Abstract
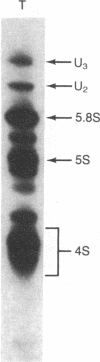
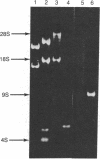
A low-molecular-weight RNA species from mouse ascites cells has been selected and purified by its intermolecular RNA X RNA hybridization capabilities. This 4.5S RNA is able to base pair with poly(A)+ mRNA sequences and with 18S rRNA. Melting experiments have shown that the intermolecular hybrids formed with this complementary low-molecular-weight RNA are of comparable stability to other RNA X RNA interactions. Analysis has shown that this hybridizing RNA is 87 nucleotides long and has an unusual sequence structure. Located near the 3' terminus is an alternating pyrimidine dinucleotide region of UUCCUUCCUU. This region along with the 3'-adjacent nucleotides form a 14-nucleotide sequence that exhibits perfect complementarity with 18S rRNA. An additional region of 10 nucleotides at the 3' terminus is perfectly homologous to a similarly located sequence in 5.8S rRNA. An obvious RNA polymerase III binding site is not found internally in this low-molecular-weight RNA sequence. The complementary and homologous character of hybridizing RNA with respect to rRNA and mRNA sequences suggests a potential regulatory role for this RNA in the coupling of ribosome and mRNA functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacq B. Sequence homologies between eukaryotic 5.8S rRNA and the 5' end of prokaryotic 23S rRNa: evidences for a common evolutionary origin. Nucleic Acids Res. 1981 Jun 25;9(12):2913–2932. doi: 10.1093/nar/9.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P., Arrand J. R. In vitro transcription of two Epstein-Barr virus specified small RNA molecules. Nucleic Acids Res. 1982 Jun 11;10(11):3407–3425. doi: 10.1093/nar/10.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Kelly J. M., Cox R. A. The nucleotide sequence at the 3'-end of Neurospora crassa 18S-rRNA and studies on the interaction with 5S-rRNA. Nucleic Acids Res. 1982 Nov 11;10(21):6733–6745. doi: 10.1093/nar/10.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Martin T. E., McCarthy B. J. Synthesis and turnover of RNA in the 30-S nuclear ribonucleoprotein complexes of mouse ascites cells. Biochim Biophys Acta. 1972 Aug 25;277(2):354–367. doi: 10.1016/0005-2787(72)90417-0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Binding of adenovirus VA RNA to mRNA: a possible role in splicing? Nature. 1980 Jun 19;285(5766):575–577. doi: 10.1038/285575a0. [DOI] [PubMed] [Google Scholar]
- Maxwell E. S., Martin T. E. Complementarity of sequences in low molecular weight RNAs to regions of messenger and ribosomal RNAs. Nucleic Acids Res. 1986 Jul 25;14(14):5741–5760. doi: 10.1093/nar/14.14.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell E. S., Maundrell K., Scherrer K. Two low molecular weight nuclear RNAs, isolated from avian erythroblast nuclear ribonucleoprotein complexes, hybridize to duck pre-messenger and globin messenger RNA. Biochem Biophys Res Commun. 1980 Dec 16;97(3):875–882. doi: 10.1016/0006-291x(80)91458-8. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Stewart A. G., Gander E. S., Morel C., Luppis B., Scherrer K. Differential translation of duck- and rabbit-globin messenger RNAs in reticulocyte-lysate systems. Eur J Biochem. 1973 Apr;34(2):205–212. doi: 10.1111/j.1432-1033.1973.tb21105.x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Walker T. A., Johnson K. D., Olsen G. J., Peters M. A., Pace N. R. Enzymatic and chemical structure mapping of mouse 28S ribosomal ribonucleic acid contacts in 5.8S ribosomal ribonucleic acid. Biochemistry. 1982 May 11;21(10):2320–2329. doi: 10.1021/bi00539a008. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Zagorska L., Van Duin J., Noller H. F., Pace B., Johnson K. D., Pace N. R. The conserved 5 S rRNA complement to tRNA is not required for translation of natural mRNA. J Biol Chem. 1984 Mar 10;259(5):2798–2802. [PubMed] [Google Scholar]