Abstract
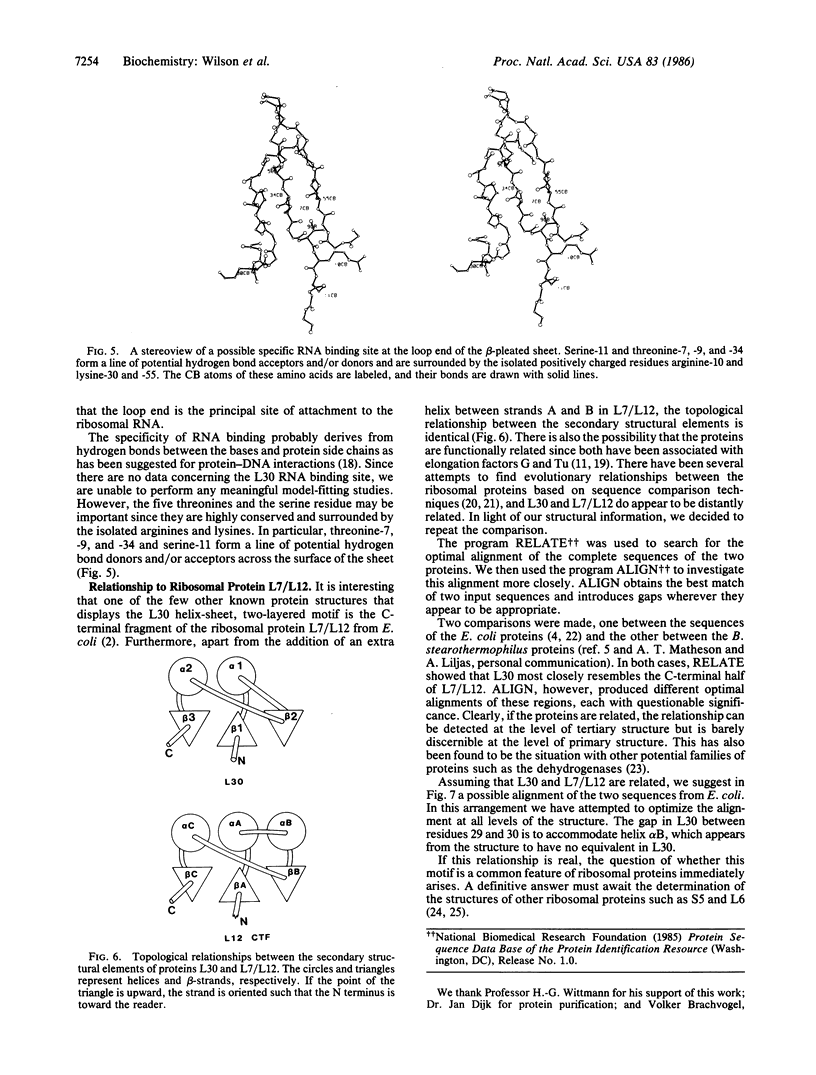
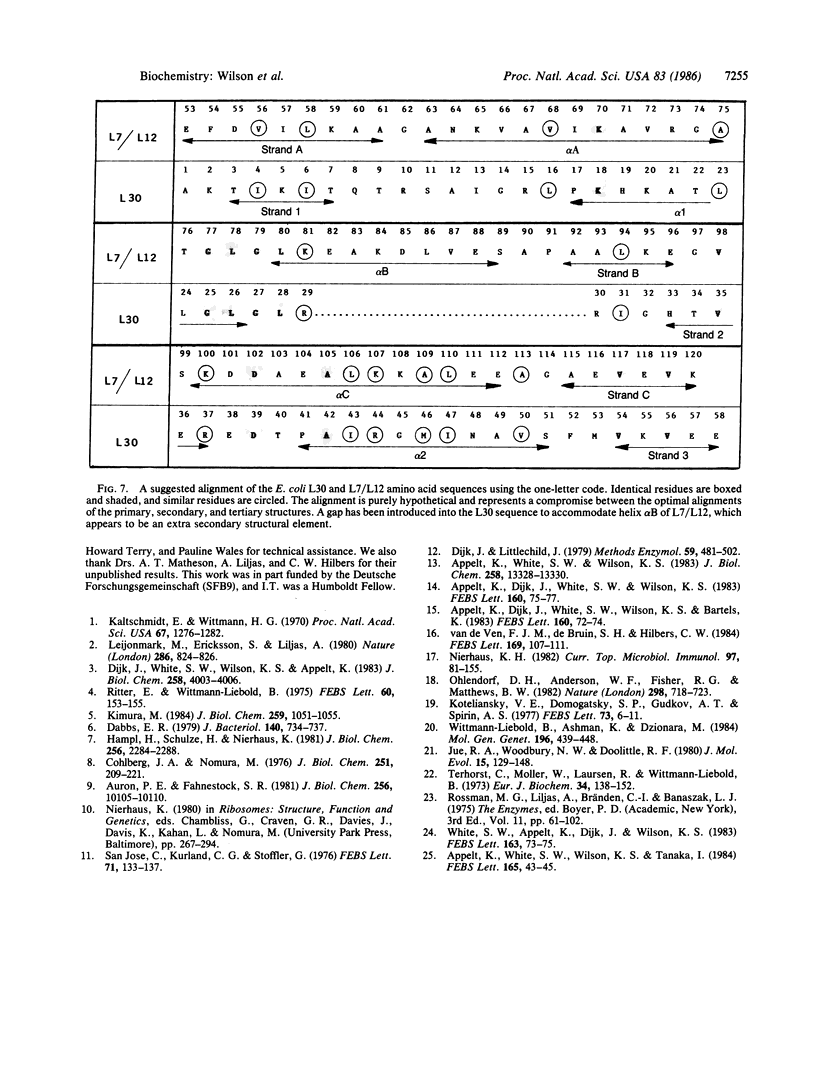
The structure of ribosomal protein L30 from Bacillus stearothermophilus has been solved to a resolution of 2.5 A. The molecule is somewhat elongated and contains two helices and a three-stranded, anti-parallel beta-pleated sheet. The protein fold, in which helices pack on the same side of the sheet, generates a simple helix-sheet, two-layered motif. It is possible to distinguish three hydrophobic patches on the molecular surface, and one end has six isolated arginine and lysine residues. It is proposed that these reflect sites of protein-protein and protein-RNA interaction, respectively. The protein fold is very similar to that of the only other known ribosomal protein structure, L7/L12 from Escherichia coli, and, based on this similarity, an attempt is made to align the amino acid sequences of the two proteins.
Full text
PDF
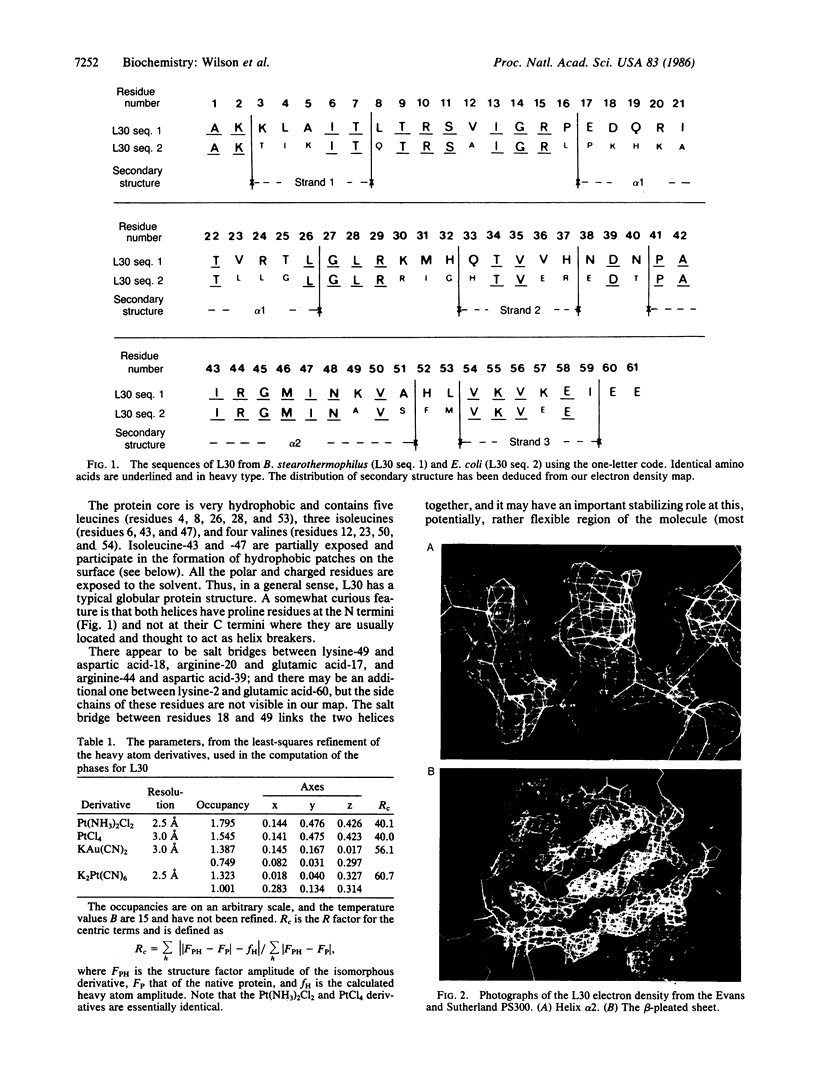
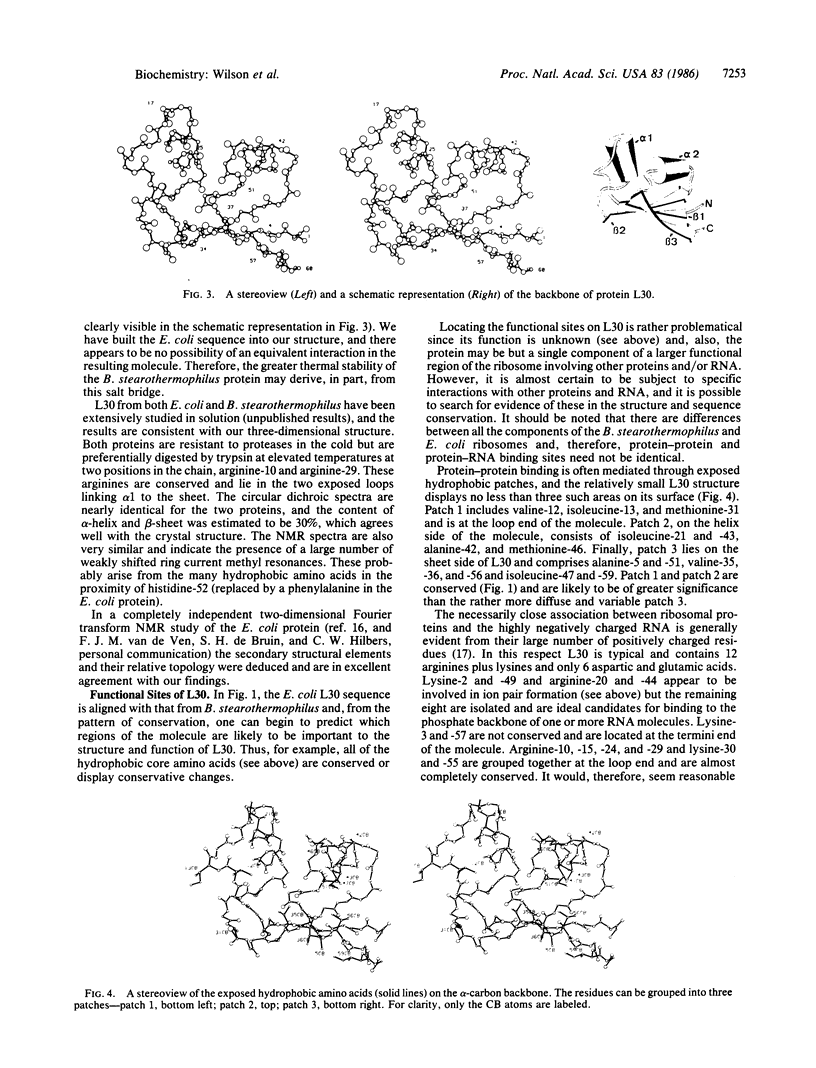
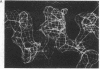
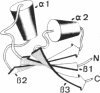
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelt K., Dijk J., White S. W., Wilson K. S. Proteins of the Bacillus stearothermophilus ribosome. Crystallization of protein L6. FEBS Lett. 1983 Aug 22;160(1-2):75–77. doi: 10.1016/0014-5793(83)80939-9. [DOI] [PubMed] [Google Scholar]
