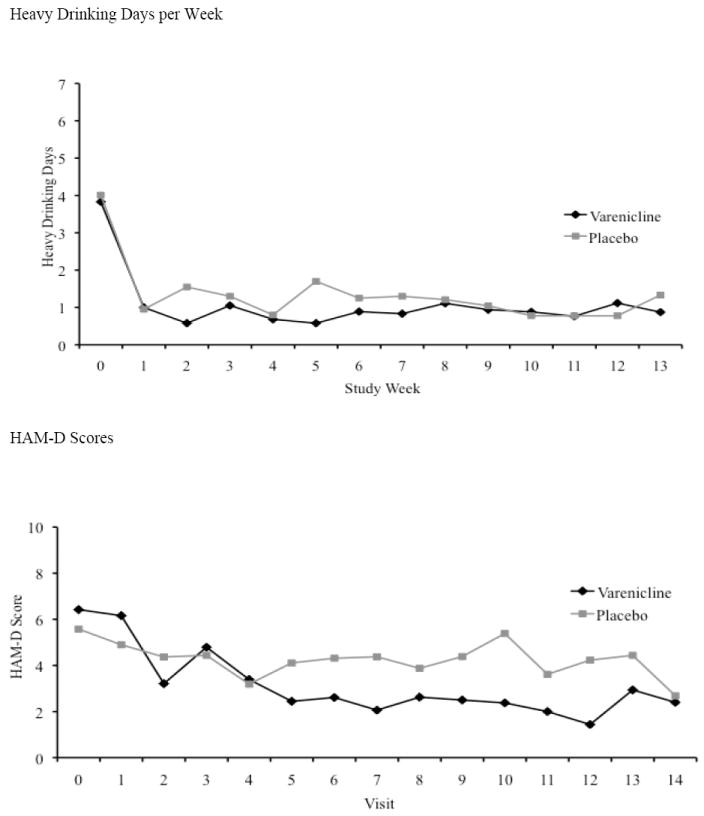

a Heavy Drinking Days
Heavy drinking days per week are shown. Timepoint 0 is baseline, and timepoints 1-13 represent the 12 weeks of treatment and the end of study assessment. Although varenicline treated subjects had lower numbers of heavy drinking days per week, the difference from placebo was not significant.
b HAM-D
Weekly HAM-D scores are shown. Timepoint 0 is baseline, and timepoints 1-14 represent the 12 weeks of treatment, and the end of study and follow-up assessments. Varenicline treated subjects have lower depression scores than placebo treated subjects during visits 5-13.
c PACS Alcohol Craving
Weekly PACS alcohol craving scores are shown. Timepoint 0 is baseline, and timepoints 1-14 represent the 12 weeks of treatment, and the end of study and follow-up assessments. Varenicline treated subjects have lower alcohol craving scores than placebo treated subjects during visits 6-14.
3.3 Cigarette Smoking Results (TLFB)
At baseline smokers in the placebo group (n =8) smoked an average of 15.01 (SD=12.42) cigarettes per smoking day, while the varenicline-treated smokers (n = 9) averaged 13.88 (SD=6.75), with no significant difference between the groups (χ2(1) = 0.03, p=0.87). During treatment, smokers in the placebo group smoked an average of 13.99 (SD=11.57) cigarettes per smoking day while the smokers in the varenicline group smoked an average of 8.79 (SD=7.13), again with no significant difference between the groups (χ2(1) = 1.26, p=0.21). Repeated measures negative binomial models comparing baseline and the treatment phase showed a significant group by time interaction for cigarettes per smoking day (χ2(1) = 4.52, p=0.03), with a greater reduction for the smokers in the varenicline group.
