Abstract
Introduction
99mTc-Duramycin is a unique radiopharmaceutical that binds specifically to phosphatidylethanolamine (PE). The current effort is to develop a single-step kit formulation for the 99mTc labeling of HYNIC-Duramycin.
Methods
A titration series of Tricine/TPPTS coligand systems were tested for an optimal formulation to produce 99mTc-Duramycin with high radiochemical purity and specific activity. The radiopharmaceutical prepared using the kit formulation was tested for PE binding specificity using polystyrene microbeads coated with different phospholipid species. Radiochemical performance of the kits was assessed after storage at –20 °C, room temperature and 37 °C. Biodistribution profile of kit-prepared 99mTc-Duramycin was characterized in healthy rats at 3, 10, 20, 60 and 180 min after intravenous injection. Binding studies were performed using the rat aortic arch and a rat model of myocardial ischemia/reperfusion, which represent scenarios of physiological and pathological PE externalization.
Results
A Tricine/TPPTS ratio of 10:1 led to a consistent production of 99mTc-Duramycin with high radiochemical purity (N 90%), whereas a higher ratio at 40:1 produced radiopharmaceuticals with incomplete substitution of Tricine coligand. 99mTc-Duramycin prepared using the single-step kit formulation retained PE-binding specificity. The kits are stable over long-term storage. The biodistribution profile of kit-prepared 99mTc-Duramycin is consistent with HPLC purified radiopharmaceutical from prior studies. Binding studies on a tissue level indicate that the radiopharmaceutical is suitable for studying biological processes that involve PE distribution and redistribution in various physiological and pathological conditions.
Conclusion
A single-step kit formulation is developed for 99mTc-labeling of HYNIC-Duramycin. The radiopharmaceutical has high radiochemical purity and specific activity, retained PE binding activities, amiable to long-term storage, and is injection-ready for in vivo applications.
Keywords: 99mTc-Duramycin, Phosphatidylethanolamine, Kit formulation
1. Introduction
The asymmetric distribution and redistribution of membrane phospholipids are increasingly recognized to play important roles in normal physiology and in pathophysiology of diseases [1,2]. Phosphatidylethanolamine (PE) is the second most abundant phospholipid species in mammalian cellular membranes. Emerging evidence indicates that the dynamics of this phospholipid may be involved in a number of important biological functions, including the membrane reorganization in cytokinesis, apoptosis, and hemostasis [3–6]. In order to examine the distribution and redistribution of this versatile phospholipid on a tissue level, it is critical to establish effective imaging agents that are suitable for routine applications.
In a proof of concept study, we demonstrated the technetium-99 m labeling of Duramycin (99mTc-Duramycin), a natural lantibiotic peptide that binds PE with high affinity and specificity [7,8]. HPLC-purified 99mTc-Duramycin exhibited favorable imaging properties, including a high target-to-background uptake ratio, high stability, fast renal clearance, and low hepatic background [7]. However, up to this point the preparation of injectable dosages of 99mTc-Duramycin necessitates HPLC purification. It would be of importance to attain a single-step kit formulation for producing high quality 99mTc-Duramycin for direct injection without additional purification steps. A number of issues remain to be addressed, including a better understanding in the coligand system, the characterization of radiochemical species, and an optimal formulation for consistent radiopharmaceutical products. Thus, given the potentially wide utility of 99mTc-Duramycin, the aim of the current study was to understand the impact of radiochemical compositions and to develop a single-step radiolabeling kit that produces high radiochemical purity with desired biodistribution and clearance profiles.
2. Materials and methods
2.1. Reagents
Duramycin (90%–95%), SnCl2 (≥99.99%) and tricine (BioUltra, ≥ 95%) were purchased from Sigma-Aldrich. Succinimidyl 6-hydrazinonicotinate acetone hydrazone (HYNIC) (≥90%) was purchased from Solulink. Trisodium triphenylphosphine-3, 3′, 3″-trisulfonate (TPPTS) (93%) was purchased from Strem Chemicals Inc. All other solvents and reagents used were of highest quality available, including acetonitrile (CHROMASOLV Plus, ≥99.9%), trifluoroacetic acid (CHROMASOLV, ≥99.0%).
