Abstract
Acute kidney injury (AKI) is classically described as a rapid loss of kidney function. AKI affects more than 15% of all hospital admissions and is associated with elevated mortality rates. Although many advances have occurred, intermittent or continuous renal replacement therapies are still considered the best options for reversing mild and severe AKI syndrome. For this reason, it is essential that innovative and effective therapies, without side effects and complications, be developed to treat AKI and the end-stages of renal disease. Mesenchymal stem cell (MSC) based therapies have numerous advantages in helping to repair inflamed and damaged tissues and are being considered as a new alternative for treating kidney injuries. Numerous experimental models have shown that MSCs can act via differentiation-independent mechanisms to help renal recovery. Essentially, MSCs can secrete a pool of cytokines, growth factors and chemokines, express enzymes, interact via cell-to-cell contacts and release bioagents such as microvesicles to orchestrate renal protection. In this review, we propose seven distinct properties of MSCs which explain how renoprotection may be conferred: 1) anti-inflammatory; 2) pro-angiogenic; 3) stimulation of endogenous progenitor cells; 4) anti-apoptotic; 5) anti-fibrotic; 6) anti-oxidant; and 7) promotion of cellular reprogramming. In this context, these mechanisms, either individually or synergically, could induce renal protection and functional recovery. This review summarises the most important effects and benefits associated with MSC-based therapies in experimental renal disease models and attempts to clarify the mechanisms behind the MSC-related renoprotection. MSCs may prove to be an effective, innovative and affordable treatment for moderate and severe AKI. However, more studies need to be performed to provide a more comprehensive global understanding of MSC-related therapies and to ensure their safety for future clinical applications.
Introduction
Acute Kidney Injury
Acute kidney injury (AKI) is classically described as a rapid and progressive loss of renal function, which persists for variable periods, resulting in an increase in markers of kidney injury.1 It is important to consider that AKI is also characterised as a wide-spectrum syndrome, with progressive and cumulative damage ranging from mild to severe forms.1,2
AKI affects more than 15% of all hospital admissions and is associated with elevated rates of mortality and morbidity. In AKI, the mortality rate can range from 15% in patients with isolated renal failure up to 50–80% in severe cases in which renal replacement therapies are needed.3,4 Even after the recovery of kidney function, some patients remain dependent on dialysis (≈13%) or have compromised renal function in the long term. Indeed, it has been reported that patients who recover from acute renal dysfunction have an increased risk for developing progressive chronic kidney disease.5–7
Pathophysiology of AKI
AKI is frequently multifactorial and can occur as a result of a fall in renal perfusion, direct insults to the renal tubule (toxic or obstructive), tubule-interstitial inflammation and oedema, or a primary reduction in the glomerular filtration rate.8 After an ischaemic injury to the kidney, structural and biochemical changes occur which result in vasoconstriction, detachment of tubular cells, luminal tubular obstruction and trans-tubular back-leakage of the glomerular filtrate.9 Additionally, morphologic changes can be observed after ischaemic damage, including the loss of cytoskeletal integrity and cell polarity (the mislocalisation of Na+/K+ ATPase and β-integrins from the basolateral to the apical membrane), loss of the brush border, breakdown of the epithelial cell barrier and disruption of the tight junctions causing apoptosis/necrosis of tubular cells.10,11
These insults to the epithelium result in the generation of inflammatory mediators, which can promote vasoconstriction and further stimulate the inflammatory process. Furthermore, infiltrating neutrophils release reactive oxygen species, proteases and myeloperoxidase, which lead to tissue damage. These substances can act synergically with leukotriene B4 and platelet-activating factor (PAF) that can further sustain the inflammation.12,13
Although injured, the kidney has great regeneration capabilities. This organ can potentially recover its parenchyma by promoting increases in the number of tubular cells after injury. Stem cell or progenitor cell populations inside the kidney can drive this process by promoting epithelial cell spreading and migration and cell de-differentiation and proliferation.9
Prevention and Conventional Treatment of AKI
Many biomarkers can identify the occurrence of AKI, including plasma creatinine and urea, urine interleukin 18 (IL-18), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), N-acetyl-β-glucosaminidase (NAG), β2-microglobulin (β2M), retinol binding protein (RBP) and microalbuminuria. However, each of these biomarkers have their own limitations, such as up-regulation during the early or late stages and influences from diet, age or sex, and individually, these markers have not been comprehensively evaluated in a large cohort of patients or during different clinical stages of AKI.14–21
Few known treatments have the potential to change the course of the disease once AKI has developed. Thus, alternative treatment strategies have been attempted to avoid AKI following the initial insult to the kidney, including adequate hydration control, pre-emptive use of antioxidants, maintenance of arterial pressure, and caution with exposure to nephrotoxic drugs, such as aminoglycosides, amphotericin B and radiological contrast agents.22 In addition, other pharmacological-based therapies (e.g. diuretics, dopamine, natriuretic peptides, N-acetylcysteine) have been evaluated for their ability to prevent AKI, although they have shown little efficacy to date.23–26 Consequently, intermittent or continuous renal replacement therapy (dialysis) is still considered to be the best treatment option for mild and severe AKI.27
In summary, although advances have occurred, there is a clear need for more effective therapies for the treatment of AKI and the use of mesynchymal cells offers an innovative approach to that end.