3.4 Effects of Baseline Smoking Status on Treatment Effects on Drinking
We reran the analyses for the four drinking outcomes described above, testing for the main effect of smoking status, and for its interaction with treatment group. There was a significant interaction between baseline smoking status and treatment on the binary absence/presence of heavy drinking outcome (χ2(1) = 3.95, p=0.047): in the group of baseline smokers, the varenicline group was less likely to have heavy drinking (beta=-2.31, exp(beta)=0.01, χ2(1) = 6.20, p=0.01), while in the non-smokers the varenicline group had slightly higher rates of heavy drinking, but the difference was not significant (beta=0.29, exp(beta)=1.34, χ2(1) = 0.14, p=0.71). The interactions between smoking group and treatment group were not significant for the number of drinking days (χ2(1) = 1.94, p=0.16), number of heavy drinking days (χ2(1) = 0.76, p=0.38), and presence of any drinking (χ2(1) = 1.81, p=0.18)
3.5 Results from the Addiction Severity Index
The mean scores (SD) on the ASI Alcohol Composite score at baseline were 0.60 (SD=0.15) for the placebo group and 0.61 (SD=0.16) for the varenicline group, with no significant difference (p = 0.90). At the end of treatment (week 13), the means were 0.25 (SD=0.22) in the placebo group and 0.22 (SD=0.23) in the varenicline group, and at the follow up visit (week 14) were 0.29 (SD=0.23) in the placebo group and 0.12 (SD=0.20) in the varenicline group. The decrease from week 0 to week 14 was significantly greater in the varenicline group than in the placebo group (t(31)=-2.54, p=0.02), but the change from week 0 to week 13 was not significantly different. (p = 0.33).
3.6 Results from Hamilton Depression Scores
The Total 24-item Hamilton Depression Scores measure examines the depression intensity. Depression Scores decreased during the trial in both groups, with varenicline-treated subjects showing significantly lower scores than placebo-treated subjects (p = 0.05). The differences between the two groups come during the second half of the trial (Figure 1b, upper panel).
3.7 Results from PACS
Similar to the HAM-D findings, PACS decreased during the trial in both groups, with vareniclinetreated subjects showing significantly lower scores than placebo-treated subjects (p = 0.01). The differences between the two groups came during the second half of the trial (Figure 1c, bottom panel).
3.8 Side Effects
Overall, the most common side effects were headache, sleep disturbances (vivid dreams, nightmares, insomnia), and GI symptoms (diarrhea, nausea, abdominal cramps, etc.). There were no differences between the two groups on the frequency of side effects as they related to the treatment. In the varenicline group, one PTSD-like episode and one report of anxiety were deemed probably related to study drug. Varenicline appears to have been safe and well-tolerated in these subjects.
4. CONCLUSIONS
This study was a small pilot, and our sole measure of alcohol use and abstinence was self-report via the TLFB. In addition, we tested only a single dose of varenicline, which is the dose used for smoking cessation, the FDA-approved indication for varenicline. The subjects for this study were not very heavy drinkers, and alcohol use in both groups was very low during the trial. Varenicline-treated subjects showed greater smoking reductions, as well as improvements in mood and craving than did placebo-treated subjects. These craving outcomes mirror those found by McKee et al in their human laboratory trial of varenicline (2009). The results of this study suggest that varenicline should be investigated in larger trials with more variability in baseline drinking levels. The findings regarding the influence of smoking status on heavy drinking among varenicline-treated subjects should also be investigated in future studies. As smokers have greater reductions in heavy drinking during varenicline treatment, varenicline may be most beneficial for those with both nicotine and alcohol dependence.
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH Grants P60-DA-005186 and P50-DA-12756. Varenicline and matched placebo provided by Pfizer under IIR # GA3051S6
The NIH and Pfizer had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Dr. Plebani wrote the first draft of the manuscript, managed the literature searches and summaries of previous related work, and directed the data analysis. Drs. Plebani, Kampman, Pettinati and O’Brien designed the study. Dr. Lynch and Mr. Rennert undertook the statistical analysis. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer G. Plebani, University of Pennsylvania Department of Psychiatry
Kevin G. Lynch, University of Pennsylvania Department of Psychiatry
Lior Rennert, University of Pennsylvania Department of Psychiatry.
Helen M. Pettinati, University of Pennsylvania Department of Psychiatry
Charles P. O’Brien, University of Pennsylvania Department of Psychiatry
Kyle M. Kampman, University of Pennsylvania Department of Psychiatry
References
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Kenward MG. Informative dropout in longitudinal data analysis (with Discussion) Appl Stats. 1994;43:43–93. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-IP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–1295. [PubMed] [Google Scholar]
- Guy W. Assessment Manual for Psychopharmacology: Publication ADM 76-338. U.S. Department of Health Education and Welfare; Washington, DC: 1976. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA. New weapon to curb smoking: no more excuses to delay treatment. Arch Intern Med. 2006;166:1547–1550. doi: 10.1001/archinte.166.15.1547. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–20. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Addiction research Foundation; Toronto: 1995. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an {alpha}4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]