2.1.1. HPLC method 1
Jupiter C18 column (90 pore size, 250×4.6 mm, Phenomenex) was used with a mobile phase system consisting of acetonitrile and water at a flow rate of 1.0 ml/min at room temperature. A baseline of 5 min at 75% Mobile Phase A (water with 0.1% trifluoroacetic acid, v/v) and 25% Mobile Phase B (acetonitrile with 0.1% trifluoroacetic acid, v/ v) was followed by a linear gradient to 45% Mobile Phase A and 55% Mobile Phase B in 35 min.
2.1.2. HPLC method 2
Jupiter C18 column (90 pore size, 250×4.6 mm) was used with a buffered mobile phase system consisting of Mobile Phase A (10 mM sodium phosphate, pH 6.7) and Mobile Phase B (100% acetonitrile) at a flow rate of 1.0 ml/min at room temperature. A baseline of 5 min at 90% Mobile Phase A and 10% Mobile Phase B was followed by a linear gradient to 10% Mobile Phase A and 90% Mobile Phase B in 30 min. The level of radioactivity in the HPLC elute was monitored by an in-line gamma counter.
2.1.3. HYNIC modification
HYNIC was covalently attached to Duramycin by reacting succinimidyl 6-hydrazinonicotinate acetone hydrazone with Duramycin at a molar ratio of 8:1, in the presence of 4 equivalents of triethylamine. The reaction was carried out in dimethylformamide (DMF) overnight at room temperature with gentle stirring. Monoconjugated HYNIC-Duramycin was purified using two rounds of HPLC purification using method 1. The molecular weight of the final product was confirmed using Matrix-assisted laser desorption/ ionization (MALDI) mass spectrometry. HYNIC-Duramycin has a molecular weight of 2188 Da and the de-protected species is 2148 Da.
2.2. Radiolabeling studies
2.2.1. Formulation 1 (Tricine alone)
To 100 μg of HYNIC-Duramycin, 300 mg of Tricine was added; with 62.5 μg of SnCl2 in a final volume of 10 ml. Aliquots of 100 μl were made in 2-ml glass vials, and freeze-dried overnight. For radiolabeling (n=3), between 2 and 2.5 mCi of 99mTc-Pertechnetate was added to each vial in a volume of about 0.3 ml. The mixture was heated to 80 °C in a lead-lined heating block for 20 min.
2.2.2. Formulation 2 (Tricine:TPPTS molar ratio 10:1)
To 100 μg of HYNIC-Duramycin, 300 mg of Tricine and 95 mg of TPPTS were added, with 62.5 μg of SnCl2 in a final volume of 10 ml. Aliquots of 100 μl were made in 2-ml glass vials, and freeze-dried overnight. For radiolabeling (n=6), between 2 and 2.5 mCi of 99mTc-Pertechnetate was added to each vial. The mixture was heated to 80 °C in a lead-lined heating block for 20 min.
2.2.3. Formulation 3 (Tricine:TPPTS molar ratio 40:1)
To 100 μg of HYNIC-Duramycin, 300 mg of Tricine and 23.7 mg of TPPTS were added, with 62.5 μg of SnCl2 in a final volume of 10 ml. Aliquots of 100 μl were made in 2-ml glass vials, and freeze-dried overnight. For radiolabeling (n=3), between 2 and 2.5 mCi of 99mTc-Pertechnetate was added to each vial. The mixture was heated to 80 °C in a lead-lined heating block for 20 min.