Mesenchymal Stem Cells and Their Potential in Regenerative Medicine
Mesenchymal stem cells (MSCs) were first identified from bone marrow by Friedenstein and colleagues in 1970 as non-haematopoietic progenitor cells that had the capacity to adhere to culture flasks and displayed fibroblast colony-forming unit activity in vitro.28
Currently, MSCs are considered multipotent mesenchymal progenitor cells that have been classically defined by their ability to adhere to plastic, self-replicate and exhibit multipotent differentiation potential to mesodermal lineages such as osteocytes, chondrocytes and adipocytes. There are numerous cell surface markers that can be used to define MSCs, including positive staining for CD105, CD73 and CD90 and the absence of CD45, CD34, CD31, CD14 (or CD11b), CD79a (or CD19) and HLA-DR.29
MSCs can be obtained from numerous tissues, including bone marrow,30 synovial membrane,31 muscle,32 cord blood,33 peripheral blood,34 synovial fluid,35 tooth pulp,36 saphenous vein,37 periosteum,38 adipose tissue,39 placenta40 and umbilical vein,41 making them very attractive for experimental investigations. In fact they can be found in most tissues, and within these tissues are believed to mainly be found in the vascular walls.42,43 The cells that most closely resemble MSCs in vivo are pericytes; these cells are characterised by the ability to maintain tissue homeostasis, to stabilise blood vessel architecture and possibly to generate adipocytes, osteocytes, chondrocytes, smooth muscle cells and fibroblasts.44,46,47 In addition, it was discovered that in situ vascular pericytes express classical MSC markers such as CD44, CD73, CD90 and CD105.45 A negative correlation between the presence of CD146 (a pericyte marker) and fibroblast-specific protein-1 (FSP-1, a fibroblast marker), suggests that fibroblasts are a more specialised MSC/pericyte sub-type.46
Therapeutically, MSCs possess great potential. These cells have the ability to ‘home’ to injured tissue and produce a number of different trophic factors such as cytokines and growth factors. These factors are specifically related to the mechanisms of immune regulation, anti-scarring, endogenous progenitor cell support, anti-apoptosis, angiogenesis and chemoattraction.48,49
MSCs have been effectively used in several experimental and clinical protocols for treating inflammatory diseases such as graft-versus-host disease, multiple sclerosis, osteogenesis imperfecta and Crohn’s disease. Thus, MSC-based therapies have the potential to be an innovative and affordable treatment to repair inflamed and damaged tissues, and may thus be of potential benefit in acute and chronic kidney diseases.50 However, while many studies have demonstrated that treatment with MSC to be efficacious in animal models of kidney injury, there has only been one multicentric study of MSC therapy for human renal disease.
In this unique work it was shown that MSC infusion concomitant with low doses of immunosuppressant drugs exhibited the same benefits to kidney transplanted patients as higher doses of immunosuppressant. This may be considered a great advantage, since it is known that the use of higher doses of immunosuppressive drugs can cause further complications to the kidney after organ transplantation. The MSC treatment promoted the recovery of the glomerular filtration rates more quickly, although no difference was seen in the final score in both groups (high dose vs low dose and MSCs).51
In this review we summarise the most important effects and benefits associated with MSC-based therapies in experimental models of renal disease and attempt to clarify the mechanisms which may underlie MSC mediated renoprotection. We discuss seven aspects which help to explain the molecular and cellular mechanisms underlying MSC renoprotection: 1) anti-inflammatory; 2) pro-angiogenic; 3) stimulation of endogenous progenitor cells; 4) anti-apoptotic; 5) anti-fibrotic; 6) anti-oxidant; and 7) promotion of cellular reprogramming. We propose that each of these functions, either individually or synergically, can induce renal protection and promote functional recovery by modulation of several kinds of molecules including cytokines, growth factors, receptors, enzymes and matrix components (Figure 1).
Figure 1.
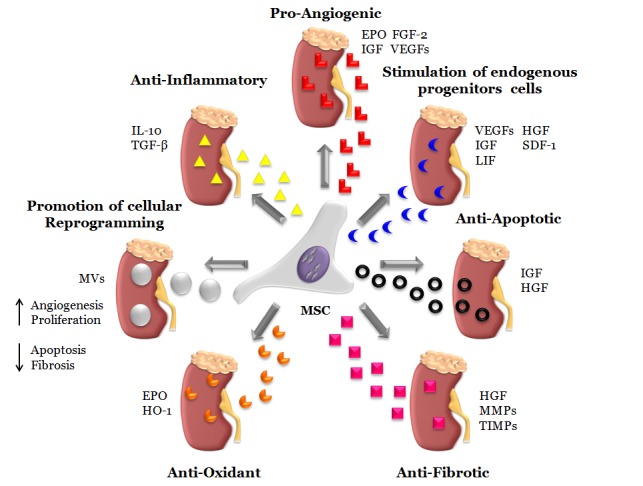
Illustration of proposed mechanisms related with mesenchymal stem cell renoprotection on acute and chronic kidney disease. Mesenchymal stem cells (MSCs) may act by a number of differentiation-independent mechanism to exert a specific renal recovery. In this present review, we propose seven main functions to explain the molecular and cellular mechanisms related to MSC renoprotection. The MSC global therapeutic effects are separated into: 1) anti-inflammatory (IL-10 and TGF-β), 2) pro-angiogenic (EPO, IGF, FGF-2 and VEGFs), 3) stimulator of progenitors endogenous cells (VEGFs, IGF, LIF, HGF and SDF-1), 4) anti-apoptotic (IGF and HGF), 5) anti-fibrotic (HGF, MMPs and TIMPs), 6) anti-oxidant (EPO and HO-1) and 7) promotion of cellular reprogramming (MVs). We suggest that all of them, either individually or synergically, could induce renal protection and functional recovery.
