Abstract
Background
Despite having the highest disease burden of HIV, Sub-Saharan Africa has limited data on HIV related kidney disease with most available data coming from the developed countries. Kidney disease is a recognised complication in HIV infected patients presenting with acute renal failure (ARF) or chronic kidney disease (CKD). This study investigated the prevalence and risk factors associated with renal dysfunction among hospitalised HIV infected patients at the University Teaching Hospital (UTH), Lusaka.
Methodology
We conducted a cross sectional study at the University Teaching Hospital Lusaka, in Zambia. Inclusion criteria were hospitalised patients aged 16years and above who consented to the study. Both HIV infected and uninfected patients were included in the study. After obtaining demographic information, study participants were screened for HIV upon their consenting for the test. A full clinical history and examination was done by study physician to determine factors associated with renal dysfunction.
Results
Of the 300 recruited hospitalised patients in this cross sectional study, 142(47%) were HIV infected. We observed a high prevalence of renal dysfunction among hospitalised HIV infected patients compared to uninfected patients (42% vs. 27%, adjusted OR 1.99, 95% CI 1.20–3.28). They had a twofold increased likelihood of developing kidney dysfunction (OR 1.96,95 CI%; 1.21–3.17). The presence of vomiting was strongly associated with renal dysfunction in both HIV positive (AOR 7.77, 95% CI 2.46-24-53) and negative (AOR4.83, 95%CI 1.40–16.66) subgroups. WHO stage III was associated with renal dysfunction in HIV infected patients. Tenofovir use, (a first line antiretroviral drug in Zambia) and hypotension were not significant factors associated with kidney disease after adjusting for other clinical parameters.
Conclusion
Renal dysfunction is significantly higher among hospitalised HIV infected compared to uninfected, however tenofovir and hypotension were not associated with renal dysfunction.
INTRODUCTION
Renal disease disproportionately affects patients living with HIV. Although Sub-Saharan Africa endures over 60% of the world's burden of HIV disease, there is limited data in the region on HIV related kidney disease, with most available data coming from the developed countries1,2. Kidney disease is a recognised complication in HIV infected patients whose presentation can be acute renal failure (ARF) or chronic kidney disease (CKD)3.
The few outpatient renal screening studies from Sub-Saharan Africa describe varying prevalence ranging from 6% to almost 50%.4 A study in Lusaka described a prevalence of renal dysfunction of 34% among HIV infected outpatients commencing Highly Active Antiretroviral Treatment (HAART).5 In hospitalised HIV infected patients, prevalence may be up to 10 times higher and carries high mortality4. This variability can be explained by differences in criterion employed in defining kidney dysfunction, study designs used and the subject population studied. This study investigated the prevalence and factors associated with renal dysfunction among hospitalised HIV infected and uninfected patients.
RESEARCH DESIGN AND METHODS
This was a cross sectional study conducted at the University Teaching Hospital, Lusaka, Zambia after obtaining approval from the University of Zambia Biomedical Ethics Committee. Enrolment of participants took place from August, 2010 to October, 2010. All consenting hospitalised patients at Adult Filter Clinic and Phase V medical admission wards were recruited to the study if they were admitted to the UTH medical admission wards, were aged 16 years or older and agreed to participate in the study. Recruitment was from Monday to Friday in the day time and the first ten eligible patients were enrolled to the study per day.
Using a data collection sheet, information on age, race, gender, and marital status was obtained. Study participants were screened for HIV after appropriate counselling and consent to testing. A full clinical history and clinical examination was then performed by study physician to assess factors associated with renal dysfunction. Blood and urine samples were collected to determine renal status. Data collected was then transcribed to a data entry programme on epi info version 3.5.1.dataset.
Inclusion Criteria
Hospitalized patients were eligible for enrolment if they were admitted to the UTH medical admission wards, were 16 years of age or older, and agreed to participate in the study. For the purpose of this study renal dysfunction was defined as rise of serum creatinine 1.5 times the upper limit of normal, i.e. ≥ 180 micromoles per litre, with or without decrement in urine output. (Zambia Laboratory Services/UTH normal values for creatinine is 60–120 micromoles per litre.)