2.2.4. Formulation 4 (Scale up)
To prepare a scaled up kit formulation, to 150 μg of HYNIC-Duramycin, 300 mg of Tricine and 95 mg TPPTS were added, with 62.5 μg of SnCl2 in a final volume of 5 ml. Aliquots of 500 μl were made in 2-ml glass vials, and freeze-dried overnight. For radiolabeling (n=6), 30 mCi of 99mTc-Pertechnetate in 0.5 ml was added to each vial. The mixture was heated to 80 °C in a lead-lined heating block for 20 min.
2.3. Stability tests
The radiolabeling kits from Formulation 2 were stored at –20 °C for more than 300 days. Sixteen kits were stored at room temperature for 7 days. Two kits were stored at 37 °C in a heating block for 7 days. In due course, each vial was labeled with 2 to 2.5 mCi 99mTc-Pertechnetate and heated at 80 °C for 20 min. The radiolabeling mixture was then subjected to radioHPLC analysis according to Method 2.
2.4. Binding assay
Polystyrene beads (2 micron from Polysciences, Inc, Warrington, PA) were coated using phosphatidylcholine (PC), PC/PE at 50/50, or PC/phosphatidylserine (PS) at 50/50. The coating process was conducted in ethyl alcohol with phospholipids dissolved at a concentration of 5 mg/ml. Coated beads were blocked using 1% BSA in phosphate buffered saline (PBS) (w/v). Binding assays were performed at room temperature, by incubating microbeads coated with various phospholipid species in the presence of 99mTc-Duramycin (Formulation 2, 0.1 mCi each). After 10 min incubation, microbeads were collected by centrifugation at 2000 g for 2 min. The microbeads were washed twice by gently resuspending in 1 ml of PBS for 2 min followed by centrifugation at 2000 g for 2 min. Radioactivity associated with microbeads in the final pellet was measured using a well counter equipped with a NaI(Tl) scintillator (Model 243, Ludlum, Measurements, Inc, Sweetwater, Texas) with energy window set at 140±15 keV.
2.5. Biodistribution study
Duramycin was radiolabeled using Formulation 2. Each Sprague Dawley rat (n=4) received a single injection via the tail vein at a dosage of 0.1 mCi. At designated time points, including 3, 10, 20, 60 and 180 min, a group of 4 rats was sacrificed. Various tissues were dissected, rinsed in saline, weighed and the amount of radioactivity was determined using gamma counting (140±15 keV) in a Ludlum Model 243 well counter with a NaI(Tl) scintillator.
2.6. Ex vivo vascular study
In prior studies, it was reported that PE may be exposed, in a physiological fashion, on the luminal surface of endothelial cells in well defined regions of major arteries such as the flow dividers [9,10]. In this study, the binding of 99mTc-duramycin was tested using the aorta of the rat which was dissected and rinsed. A solution of 99mTc-Duramycin (Formulation 2, 0.2 mCi) in Hanks Balanced Salt Solution (HBSS) was infused into the aorta for 3 min at 3 ml/min using a syringe pump. After washing with 10 ml of HBSS, the tissue was frozen in liquid nitrogen. Cryo-sections (20 micron) of the aortic arch were prepared and mounted on glass slides. The issue sections were air-dried for approximately 1 h, then gently covered using a plastic wrap and firmly pressed against a storage phosphor screen (GE Healthcare, Piscataway, NJ). After exposure, digital autoradiographs were obtained using a Cyclone scanner (Perkin Elmer, Waltham, MA).