EPO, Erythropoietin; FGF-2, Fibroblast growth factor 2; HGF, Hepatocyte growth factor; HO-1, Heme oxygenase-1; IGF, Insulin-like growth factor; IL-10, Interleukin 10; LIF, Leukemia inhibitory factor; MMPs, Matrix metalloproteinases; MV, microvesicle; SDF-1, Stromal cell-derived factor-1; TGF-β, Transforming growth factor beta; TIMPs, Tissue inhibitors of metalloproteinase; VEGF, Vascular endothelial growth factor.
Mechanisms Associated with MSC Prevention of Renal Dysfunction after AKI
The efficacy of MSCs in promoting renal recovery after an acute injury was first demonstrated in 2004 by Mongi et al.52 The authors showed that MSCs were able to migrate to the damaged tissue and restore kidney structure and function.52 Additional studies have since been performed to evaluate the potential of MSC-based therapy in several experimental conditions of renal failure. The results of these experiments have identified some potential mechanisms that explain the MSC renoprotective process.
MSCs are believed to act through various differentiation-independent mechanisms to exert specific renal recovery. Essentially, MSCs can secrete a pool of cytokines and chemokines, interact through cell-to-cell contacts and release bioagents such as vesicles to orchestrate renal protection (Table 1).
Table 1.
The global profile of mesenchymal stem cell (MSC)-derived molecules associated with renoprotection.
| MSC-related molecules | Biologic Function | Source of MSC | References |
|---|---|---|---|
| IL-10, TGF-β | Anti-Inflammatory | Mouse and Rat | 55, 57, 58, 61 |
| EPO, FGF-2, IGF, PDGFR and SM22 α | Pro-Angiogenic | Mouse and Rat | 70, 72, 71, 74 |
| VEGF, -D, HGF, IGF, SDF-l and LIF | Stimulation of Endogenous Progenitor Cells | Mouse, Rat and Human | 57, 80, 81 |
| IGF, HGF and VEGFs | Anti-Apoptotic | Mice and Human | 80, 90 |
| HGF, MMPs and TIMPs | Anti-Fibrotic | Rat | 88 |
| EPO and HO-l | Anti-Oxidant | Mouse | 70, 95 |
| MVs, mRNAs and miRNAs | Promotion of Cellular Reprogramming | Mice and Human | 103, 104, 105, 106 |
EPO, Erythropoietin; FGF-2, Fibroblast growth factor 2; HGF, Hepatocyte growth factor; HO-1, Heme oxygenase-1; IGF, Insulin-like growth factor; IL-10, Interleukin 10; LIF, Leukemia inhibitory factor; MMPs, Matrix metalloproteinases; MV, microvesicle; PDGFR, Platelet-derived growth factor receptor; SDF-1, Stromal cell-derived factor-1; SM22α, 22-kDa smooth muscle cell marker; TGF-β, Transforming growth factor beta; TIMPs, Tissue inhibitors of metalloproteinase; VEGF, Vascular endothelial growth factor.
Anti-Inflammatory/Immunomodulatory Properties of MSCs in AKI
Inflammatory cells are known to participate in the early stages of acute renal failure. Macrophages, neutrophils and T cells can actively promote the development of AKI.12,53,54 MSCs have powerful immunomodulatory effects on immune cells through cell-to-cell contacts (specifically through the interaction of PDL-1, HLA-G5 and CTL-A4) and by secretion of molecules such as cytokines, chemokines and growth factors (e.g. TSG-6, LIF, HGF, TGF-β, CCL-2, IL-6, IL-10, PGE-2, IDO, iNOS and HLA-G5).48,49,55,56 These potential effects of MSCs can down-regulate several types of inflammatory cells, such as CD4+, CD8+, NK, and B cells, macrophages, and dendritic cells, while up-regulating others such as regulatory T cells to further decrease the AKI-associated inflammation and restore renal function.49
Several studies have used experimental models of AKI to demonstrate this phenomenon. In these studies, MSC treatment has been associated with the classical immune response shift from Th1 to Th2 in models in which IFN-gamma-related molecules play a detrimental role. The renal protection was confirmed by the substantial recovery of renal function and the down-regulation of key pro-inflammatory molecules (TNF-α, IL-1α, IL-1β, IFN-γ and IL-6), adhesion molecules (ICAM-1) and chemokines (CXCL-2, MIP-2, G-CSF, GM-CSF, KC, MCP-1, MIP-3α, NGF-β and MSP), as well as some receptors such as CD68 and CD136. On the other hand, molecules associated with the Th2 immune response such as IL-10 and IL-4 were all up-regulated after MSC infusion.57–63
Furthermore, kidney derived-MSCs have been shown to play an important role in the inhibition of specific inflammatory cells such as B cells, T cells and dendritic cells.64 When co-cultivated with dendritic cells, MSCs promoted changes in the differentiation and maturation of the dendritic cells, causing a significant decrease in expression of class II MHC molecules, an increase in CD80 expression and IL-10 production, and importantly, the MSCs impaired the ability of dendritic cells to stimulate T cell proliferation. In addition, the MSC-modulated dendritic cells significantly reduced B cell activation, proliferation and antibody production (IgM/IgG) in allogeneic co-culture assays.65 These data are relevant given it is well known that dendritic cells and B cells play an important role in AKI. The cytoprotective action of MSCs on AKI models may be partly related to their effects on these immune cells.