Statistical analysis
Using a prevalence of renal dysfunction of 20% among hospitalized HIV infected patients and 8% among non HIV infected, sample size was calculated using the Pocock's formula. A minimum sample of 141 HIV positive and 141 HIV-negative patients was considered adequate to detect a difference with an alpha of 0.05. HIV positive and uninfected HIV patients were enrolled until there were at least 141 in each group. We used frequency tables to describe both categorical and quantitative variables. Because all continuous variables (other than age) had a non-Gaussian distribution, these variables were described by median and interquartile ranges. Intergroup differences in medians of the variables were compared using Kruskal-Wallis test for non-parametric variables. For categorical variables, frequencies, proportions and percentages were used to discuss participants and chi-square test was used to assess association between the variables. Adjusted odds ratios were calculated by multivariable logistic regression in order to determine factors independently associated with renal dysfunction among HIV infected and HIV uninfected patients. A p value of less than 0.05 was taken as statistically significant.
RESULTS
Baseline characteristics
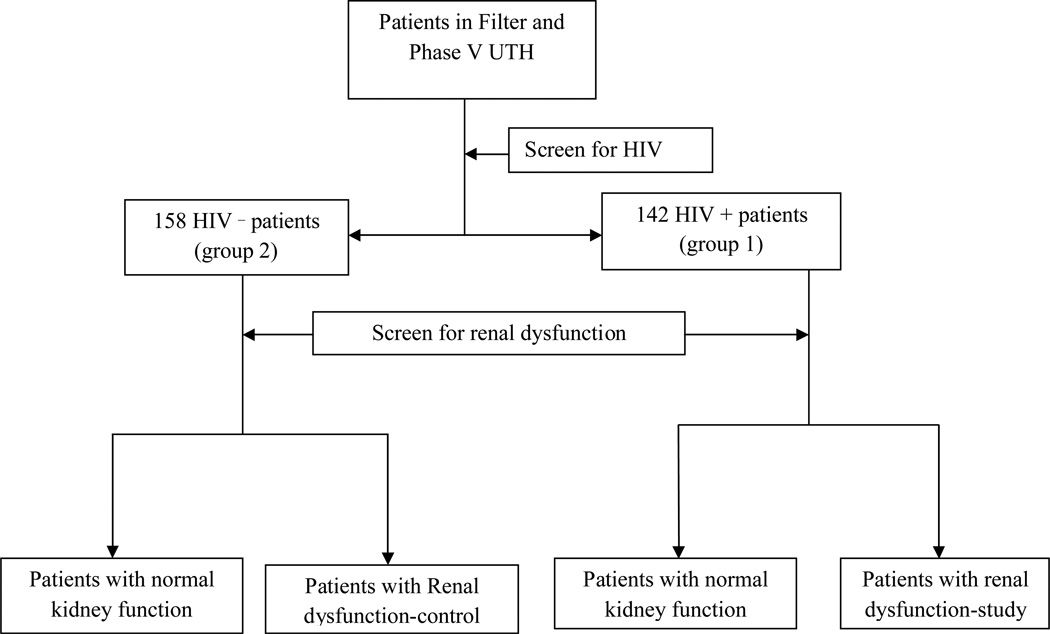
Of the 300 hospitalised patients, 154 (51%) were males. Ages ranged from 16 to 91 years with mean of 40.1 (SD 16.4). 142(47%) tested positive for HIV. See Table 1.
Table 1.