2.7. Planar imaging of acute cardiac cell death
A rat model of myocardial ischemia and reperfusion was used to create a pathological scenario, where there is a high level of PE externalization [11]. Sprague–Dawley rats (male, 200–250 g, n=4) were anesthetized with sodium pentobarbital (50 mg/kg body weight) intraperitoneally. After tracheotomy and intubation, respiration was maintained using a rodent ventilator. The proximal left anterior descending coronary artery (LAD) was occluded using a 6.0 suture just below the left atrial appendage. For sham operation, the suture was only passed underneath the LAD without ligation. The presence of acute ischemia/reperfusion was confirmed by the pale appearance in the area-at-risk region. The coronary ligation (30 min) was followed by reperfusion. The chest wall was then closed by suturing in layers and ventilation was maintained until the rat could regain spontaneous respiration. 99mTc-Duramycin (Formulation 2, 1 mCi) was injected via the tail vein at 2 h after reperfusion. Static whole-body anterior planar imaging was performed at 40 min after injection using an XRT gamma camera (General Electric) with an energy window at 140±15 keV, matrix size 128×128, field of view 22.5×22.5 cm, with 1 million counts. Sham operated animals were imaged using the identical protocol.
3. Results
3.1. HYNIC modification
According to HPLC chromatogram, the yield of mono-conjugated HYNIC-Duramycin was about 55%. In aqueous environment, the hydrazone of HYNIC is de-protected spontaneously by losing a molecule of acetone. This process was monitored by HPLC and confirmed using Mass Spectrometry, where the hydrazone form of HYNIC-Duramycin was validated.
3.2. Radiolabeling studies
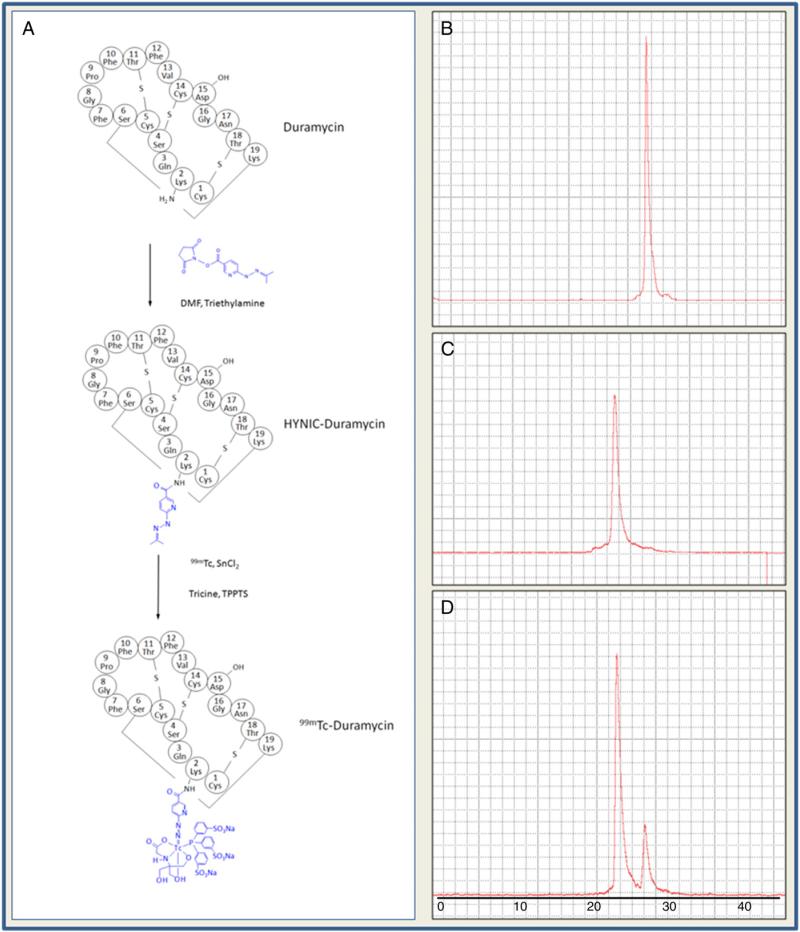
To understand the impact of Tricine-to-TPPTS ratios on the radiochemistry for labeling HYNIC-Duramycin, a titration series were prepared as individual kits with Tricine alone, Tricine:TPPTS molar ratio 10:1, and Tricine:TPPTS molar ratio 40:1. According to radioHPLC chromatograms, Tricine alone as coligand resulted in a single peak at 26 min (Fig. 1). When Tricine-to-TPPTS ratio is at 10:1, a single radiological peak appeared at 22 min with radiochemical purity greater than 90% (94.6%±2.2%, n=6) (Fig. 1). When a higher Tricineto-TPPTS content (40:1) is used, two peaks were generated at 22 and 26 min respectively (Fig. 1). Presumably, the latter species is composed of Tricine coligand alone as a result of incomplete TPPTS substitution. The radiochemical properties of individual studies from each formulation are summarized in Table 1.