Taken together, these findings suggest numerous possible immune regulatory effects by which MSCs may modulate kidney inflammation progression and change the outcome of AKI (Figure 1, Table 1, Table 2).
Table 2.
The global profile of molecules up-/down-regulated by mesenchymal stem cell (MSC) treatments in experimental models of acute and chronic kidney injury.
| Molecules up-regulated by MSC | Molecules down-regulated by MSC | Global Physiological Role |
|---|---|---|
| IL-10, IL-4, and TGF-β | TNF-α, IL-1α, IL1-β, IFN-γ, IL-6, CXCL-2, MIP-2, G-CSF, GM-CSF, KC, MCP-1, MIP-1, MIP-3α, NGF-β, MSP, ICAM-1, CD68 and CD136 | Modulation of inflammatory immune cells and generation/expansion of regulatory immune cells |
| VEGFs, PDGFR and SM22 α | Angiogenesis and support to vasculature | |
| FGF-2, TGF-α, BMP-7, VEGFs, HGF and Met | Stimulus to proliferation and angiogenesis | |
| BCL-2, and BCL-XL | BCL-XS, Bad, Caspase-3 and -7 | Control of Apoptosis |
| BMP-7, Smad-7, HGF, NCAM, Pax-2 | α-SMA, FSP-1, Vimentin, Collagen-1, -3, Fibronectin, Timp-1 and Smad-3 | Extracellular matrix remodelling |
| HO-1, SOD, GSH-Px, GSH-Rx and NQO1 | iNOS, eNOS, 8-OHdG and MDA | Control of oxidative and nitrosative stress |
Bad, Bcl-2-associated death promoter; BCL-2, B-cell CLL/lymphoma 2; BCL-XL, B-cell lymphoma-extra large; BCL-XS, B-cell lymphoma-extra small; BMP-7, Bone morphogenetic protein 7; CXCL-2, Chemokine (C-X-C motif) ligand 2; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; eNOS, Endothelial nitric oxide synthase; EPO, Erythropoietin; FGF-2, Fibroblast growth factor 2; FSP-1, Fibroblast-specific protein-1; G-CSF, Granulocyte colony-stimulating factor; GM-CSF, Granulocyte macrophage colony-stimulating factor; GSH-Px, Glutathione peroxidase; GSH-Rx, Glutathione reductase; HGF, Hepatocyte growth factor; HO-1, Heme oxygenase-1; ICAM-1, Intercellular adhesion molecule 1; IGF, Insulin-like growth factor; IL-10, Interleukin 10; IL-4, Interleukin 4; IL-6, Interleukin 6; IL-1β, Interleukin 1β; IL-1α, Interleukin 1α; IFN-γ, Interferon-gamma; iNOS, Inducible nitric oxide synthase; KC, keratoconus; LIF, Leukemia inhibitory factor; MCP-1, Monocyte chemoattractant protein-1; MDA, Malondialdehyde; Met, hepatocyte growth factor receptor; MIP-1, Macrophage inflammatory protein 1; MIP-2, Macrophage inflammatory protein 2; MIP-3α, Macrophage inflammatory protein 3α; MMPs, Matrix metalloproteinase; MSP, Macrophage-stimulating protein; MV, microvesicle; NCAM, Neural cell adhesion molecule; NGF-β, Nerve growth factor; NQO1, NAD(P)H dehydrogenase quinone 1; Pax-2, Paired box gene 2; PDGFR, Platelet-derived growth factor receptor; SDF-1, Stromal cell-derived factor-1; Smad-3, Mothers against decapentaplegic homolog 3; Smad-7, Mothers against decapentaplegic homolog 7; α-SMA, Alpha-smooth muscle actin; SM22α, 22-kDa smooth muscle cell marker; SOD, Superoxide dismutase; TGF-α, Transforming growth factor α; TGF-β, Transforming growth factor β; TIMP-1, Tissue inhibitor of metalloproteinase 1; TNF-α, Tumor necrosis factor-alpha; VEGF, Vascular endothelial growth factor.