Baseline characteristics of HIV positive and HIV negative subjects
| Total (n=300) |
HIV+ (n=142) |
HIV− (n=158) |
P | |
|---|---|---|---|---|
| Age, mean (SD)* | 40.1 (16.4) | 35.6 (9.5) | 44.1 (20) | 0.005 |
| Male | 154 (51) | 80 (56) | 74 (47) | 0.10 |
| Diarrhea | 39 (13) | 27 (19) | 12 (8) | 0.003 |
| Vomiting | 49(16) | 32(23) | 17(11) | 0.006 |
| Anuria | 13 (4) | 6 (4) | 7 (4) | 0.93 |
| Diabetes | 20 (7) | 4 (3) | 16 (10) | 0.01 |
| Hypertension | 41(14) | 6 (4) | 35 (22) | <0.001 |
| SBP mean (SD)* | 113(30) | 105(17) | 120(36) | <0.001 |
| DBP mean SD | 71(19) | 66(13) | 75(22) | <0.001 |
| Pulse mean (SD) | 95(12) | 97(12) | 93(12) | 0.0038 |
| Temperature mean (SD) | 37(1) | 37(1) | 37(1) | 0.02 |
| MAP <65mmHg | 32(10.7) | 21(14.8) | 11(7) | 0.028 |
| Nephrotoxic drugs | ||||
| NSAIDs | 86(29) | 28(20) | 58(37) | <0.001 |
| ACEI/ARB | 54(18) | 2(1.4) | 52(33) | <0.001 |
| Septrin | 111 (37) | 110 (78) | 1 (0.6) | < 0.001 |
| Tenofovir | 51(17) | 51(35.9) | 0 | <0.001 |
| Amphotericin B | 6(2) | 6(4.2) | 0 | 0.009 |
| Heart failure, | 29(9.7) | 1(0.7) | 28(17.7) | <0.001 |
| CVA, | 19(6.3) | 2(1.4) | 17(10.8) | <0.001 |
| Sepsis, | 91(30.3 | 62(43.7) | 29(18.4) | <0.001 |
| Malignancy, | 16(5.3) | 10(7) | 6(3.8) | 0.212 |
| Anemia <8g/dl | 102(34) | 57(40) | 45(28) | 0.033 |
| Creatinine >180 micromole/l | 103 (34) | 60 (42) | 43 (27) | 0.006 |
Variables expressed as SD otherwise in %
The prevalence of renal dysfunction among HIV positive subjects was 42% compared to 27% among HIV negative participants (AOR 1.99, 95% CI 1.20–3.28). HIV infected patients were younger than the HIV-negative subjects. (35.6 (SD 9.5) vs.44.1 (SD 20.0), p=0.05). HIV negative patients were significantly more likely to have hypertension (22% vs.4%, p<0.001)
Heart failure and CVA were common in HIV negative patients (p<0.001), while sepsis and anemia were more common in HIV infected patients. Regarding nephrotoxic drugs,70% of HIV reactive patients were taking septrin (used for prophylaxis of opportunistic infections) compared to only 0.6% of HIV uninfected patients(p<0.001). Only 3 of the 300(1%) were using herbal medications prior to admission.
Factors associated with renal dysfunction
Of the total 142 HIV positive patients, 2(1.4%) were in stage 1, 86(60%) were in stage III and 54(38%) in stage IV of HIV infection. The most common stage IV conditions were extra pulmonary tuberculosis (EPTB) in 18, kaposi's sarcoma in 9 and cryptococcal meningitis in 8. Interestingly, WHO stage III patients had a higher prevalence of renal dysfunction than those with stage IV HIV disease (AOR 0.18, 95% CI 0.06–0.53). Of the 61patients on HAART, 52 (85.2%) were on tenofovir based regimen, 6(9.8%) on AZT (zidovudine) based regimen and 3(4.9%) on stavudine based regimen. Tenofovir was not associated with renal dysfunction among hospitalised patients (1.03; 0.45–2.37, 95%CI). Other drugs not associated with renal dysfunction were amphotericin B (OR 1.49; 0.26–8.39, 95% CI) and septrin (OR 1.09; 0.49–2.42, 95% CI). See table 2. In multivariable analysis, history of vomiting and WHO stage III were significant characteristics associated with kidney dysfunction among HIV positive participants.
Table 2.