Fig. 1.
Radiochemistry. A, schematic drawing of HYNIC modification of Duramycin, followed by 99mTc-labeling in a Tricine/TPPTS coligand system. B, radiochromatogram of 99mTc labeled HYNIC-Duramycin using Tricine alone as coligand. C, radiolabeling with Tricine/TPPTS at 10:1 molar ratio. D. radiolabeling with Tricine/TPPTS at 40:1 molar ratio.
Table 1.
Radiochemical properties of labeling products using different radioligand compositions.
| Radioligand composition | Radiochemical purity | Number of radiochemical species | Specific activity (MBq/μg) |
|---|---|---|---|
| Tricine alone | 96.6±1.7% (n = 3) | 1 | 90.1 ±2.8 |
| Tricine:TPPTS 10:1 | 94.6±2.0% (n = 6) | 1 | 89.9 ±1.9 |
| Tricine:TPPTS40:1 | 95.4±0.9% (n = 3) | 2 | 90.6 ±0.9 |
| Scale up | 95.8±1.2% (n = 3) | 1 | 91.4± 1.1 |
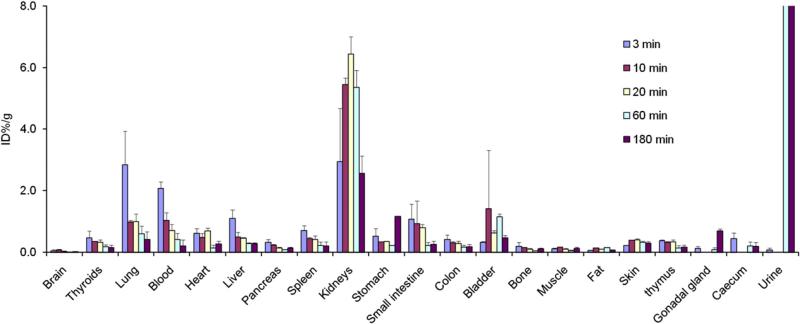
Combining the above results, we determined that an optimal formulation for the 99mTc-labeling of HYNIC-Duramycin is as detailed in Formulation 2 (Tricine:TPPTS molar ratio 10:1). At the current formulation, the specific activity of the radiolabeled product is at 89.9 ±2.0 MBq/μg (n=6) peptide. According to binding studies using phospholipid-coated microbeads, radiolabeled Duramycin retained its binding specificity, where greater than half of radioactivity bound to PE-coated microbeads (53.9%±9.1%, n=3), whereas that associated with PC or PS-coated microbeads was near background. Quantitative biodistribution studies indicate that the behaviors of this radiopharmaceutical, as a product of a single-step labeling kit, were consistent with the original radiopharmaceutical that was refined after HPLC purification (Fig. 2) [7]. More specifically, at 3 min after intravenous injection, the level of radioactivity in perfusion-rich tissues, including the lungs (2.84%ID/g), myocardium (0.62%ID/g), liver (1.10%ID/g), spleen (0.72%ID/g), was significant. The presence of radioactivity mainly reflected blood pool signal, which rapidly receded with time. Radioactivity in the blood was 2.08, 1.03, 0.72, 0.42 and 0.21%ID/g at 3, 10, 20, 60 and 180 min after injection, respectively. Within 60 min after injection, the values were decreased to 0.60%ID/g (lung), 0.14%ID/g (myocardium), 0.28%ID/g (liver), and 0.21%ID/g (spleen). The radiopharmaceutical efficiently cleared from the renal/urinary system. The uptake in other tissues and organs was relatively low, including the brain, pancreas, gastrointestinal tract, bone, muscle, fat and skin.