Pro-Angiogenic Potential of MSCs on AKI
An adequate blood supply is fundamental for the recovery of renal function, and MSC-derived factors may restore renal vasculature and perfusion after kidney injury. The pro-angiogenic properties of MSCs have been reported to be closely associated with the recovery of damaged tissues.66,67 MSCs can promote angiogenesis in two ways; firstly by acting as pericyte-like cells to support the new vasculature and secondly by secreting molecules strongly associated with the angiogenesis process including vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), placental growth factor (PIGF), monocyte chemoattractant protein-1 (MCP-1), platelet-derived growth factor (PDGF) and fibroblast growth factor 2 (FGF-2).48,68,69
The abilities of MSCs to support angiogenesis and express markers of supportive vascular cells in a renal context was first demonstrated in 2006 when MSCs were co-cultivated with ureteric bud and kidney epithelial cells. It was observed that MSCs were positive for PDGF receptor and SM22α (pericyte markers) and their infusion increased the capillary density in vivo, as assessed by the quantification of blood vessel formation in a matrigel plug system.70 In addition, the MSCs preferably migrated to the peritubular capillaries when injected into the subcapsular space of ischaemic kidneys.70 This study also examined whether a hypoxic microenvironment, which is necessary for angiogenesis, could enhance pro-angiogenic properties in MSCs. The MSCs seeded in hypoxic (2% O2) or anoxic (<1% O2) conditions, had an increase in the erythropoietin (EPO) and VEGF levels (two powerful pro-angiogenic factors) concomitant with the maintained expression of α-SMA and vimentin, characterising the acquisition of a pericyte-like phenotype.70
Furthermore, another study has shown that MSCs exerted robust angiogenesis effects when cultivated in matrigel plugs together with endothelial cells plus angiogenic factors (VEGF and FGF-2). To evaluate their angiogenic abilities in vivo, these MSCs were transplanted into mice with acute renal ischaemia. The MSCs selectively grafted onto damaged areas and supported functional recovery and tubular regeneration, induced epithelial proliferation and decreased apoptosis. MSC-treated mice also displayed a five-fold increase in VEGF expression in the renal tissue, which was associated with better preservation of the peritubular capillaries.71 Other studies, again in a mouse model of renal injury, showed that MSCs genetically modified to express erythropoeitin (EPO) and IGF-1, not only rectified the anaemia associated with renal failure, but also stimulated angiogenesis.72
Other work, using a model of acute renal injury induced by cisplatin, also demonstrated that the infusion of MSCs improved renal functional parameters, increasing the endothelial cell density and the capillary lumen volume, with no perceptible ultrastructural peritubular changes such as cytoplasmic swelling or retraction.73 Finally, when injected into mice with renal ischaemia, animals treated with normal MSCs showed elevated renal microvessel density scores when compared to animals treated with either VEGF-silenced MSCs or a vehicle control.74
In all, these encouraging results suggest that MSCs exhibit various pro-angiogenic properties to promote the recovery of damaged blood vessels, through production of angiogenic factors (VEGFs, FGF-2, IGF, EPO) and by acting as pericyte-like cells (providing structural support), thereby contributing to the maintenance of blood perfusion and to the stabilisation of the vasculature (Figure 1, Table 1, Table 2).
MSCs as Stimulators of Endogenous Progenitor Cells
The kidney has remarkable regenerative capacities, which are principally attributed to a large population of resident progenitor cells.75 These special renal cells, which are responsible for tissue repair, have been found in the renal papilla,76 tubular epithelium,77 and Bowman’s capsule.78 An increase in the number of these resident progenitor cells (endothelium progenitor cells, hematopoietic progenitor cells, and mesodermal-derived progenitor cells) has been observed after renal damage; however, after the restoration of renal function, these cells tend to decrease back to their basal levels.79
MSCs have the ability to secret several growth factors associated with the stimulation of progenitor cells (e.g. SCF, LIF, angiopoetin-1, M-CSF, HGF, IGF, SDF-1), which can then assist in the proliferation and generation of new endogenous cells by promoting renal repair.48,80,81
HGF is known to be a potent mitogen for many cells (e.g. hepatocytes, endothelial and epithelial cells). This growth factor and its receptor (Met) increase transiently after injury, and it has been reported that the HGF/Met binding causes autophosphorylation and the activation of tyrosine kinase activity (PI3Ks and MAPKs). Thus, it has been suggested that HGF and Met may play an important role in promoting the regeneration of injured kidneys.82
In 2011, Rampino and colleagues explored the HGF/Met (HGFR) axis in AKI and verified de novo the intense expression of HGF in the kidneys of rats with anti-Thy1-induced renal disease after MSC injection. Likewise, treatment with MSCs induced a striking increase in Met expression in kidney sections. Curiously, however, MSC injection into healthy rats did not result in the up-regulation of HGF.62
To identify the precise molecule responsible for MSC-induced renal protection by the stimulation of endogenous cell proliferation, several studies have utilised techniques that inactivate a target via a specific-antibody or inhibitor, silence mRNA (small interfering-RNA, siRNA), or overexpress a specific molecule.
Although exposure to nephrotoxic drugs (cisplatin) markedly reduced tubular epithelial cell viability, in vitro analyses revealed that co-culture with MSCs provided a renoprotective effect by decreasing of apoptosis and promoting tubular cell proliferation. Conversely, tubular cell proliferation was attenuated when IGF-1, which is expressed at elevated levels on MSCs, was blocked using an antibody specific for this molecule. Knockdown of IGF-1 expression in MSCs using siRNA also resulted in an elevated apoptosis index and a prominent reduction in the proliferation rate of tubular epithelial cells when co-cultivated with MSCs.80 In a murine model of cisplatin-induced AKI, the administration of MSCs treated with IGF-1 siRNA also limited their protective effect on tubular structure (necrosis index and tubular casts) and renal function as assessed by creatinine and urea.
Thus, the expression of keys molecules such as VEGF, HGF, IGF, SDF-1 and LIF suggests that MSCs can either directly or indirectly induce resident endogenous progenitor cells to proliferate and stimulate functional renal repair (Figure 1, Table 1, Table 2).