- Appelt K., Dijk J., White S., Wilson K., Bartels K. Proteins of the Bacillus stearothermophilus ribosome. A low resolution crystal analysis of protein L30. FEBS Lett. 1983 Aug 22;160(1-2):72–74. doi: 10.1016/0014-5793(83)80938-7. [DOI] [PubMed] [Google Scholar]
- Appelt K., White S. W., Wilson K. S. Proteins of the Bacillus stearothermophilus ribosome. Crystallization of proteins L30 and S5. J Biol Chem. 1983 Nov 10;258(21):13328–13330. [PubMed] [Google Scholar]
- Auron P. E., Fahnestock S. R. Functional organization of the large ribosomal subunit of Bacillus stearothermophilus. J Biol Chem. 1981 Oct 10;256(19):10105–10110. [PubMed] [Google Scholar]
- Cohlberg J. A., Nomura M. Reconstitution of Bacillus stearothermophilus 50 S ribosomal subunits from purified molecular components. J Biol Chem. 1976 Jan 10;251(1):209–221. [PubMed] [Google Scholar]
- Dabbs E. R. Selection for Escherichia coli mutants with proteins missing from the ribosome. J Bacteriol. 1979 Nov;140(2):734–737. doi: 10.1128/jb.140.2.734-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk J., Littlechild J. Purification of ribosomal proteins from Escherichia coli under nondenaturing conditions. Methods Enzymol. 1979;59:481–502. doi: 10.1016/0076-6879(79)59109-5. [DOI] [PubMed] [Google Scholar]