Fig. 2.
Biodistribution. Tissue uptake of 99mTc-Duramycin prepared using Formulation 2 (Tricine/TPPTS 10:1), at 3, 10, 20, 60 and 180 min after intravenous injection in healthy rats (n=4 each time point).
In a scaled-up study, 15 μg of HYNIC-Duramycin is labeled with 30 mCi of 99mTc. The radiopharmaceutical retained the radiochemical purity (94.3%±2.7%, n=5) and specific activity (89.6±2.6 MBq/μg, n=5) in a single-step labeling kit. The labeled radiopharmaceutical can be sterile filtered using 0.22 μm membranes consisting of either mixed cellulose ester membrane (CEM) or polyvinylidene fluoride (PVDF). No significant retention on the filter membranes was detected after sterile filtration.
3.3. Stability tests
The radiolabeling kit (Formulation 2) remained stable at the latest testing point (more than 300 days storage at –20 °C). This was demonstrated by a lack of significant changes in radiochemical purity (94.3%±2.7%, n=5) (Table 2). Additionally, short-term storage at room temperature or 37 °C did not adversely affect the quality of the kits, with radiochemical purity at 97.1% ± 2.0%, (n = 3) and 93.8%±1.4, respectively.
Table 2.
Radiochemical properties of labeled product after storage at different conditions.
| Storage condition and duration | Radiochemical purity (%) | Specific activity (MBq/μg) |
|---|---|---|
| – 20 °C, > 300 days | 94.3 ±2.7 (n = 5) | 89.6 ±2.6 |
| Room temperature, 7 days | 97.1 ±2.0 (n = 3) | 92.3 ± 1.9 |
| 37 °C, 7 days | 93.8 ±1.4 (n = 3) | 89.1 ± 1.4 |
3.4. Ex vivo vascular study
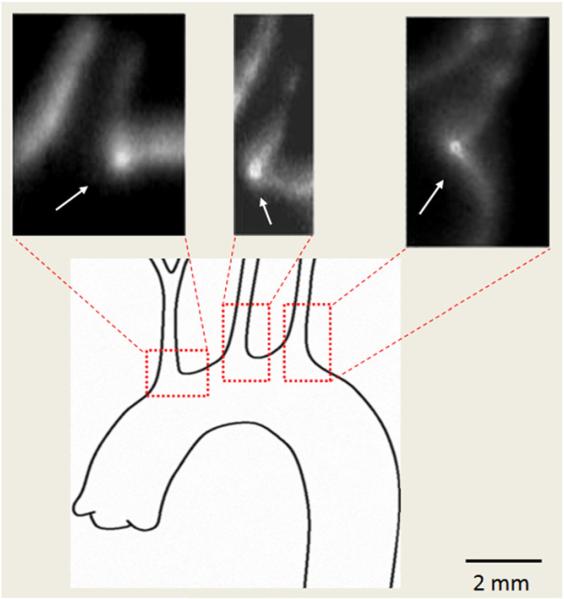
Consistent with prior findings, intense radioactivity uptake was present at flow dividers that bifurcate the blood flow in the rat aortic arch (Fig. 3) [9,10]. Even though higher energy isotopes such as Tc-99 m with a 140 keV energy result in poorly defined autoradiographs, but it is apparent that signals from all three flow bifurcation regions were well above background. These data demonstrate the binding of 99mTc-Duramycin to physiologically exposed PE at the luminal surface of the endothelium.
Fig. 3.
Ex vivo vascular binding. Autoradiographs demonstrate the focal binding of 99mTc-Duramycin at flow bifurcations in cryosections of the rat aortic arch (arrows).