Anti-Apoptotic Effects of MSCs on AKI
AKI is characterised by apoptosis of the tubular epithelial cells. The anti-apoptotic effect of MSCs could be a very useful tool for preventing cell death in scenarios involving tissue injury. In addition, MSCs have the ability to secrete an abundant number of anti-apoptotic factors such as VEGF, HGF, IGF-1, stanniocalcin-1, TGF-β, FGF-2 and GM-CSF.48
Experimental models of AKI have demonstrated some renoprotective effect of MSCs on tissue apoptosis. Animals treated with MSCs showed a reduced apoptosis index, as evaluated using terminal transferase-mediated dUTP nick-end labelling (TUNEL) assays. Moreover, these MSC-treated mice displayed an increase in anti-apoptotic molecules (BCL-2 and BCL-XL) and a decrease of pro-apoptotic molecules (BCL-XS), as assessed by gene expression analysis.57
Changes in the anti-/pro-apoptotic balance have been observed in renal tissues after therapy with MSCs. In a study using ischaemia/reperfusion-mediated AKI, our group reported that MSC injection enhanced renal recovery (reduction of creatinine and intense proliferation) by decreasing apoptosis, with a marked up-regulation of the BCL-2/Bad ratio.58
In search of a specific molecule behind the anti-apoptotic effect of MSCs, a study reported that IGF-1 may play a fundamental role in promoting this protection. In vitro analysis demonstrated that the percentage of apoptotic cells positive for caspases 3 and 7 (apoptosis markers) and the level of incorporation of propidium iodide (necrosis marker) significantly increased after cisplatin treatment, but the addition of MSCs at the co-cultures notably reduced the number of dead cells when compared with control values. To investigate the role of MSC-derived IGF-1 in limiting tubular apoptosis, IGF-1 was genetically silenced using siRNAs in MSCs. The IGF-1 knockdown MSCs were co-cultured with tubular cells and after incubation these cells shown to be ineffective in protecting against cisplatin-induced apoptosis.80
Subsequently, another study has reported the role of HGF and VEGF molecules in the anti-apoptotic effect of MSCs. MSCs secreting high levels of HGF/VEGF were transplanted in a model of folic acid-induced AKI. MSC infusion ameliorated the renal functional parameters (creatinine, urea and histopathological score) and provided a reduced DNA fragmentation score (TUNEL) in kidney parenchyma, suggesting that HGF and VEGF may have participated in apoptosis protection.83
These findings show that MSCs exert an anti-apoptotic effect possibly via the secretion of IGF, HGF and VEGF, which may contribute to the reduced initial apoptosis and allow tissue to regenerate and re-establish the renal physiological parameters (Figure 1, Table 1, Table 2).
Anti-Fibrotic Mechanisms of MSCs on AKI
Fibrosis is a secondary event following acute kidney injury and MSCs can secrete or up/down-regulate numerous molecules including FGF-2, HGF and adrenomedullin which can influence fibrosis.48
Our group has demonstrated that MSC administration can up-regulate key molecules such as HO-1, BMP-7, Smad7, and HGF, which have classical anti-fibrotic properties, in an experimental model of CKD. The up-regulation of these markers was associated with a reduction in the renal fibrosis score, as evaluated using the Sirius red matrix marker and Masson’s trichrome stain. In addition, MSCs still promoted the intense modulation of the fibrosis network molecules, including the up-regulation of E-cadherin and the down-regulation of epithelial-mesenchymal transition markers (α-SMA, FSP-1 and Vimentin) beyond of extracellular matrix markers (collagen 1–3, fibronectin, Timp-1, Samd-3).84
We also evaluated the anti-fibrotic effect of MSCs in a unilateral severe ischaemia model. First we verified, after MSC infusion, the presence of reduced renal dysfunction and an increase in tubular regeneration 24 hours after injury. As predicted for this model, the kidneys of untreated mice shrank at six weeks, while the kidneys of MSC-treated animals remained at a normal size and displayed less matrix deposition and decreased staining for FSP-1 and type I collagen (fibrosis markers). In another set of experiments, mice were treated at six weeks when fibrosis was already established. It was possible to observe that MSC infusion ameliorated the renal functional parameters and reduced tissue fibrosis, including low expression of fibrosis markers such as type I collagen and vimentin mRNAs.85
Other groups using different experimental models of CKD have reported similar findings. All of these studies showed that MSC administration decreased fibrotic markers (i.e. α-SMA, FSP-1, collagen, and Masson staining) and induced the overexpression of renoprotective molecules such as HGF and VEGF.83,86 To evaluate the efficacy of different MSC delivery routes to revert fibrosis, MSCs were injected into the kidney subcapsular space using the 5/6 nephrectomy model. In this model after either 15 or 30 days, untreated rats displayed continued hypertension, which was partially reduced after MSC infusion. Moreover in the same study, a significant reduction in the glomerulosclerosis index and improvements in renal functions were also observed at 30 days after MSC injection.87
Interestingly, HGF was predicted to play a pivotal role in the anti-fibrotic mechanism of MSCs. To verify this effect, MSCs were genetically engineered to overexpress HGF. These MSCs expressing HGF were then injected in animals with unilateral ureteral obstruction, and the tissue expression of α-SMA was found to be significantly reduced compared to animals treated with untransfected MSCs.88
Although the precise mechanism remains unclear, some evidence suggests that the modulation of the matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinases (TIMP) balance may also be essential for MSC-induced fibrosis regression.89,90 Thus, MSCs could be a powerful tool to revert renal fibrosis by endogenous production of HGF and FGF-2. However, more studies are required to clarify the role of MSCs in renal fibrosis (Figure 1, Table 1), before its use for the treatment of chronic, end-stage kidney disease is feasible.