- Dijk J., White S. W., Wilson K. S., Appelt K. On the DNA binding protein II from Bacillus stearothermophilus. I. Purification, studies in solution, and crystallization. J Biol Chem. 1983 Mar 25;258(6):4003–4006. [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Jue R. A., Woodbury N. W., Doolittle R. F. Sequence homologies among E. coli ribosomal proteins: evidence for evolutionarily related groupings and internal duplications. J Mol Evol. 1980 May;15(2):129–148. doi: 10.1007/BF01732666. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Proteins of the Bacillus stearothermophilus ribosome. The amino acid sequences of proteins S5 and L30. J Biol Chem. 1984 Jan 25;259(2):1051–1055. [PubMed] [Google Scholar]
- Koteliansky V. E., Domogatsky S. P., Gudkov A. T., Spirin A. S. Elongation factor-dependent reactions of ribosomes deprived of proteins L7 and L12. FEBS Lett. 1977 Jan 15;73(1):6–11. doi: 10.1016/0014-5793(77)80003-3. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Eriksson S., Liljas A. Crystal structure of a ribosomal component at 2.6 A resolution. Nature. 1980 Aug 21;286(5775):824–826. doi: 10.1038/286824a0. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. Structure, assembly, and function of ribosomes. Curr Top Microbiol Immunol. 1982;97:81–155. doi: 10.1007/978-3-642-68318-3_3. [DOI] [PubMed] [Google Scholar]