3.5. Planar imaging of acute cardiac cell death
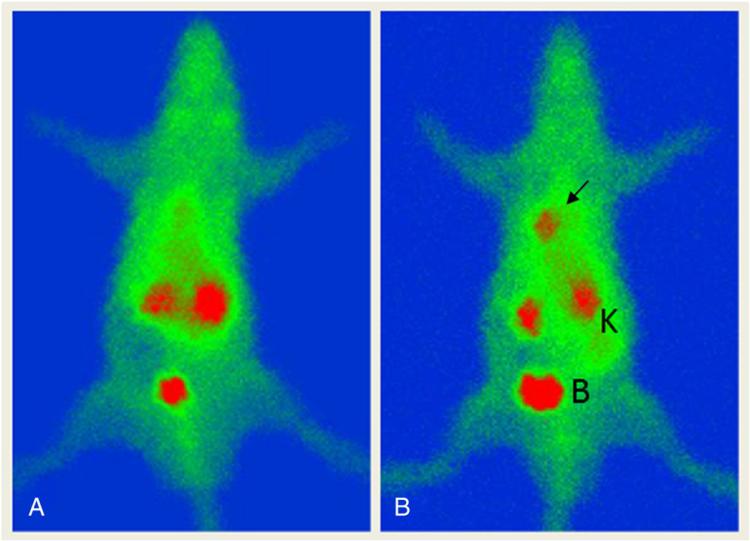
Prominent radioactivity uptake is seen at the infarct site, where there is a high level of pathologically-induced PE (Fig. 4). Alternatively, no significant signal is present in the heart region of sham operated animal. The radiotracer is cleared mainly via the renal/urinary tract, whereas the general background is relatively low, including the hepatic region. The biodistribution profile and clearance pattern are consistent with quantitative measurements made by gamma counting using dissected tissues (Fig. 2).
Fig. 4.
Whole-body planar imaging of cardiac cell death using 99mTc-Duramycin prepared from a single-step kit formulation (Tricine/TPPTS 10:1). There is prominent focal uptake of 99mTc-Duramycin in cardiac tissues damaged by ischemia and reperfusion (arrow), whereas no such binding occurs in sham operated animal (left). The radiotracer clears via the renal/urinary system with low systemic background. Kidneys and the bladder are marked by “K” and “B”, respectively.
4. Discussion
The current study establishes a robust and practical kit formulation for 99mTc-labeling of HYNIC-Duramycin. The benefits of this kit formulation include: 1) single-step preparation; 2) high radiochemical purity and specific activity; and 3) stable for a minimum of 300 days at room temperature, a minimum of 7 days at room temperature and a minimum of 7 days at 37 °C, allowing long-term storage and wide spread distribution.
There are two solvent-exposed primary amines on Duramycin, with one at Lys-2 and the other at the N-terminal. Accordingly, two mono-conjugated HYNIC-Duramycin species should be generated. However, due to the close physiochemical properties, the two monoconjugated chemical species could not be separated using the current chromatographic conditions.
In terms of radiochemistry, we examined the range of coligand concentrations in order to better characterize the dynamics of the chelation system for the 99mTc-labeling of HYNIC-Duramycin [7,12]. According to radioHPLC chromatograms, we determined that a TPPTS concentration, at a molar ratio of 10:1 (tricine:TPPTS), favors a consistent substitution of tricine coligand. The radiopharmaceutical produced using the single-step formulation retained PE binding specificity, while its in vivo biodistribution and clearance properties were in agreement with the radioHPLC-purified preparation in prior studies [7].
In scale-up experiments, the data indicate that the radiopharmaceutical preparation can be adapted to a clinical dose level without compromising its radiochemical purity or specific activity, while maintaining its single-step labeling process. Stability studies demonstrated that the kits retained their radiolabeling properties after long-term storage. The radiolabeled product can be sterile filtered with commonly used syringe filters (CEM, PVDF). Additionally, this formulation is sufficiently stable for room temperature handling such as during shipment or in transit.