Anti-Oxidant Role of MSCs During AKI Progression
Oxidative stress is a classical mechanism involved in early inflammation, and reactive oxygen and nitrogen species have been implicated in the pathogenesis of AKI. Superoxide anion, nitric oxide and hydrogen peroxide are generated during kidney injury, and association between these species can generate peroxynitrite, which is considered to be a key oxidant species that is directly involved in protein oxidation and renal failure.91
A limited number of studies have shown that MSCs can secrete elevated levels of heme oxygenase-1 (HO-1) and EPO, which are considered to be potent anti-oxidant molecules.92,93 Heme oxygenase degrades heme to biliverdin, iron, and carbon monoxide; there are numerous forms of the enzyme, and HO-1 is an inducible isoform responsive to numerous stressors including oxidation. Expression of HO-1 can regulate inflammatory and immune responses as well as oxidative stress, confer anti-apoptotic protection94 and MSCs isolated from HO-1 knockout animals showed reduced expression and secretion of several important growth and pro-angiogenic factors, including SDF-1, VEGF, and HGF, compared to MSCs derived from normal mice. Moreover, conditioned medium with HO-1 knockout MSCs was unable to restore the functional and morphological changes associated with kidney injury.95 In addition, molecules associated with the release of free radicals, such as the inducible nitric oxide synthases (iNOS), endothelial nitric oxide synthases (eNOS) and 8-hydroxy-2-deoxyguanosine (8-OHdG), are decreased after MSC administration in an ischaemic AKI model.57,96
The ability of MSCs to inhibit oxidative damage was further confirmed using an experimental model of cisplatin-induced AKI. The nitration of tyrosine residues is considered to be a marker of protein oxidation, and the expression level of nitrotyrosine was significantly increased in mice with cisplatin-induced AKI. Conversely, a marked reduction in nitrotyrosine staining was observed when mice were treated with MSCs. Furthermore, this protection was correlated with an increase pAkt expression in renal tissues, suggesting that pAkt signalling pathway could have participation on MSC anti-oxidant effect.97
MSC treatment can modulate the level of anti-oxidative molecules in the renal parenchyma after kidney injury with AKI mice injected with MSCs displaying higher expression levels of NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione reductase (GSH-Rx) and glutathione peroxidase (GSH-Px) when compared with control or untreated groups. Futhermore, the authors verified that the global oxidative index had decreased after MSC treatment.63 The antioxidant/oxidant balance may also be modulated by MSC administration in post-ischemic kidneys. MSC infusion significantly improved the activity of superoxide dismutase (SOD), a key molecule responsible for reducing oxidative stress, and increased GSH-Px expression, a potent antioxidant enzyme, in renal tissues. Treatment with MSCs also resulted in a significant reduction in the levels of malondialdehyde (MDA), which is associated with renal injury.98
In summary, MSCs can control the antioxidant/oxidant balance after kidney injury, potentially via HO-1 and EPO, contributing both to lower oxidative stress and to functional renal recovery (Figure 1, Table 1, Table 2).
Role of MSC Microvesicles in Cellular Reprogramming in AKI
MSCs also play a prominent role in the regulation of endogenous gene expression by promoting cellular reprogramming. These cells, via the secretion of small organelles or vesicles, may have a direct role in modulating gene expression in injured tissues or organs. These secreted microvesicles are loaded with miRNAs (small non-coding RNA molecules, about 21–25 nucleotides in length), which are potent epigenetic and non-epigenetic regulators. Once inside of target cells (such as damaged cells), these miRNAs can change the cellular genomic programming by modulating gene expression through translational repression of specific mRNAs targets or by up/down-regulating the expression of other miRNAs.99,100
As previously mentioned, some studies using models of renal disease have shown that medium conditioned with MSCs contains bioactive molecules such as HGF, IGF and VEGF, which could contribute to the growth and survival of endothelial and epithelial tubular cells and thus promote renal angiogenesis and regeneration.74,101 Indeed, in some cases, communication between the damaged renal cells and the MSCs can be critically important for the MSCs to induce their renoprotective effects. Co-culture of damaged renal cells and MSCs yields a conditioned medium which is more beneficial in promoting tubular cell proliferation and protection than conditioned medium generated by culture of MSCs in isolation.102
In 2009, Bruno and co-workers provided the first evidence that MSCs release microvesicles loaded with small RNAs, which might exert a renoprotective effect. First, the authors showed that MSC-derived microvesicles expressed MSC markers and had both proliferative and anti-apoptotic effects on tubular cells in vitro. After, these microvesicles were injected in glycerol-mediated AKI animals and promoted the recovery of renal function, as indicated by elevated indices of tissue proliferation index. Moreover, the renoprotective effects of MSC-derived microvesicles were abrogated both in vitro and in vivo when the vesicles were pre-treated with RNases.103 To test the occurrence of the horizontal transfer of small RNAs between MSCs and damaged renal cells, microvesicles were isolated from human MSCs that contain the specific human reporter genes POLR2E and SUMO-1. These genes, which are normally present only in human cells, were also found in murine cultures of epithelial cells and renal parenchyma of the treated mice, possibly by a microvesicle-mediated transfer mechanism.103
Additionally, another study reported that both IGF and its receptor (IGFR) are expressed in MSCs, but only IGFR was found in MSC-derived microvesicles. The co-culture of renal cells with MSC-derived microvesicles promoted IGFR up-regulation in tubular cells concomitant with elevated proliferation rates. Subsequently, it was verified that specifically inhibiting IGFR mRNA blocked this proliferative effect.104 Other studies demonstrated that microvesicle treatment improved renal functional parameters and fibrosis.105,106
Multiple infusions of microvesicles seemed to be more effective at conferring renal protection than one isolated intervention.105 In this context is important to know if MSC-derived microvesicles possess similar protective properties to MSCs. One study has demonstrated the additional pro-angiogenic effects of MSC-derived microvesicles. These microvesicles were internalised into endothelial cells and promoted their proliferation in a dose-dependent manner. Further, the in vitro administration of microvesicles enhanced the ability of the endothelial cells to form a capillary-like network, which is critical to promote angiogenesis. In addition, treatment with MSC-derived microvesicles in rat with hindlimb ischaemia also induced significant improvement in the blood flow recovery index compared to the vehicle control group.107
Finally, these preliminary studies support the evidence that MSC-derived microvesicles can have similar effects to MSCs by promoting proliferation and angiogenesis and inducing anti-apoptotic and anti-fibrotic effects, which may act synergically to confer renal protection. Although the precise mechanisms involved in microvesicle-mediated renoprotection remain unknown, small regulatory molecules such as growth factors, transcription factors and miRNAs appear to be intimately involved (Figure 1, Table 1). We believe that further studies will clarify and provide more information about these concepts and we hope that microvesicle therapy can be widely exploited in several kinds of situations and inflammatory diseases including AKI.