- Ohlendorf D. H., Anderson W. F., Fisher R. G., Takeda Y., Matthews B. W. The molecular basis of DNA-protein recognition inferred from the structure of cro repressor. Nature. 1982 Aug 19;298(5876):718–723. doi: 10.1038/298718a0. [DOI] [PubMed] [Google Scholar]
- Ritter E., Wittmann-Liebold B. The primary structure of protein L30 from Escherichia coli ribosomes. FEBS Lett. 1975 Dec 1;60(1):153–155. doi: 10.1016/0014-5793(75)80440-6. [DOI] [PubMed] [Google Scholar]
- San José C., Kurland C. G., Stöffler G. The protein neighborhood of ribosome-bound elongation factor Tu. FEBS Lett. 1976 Nov 15;72(1):133–137. doi: 10.1016/0014-5793(76)80915-5. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Möller W., Laursen R., Wittmann-Liebold B. The primary structure of an acidic protein from 50-S ribosomes of Escherichia coli which is involved in GTP hydrolysis dependent on elongation factors G and T. Eur J Biochem. 1973 Apr 2;34(1):138–152. doi: 10.1111/j.1432-1033.1973.tb02740.x. [DOI] [PubMed] [Google Scholar]
- White S. W., Appelt K., Dijk J., Wilson K. S. Proteins of the Bacillus stearothermophilus ribosome. A 5 A structure analysis of protein S5. FEBS Lett. 1983 Oct 31;163(1):73–75. doi: 10.1016/0014-5793(83)81166-1. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Ashman K., Dzionara M. On the statistical significance of homologous structures among the Escherichia coli ribosomal proteins. Mol Gen Genet. 1984;196(3):439–448. doi: 10.1007/BF00436191. [DOI] [PubMed] [Google Scholar]