Growing evidence indicates that the dynamics of phospholipid membrane are fundamentally important. However, to date, the underlying biological mechanism that regulates the trans-bilayer flip-flop of phospholipids remains largely unknown. The trans-bilayer movement of major phospholipid species, such as PE, is likely to play vital roles in physiological settings, including cytokinesis, cell mobility, coagulation, and sperm capacitation [3–6]. To this end, the current data provide further evidence on the potential physiological distribution of PE at the luminal endothelial surface [9,10]. In pathological scenarios, the redistribution of membrane phospholipids can take place in thrombotic events and/or cell death. Likewise, we demonstrate that the radiopharmaceutical prepared using the single-step kit formulation is suitable for diagnostic imaging applications for tissue degeneration. Ultimately, the availability of phospholipidspecific imaging agents will aid the characterization of membrane dynamics, which will contribute to the understanding of fundamental membrane biology and to clinical translation.
5. Conclusions
Overall, a single-step labeling kit for 99mTc-Duramycin is an enabling technical development, which will allow the distribution and applications of this imaging agent in a broad range of imaging studies.
Acknowledgments
The authors would like to thank Xiaoguang Zhu, MD, for his technical assistance. Funding support from the National Institute of Health (1R01HL102085) is gratefully appreciated.
References
- 1.Yamaji-Hasegawa A, Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. 2006;29:1547–53. doi: 10.1248/bpb.29.1547. [DOI] [PubMed] [Google Scholar]
- 2.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, et al. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci U S A. 1996;93:12867–72. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maulik N, Kagan VE, Tyurin VA, Das DK. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes reperfusion-induced apoptosis. Am J Physiol. 1998;274:H242–8. doi: 10.1152/ajpheart.1998.274.1.H242. [DOI] [PubMed] [Google Scholar]
- 5.de Vries KJ, Wiedmer T, Sims PJ, Gadella BM. Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod. 2003;68:2122–34. doi: 10.1095/biolreprod.102.012500. [DOI] [PubMed] [Google Scholar]
- 6.Smirnov MD, Safa O, Regan L, Mather T, Stearns-Kurosawa DJ, Kurosawa S, et al. A chimeric protein C containing the prothrombin Gla domain exhibits increased anticoagulant activity and altered phospholipid specificity. J Biol Chem. 1998;273:9031–40. doi: 10.1074/jbc.273.15.9031. [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Li Z, Bugenhagen S. 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J Nucl Med. 2008;49:1345–52. doi: 10.2967/jnumed.107.048603. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi F, Nagashima K, Terui Y, Kawamura Y, Matsumoto K, Itazaki H. The structure of PA48009: the revised structure of duramycin. J Antibiot (Tokyo) 1990;43:1421–30. doi: 10.7164/antibiotics.43.1421. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Wells CW, Esmon CT, Zhao M. Phosphatidylethanolamine at the endothelial surface of aortic flow dividers. J Thromb Haemost. 2009;7:227–9. doi: 10.1111/j.1538-7836.2008.03193.x. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Wells CW, North PE, Kumar S, Duris CB, McIntyre JA, et al. Phosphatidylethanolamine at the luminal endothelial surface—implications for hemostasis and thrombotic autoimmunity. Clin Appl Thromb Hemost. 2011;17:158–63. doi: 10.1177/1076029609350620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post JA, Verkleij AJ, Langer GA. Organization and function of sarcolemmal phospholipids in control and ischemic/reperfused cardiomyocytes. J Mol Cell Cardiol. 1995;27:749–60. doi: 10.1016/0022-2828(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DS, Liu S, Barrett JA, et al. New and versatile ternary ligand system for technetium radiopharmaceuticals: water soluble phosphines and tricine as coligands in labeling a hydrazinonicotinamide-modified cyclic glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconjug Chem. 1997;8:146–54. doi: 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]