Factors associated with renal dysfunction among HIV positive participants
| Renal Dysfunction N=60 |
No renal dysfunction N=82 |
Crude OR (95% CI) |
|
|---|---|---|---|
| HTN | 2(3.3) | 4(4.9) | 1.48 (0.26–8.39) |
| DM | 2(3.3) | 2(2.4) | 1.37 (0.19–10.8) |
| Diarrhoea | 16(26.7) | 11(13.4) | 2.35 (0.99–5.52) |
| Vomiting | 24(40) | 8(9.8) | 6.17 (2.52–15.1) |
| Nephrotoxic Drugs | |||
| NSAIDS | 12(20) | 16(19.5) | 1.03 (0.45–2.37) |
| Tenofovir | 23(38) | 28(34) | 1.19 (0.59–2.39) |
| Amphotericin | 2(3.3) | 4(4.9) | 1.49 (0.26–8.39) |
| Septrin | 47(78.3) | 63(76.8) | 1.09 (0.49–2.42) |
| CD4 count | * | ||
| >350cell/ml | 5(8) | 10 (12) | 1.0 |
| 200–350 | 5(8) | 10 (12) | 0.68 (0.14–3.07) |
| <200cells/ml | 50(83) | 62 (76) | 1.29 (0.40–4.19) |
| WHO | |||
| III | 43(72) | 43(53) | 1.0 |
| IV | 16(27) | 38(46) | 0.42 (0.20–0.86) |
| Sepsis | 23(38.3) | 39(47.6) | 0.68 (0.34–1.35) |
| Malignancy | 2(3.3) | 8(9.8) | 0.32 (0.06–1.55) |
| MAP < 65 | 14(23.3) | 7(8.5) | 3.26 (1.22–8.67) |
| Anaemia† | 26(43) | 31(37.8) | 1.26 (0.64–2.47) |
Anaemia defined as Hb < 8 g/l
Among uninfected HIV participants, history of hypertension and vomiting and use of ACEI were significant factors associated with the development of kidney dysfunction. Of 54 patients on ACE/ARBs, 52(96%) were HIV uninfected patients with heart failure. Despite being protective in HIV positive patients, only 2(4) HIV infected patients were on ACEI/ARB.
DISCUSSION
Prevalence of renal dysfunction in our study was 42% among hospitalised HIV positive infected patients compared to 27% among uninfected patients. In a few in-patient studies in Africa, renal dysfunction ranged from 17% to almost 30% in HIV positive patients4, 9. This is similar to Lopez at al. study that found 47% renal dysfunction among critically ill hospitalised patients. The subject populations in Lopez's study were critically ill and hence the slight increase in total number of renal dysfunction cases6. Franceschini et al. also found increased renal dysfunction among hospitalised HIV infected in both pre-HAART and post HAART patients with a threefold increased risk of developing kidney disease7, 8, 17
Low mean arterial pressure appeared to be a risk factor for renal dysfunction but after adjusting for other co-variables it was found to be statistically not significant unlike previous studies done in Europe and Africa. This may be explained by the small sample size recruited in our study. In a study by Valerie et al. among hospitalized patients, 38% of renal dysfunction was due to volume depleting conditions. The causes of low mean arterial pressure included sepsis with shock, chronic gastroenteritis and adrenal insufficiency10, 11, 12, 13.
WHO stage III was a significant factor associated with renal dysfunction in our study unlike previous studies that found both stages III and IV as contributing characteristics 5. Other studies found WHO stage IV renal protective similar to our study findings5, 18.This may be explained that patients in Zambia delay in seeking treatment.
More patients on tenofovir based regimen had kidney disease; however this was not statistically significant in our study. This is in keeping with many other studies. Izzedine et al study found no differences in tenofovir based regimen compared to non-tenofovir based regimen8, 18.
Mauss et al. 2005 found renal impairment among patients taking tenofovir compared with patients not receiving tenofovir. Patients on tenofovir had higher proteinuria while their GFR remained the same. Cohort and case control studies found that renal failure associated with tenofovir was not more common than with other ARV regimens13, 14, 15. The small sample size of patients on tenofovir in our study and criterion used in defining kidney dysfunction may also explain the difference.
In our study, a CD4 count below 200 cells was not associated with renal dysfunction among hospitalised HIV infected patients unlike previous studies. In other studies, a low CD4 count was a predictor of renal dysfunction and likelihood of kidney dysfunction increased with CD4 count <100 cells/mm14,15. Limited sample size of HIV hospitalised patients in our study may as well explain the difference.
History of hypertension was a significant factor for development of renal dysfunction among hospitalised HIV uninfected patients similar to other studies in Europe and Africa10. The likelihood of developing renal dysfunction among hypertensive patients was almost five fold after adjusting for other co-variable. Hypertension is both a cause and complication of renal dysfunction and determines prognosis16. HIV infection was associated with reduced prevalence of hypertension in our study similar to other studies19, 21, 22. Primary kidney disease is responsible for almost 4% hypertension in the general populations16, 19, 20.
Vomiting was strongly associated with kidney disease in both HIV positive and HIV negative patients. Vomiting and nausea are common clinical presentations of kidney disease10. It is likely that the vomiting in our patients was a sign of uremia and not a primary cause of kidney dysfunction. Based on our findings, renal dysfunction should be suspected in any adult patients presenting to the hospital with vomiting particularly if the patient is HIV positive. Our study has several limitations. Cross sectional studies by their nature are unable to measure risk nor confirm causal relationship among variables. We did not have data regarding patient's baseline renal function, so we were unable to distinguish between acute kidney injury from chronic kidney disease.
CONCLUSION
HIV infection is associated with increased prevalence of renal dysfunction in hospitalised patients. The presence of vomiting is strongly associated with renal dysfunction in HIV positive and negative patients. Hypertension and ACE inhibitor usage were associated with renal dysfunction in uninfected HIV patients.
Figure 1.
Patient enrolment
Table 3.
Factors associated with renal dysfunction in HIV+ multivariable logistic regression
| Term | Odds Ratio | 95%CI |
|---|---|---|
| AGE* | 1.02 | (0.97–1.06) |
| Amphoterin B | 3.60 | (0.43–30.19) |
| CD4 <200 | 0.46 | (0.09–30.19) |
| CD4 200–350 | 0.52 | (0.14–1.98) |
| CD4 >300 | 1 | |
| Diarrhoea | 0.48 | (0.12–1.91) |
| Tenofovir | 2.13 | (0.39 –11.39) |
| MAP<65mmHg | 2.50 | (0.73–8.58) |
| NSAIDS | 1.94 | (0.69–5.46) |
| Sepsis | 0.26 | (0.09– 0.70) |
| Vomiting | 7.77 | (2.46–24.53) |
| WHO Stage III | 1 | |
| WHO Stage IV | 0.18 | (0.06–0.53) |
| Any HAART | 0.49 | (0.09–2.55) |
OR for age is the increasing odds per year of life.
REFERENCES
- 1.UNAIDS. On this day World AIDS day in 2008/20years after the first one/several milestones have been reached. World AIDS day report. 2009 2009. [Google Scholar]
- 2.Wools-Kaloustian Will there be an epidemic of HIV related chronic kidney disease in Sub-Saharan Africa? Kidney international. 2008:74–845. doi: 10.1038/ki.2008.326. [DOI] [PubMed] [Google Scholar]
- 3.Fabian J, Naicker S. HIV Infection and the kidney. Southern African Journal HIV medicine. 2008:9–12. [Google Scholar]
- 4.Fabian J, Naicker S. Nat.Rev.Nephrol. 2009;5:591–598. doi: 10.1038/nrneph.2009.141. [DOI] [PubMed] [Google Scholar]
- 5.Mulenga B, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV infected HIV adults on antiretroviral therapy in Lusaka, Zambia. AIDS. 2008;22:1–7. doi: 10.1097/QAD.0b013e328307a051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez JA, Fernandez J, Jorge S, et al. Acute renal failure in critically ill HIV-Infected patients. Crit Care. 2007;11(1):404. doi: 10.1186/cc5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzedine H, Mariane H, Parazella M. The Nephrotoxic effects of HAART. Rev.Nephrol. 2009;5:563–573. doi: 10.1038/nrneph.2009.142. [DOI] [PubMed] [Google Scholar]
- 8.Rao Fredman EA. Outcome of severe renal failure in patients with Acquired Immune deficiency Syndrome. Am J Kidney Dis. 1995;25:390–398. doi: 10.1016/0272-6386(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 9.Emem C, Arogundade F, Sanusi K, et al. Renal diseases in HIV Seropositive patients in Nigerian: an assessment of prevalence, clinical features and risk factors. Nephrol.Dial.Transplant. 2008;23:741–746. doi: 10.1093/ndt/gfm836. [DOI] [PubMed] [Google Scholar]
- 10.Asudani D, Patel R, Corser J. Renal Diseases in HIV Infection. Internet Journal of Internal Medicine. 2004;5(1) [Google Scholar]
- 11.Perazella Mark. Acute Renal Failure with HIV infection: Hospital physician. 2001:30–41. [Google Scholar]
- 12.Valeri A, Neursy AJ. Acute and chronic renal disease in hospitalised AIDS patients. Clin Nephrol. 1991;35:110–118. [PubMed] [Google Scholar]
- 13.Gitman MD, Hirscherick D, Beskin CH, et al. Tenofovir induced Kidney Injury. Expert Opin Drug Saf. 2007;5:156–162. doi: 10.1517/14740338.6.2.155. [DOI] [PubMed] [Google Scholar]
- 14.Padilla S, Gutierrea F, Masia M, et al. Low frequency of renal function impairment during one-year of therapy with Tenofovir-containing regimens in the real-world: a case-control study. AIDS Patient Care STDS. 2005;19:421–424. doi: 10.1089/apc.2005.19.421. [DOI] [PubMed] [Google Scholar]
- 15.Harris M. Nephrotoxicity associated with antiretroviral therapy in HIV infected patients. Expert Opin. Drug Saf. 2008;7(4):389–400. doi: 10.1517/14740338.7.4.389. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim F, Naftalin C, Cheserem E, et al. Low CD4 count and impaired renal function (eGFR) are independent risk factors for ARF in HIV infected patients. Conference report on Retroviruses and Opportunistic infections; CROI 2010. [Google Scholar]
- 17.Franceschini N, Napravnik S, Eron J, et al. Incidence and etiology of acute renal failure among ambulatory HIV infected patients. Kidney International. 2005;67:1526–1531. doi: 10.1111/j.1523-1755.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 18.Peter PJ. Antiretroviral therapy improves renal function among HIV infected Ugandans. Kidney Int. 2008;74:925–929. doi: 10.1038/ki.2008.305. [DOI] [PubMed] [Google Scholar]
- 19.Whitworth JA. Progression of Renal Failure- The role of Hypertension. Ann. Acad.Med Singapore. 2005;34:8–15. [PubMed] [Google Scholar]
- 20.Palmer BF. Renal dysfunction complicating the treatment of Hypertension. N Eng J Med. 347(16):1256–1261. doi: 10.1056/NEJMra020676. [DOI] [PubMed] [Google Scholar]
- 21.Bergeson B, Sandvik L, Dunlop O, et al. Prevalence of Hypertension in HIV positive patients on HAART compared to HIV negative controls; results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infec Dis. 2003:731–736. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 22.Oliver J, Markus B, Tilmann D, et al. Hypertension in HIV infected patients and its impact on renal and cardiovascular integrity. Nephrology Dialysis Transplantation. Vol 19(issue):2250–2258. doi: 10.1093/ndt/gfh393. [DOI] [PubMed] [Google Scholar]