Conclusion
Over the past few years, MSC-based therapies have been extensively studied as a potential treatment for several inflammatory diseases. However, our understanding of the regulatory effects and mechanisms of action of MSCs or MSC-derived bioagents remain to be fully elucidated, and this current lack of understanding is a limiting factor to the utilization of this therapy in clinical practice.
Although promising results have been obtained using MSC therapies in experimental kidney diseases, these studies were all performed in small animals such as mice and rats, and autologous studies conducted with large-animal models have not exhibited the same reparative properties.108 In the first human clinical trial using MSCs as part of a cocktail of suppressive agents during kidney transplantation, adding MSCs was not more effective than treating the control group with standard doses of immunosuppressive agents. Although the combination of MSCs with a low dose of immunosuppressive drugs had some advantages (as a faster, but not better, improvement in glomerular filtration rates), this treatment has not yet yielded significant enough results to justify it replacing classical intervention.51
In addition, while MSCs have the ability to target and migrate to damaged tissues, a property that is relevant to their therapeutic use, evidence indicates that long-term MSC engraftment after systemic or local administration rarely occurs. Thus, a better understanding of the mechanisms behind tissue-specific homing is fundamental to establish how MSCs act dynamically in vivo. It is also important to understand how the numerous trophic factors secreted by MSCs interact in vivo to promote protection without causing side effects such as uncontrolled angiogenesis. In addition, the question of how and why many MSC features are niche- and species-specific (e.g. derived from fat, kidney, umbilical cord or bone marrow or obtained from rat, mice, or human) cannot be left unanswered.
Lastly, the viability, safety and efficacy of fresh and cryopreserved MSCs must be established prior to their routine use in clinical practice. Encouragingly, one experimental study on kidney disease found no difference in the therapeutic potential between fresh and cryopreserved MSCs.60
In summary, despite the remaining challenges, MSC-based therapies have great potential for clinical applications due to the potentially large repertoire of regulatory agents that they can secrete beyond their cell-to-cell contact interactions. Taken together, these properties of MSCs may provide an effective, innovative and affordable therapy for the treatment of acute and chronic renal diseases. Nevertheless, more studies need to be performed to provide a more comprehensive understanding of MSC-related therapies in order to ensure their safety for future clinical applications.
Footnotes
Competing Interests: None declared.
References
- 1.Warnock DG. Towards a definition and classification of acute kidney injury. J Am Soc Nephrol. 2005;16:3149–50. doi: 10.1681/ASN.2005090934. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liaño F, Pascual J, Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–9. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–85. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 7.Wald R, Quinn RR, Adhikari NK, Burns KE, Friedrich JO, Garg AX, et al. University of Toronto Acute Kidney Injury Research Group Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125:585–93. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–78. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 10.Molitoris BA. Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton. Am J Physiol. 1991;260:F769–78. doi: 10.1152/ajprenal.1991.260.6.F769. [DOI] [PubMed] [Google Scholar]
- 11.Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol. 1998;275:C711–31. doi: 10.1152/ajpcell.1998.275.3.C711. [DOI] [PubMed] [Google Scholar]
- 12.Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 13.Grino JM. BN 52021: a platelet activating factor antagonist for preventing post-transplant renal failure. A double-blind, randomized study. The BN 52021 Study Group in Renal Transplantation. Ann Intern Med. 1994;121:345–7. doi: 10.7326/0003-4819-121-5-199409010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36(Suppl):S152–8. doi: 10.1097/CCM.0b013e318168c613. [DOI] [PubMed] [Google Scholar]
- 15.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 17.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 18.Schaub S, Wilkins JA, Antonovici M, Krokhin O, Weiler T, Rush D, et al. Proteomic-based identification of cleaved urinary beta2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am J Transplant. 2005;5:729–38. doi: 10.1111/j.1600-6143.2005.00766.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf MW, Boldt J. Kidney specific proteins: markers for detection of renal dysfunction after cardiac surgery? Clin Res Cardiol Suppl. 2007;2:S103–7. [Google Scholar]
- 20.Roberts DS, Haycock GB, Dalton RN, Turner C, Tomlinson P, Stimmler L, et al. Prediction of acute renal failure after birth asphyxia. Arch Dis Child. 1990;65(10 Spec):1021–8. doi: 10.1136/adc.65.10_spec_no.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti M, Moutereau S, Zater M, Lallali K, Durrbach A, Manivet P, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med. 2006;44:288–91. doi: 10.1515/CCLM.2006.050. [DOI] [PubMed] [Google Scholar]
- 22.Venkataraman R. Can we prevent acute kidney injury? Crit Care Med. 2008;36(Suppl):S166–71. doi: 10.1097/CCM.0b013e318168c74a. [DOI] [PubMed] [Google Scholar]
- 23.Bagshaw SM, Delaney A, Jones D, Ronco C, Bellomo R. Diuretics in the management of acute kidney injury: a multinational survey. Contrib Nephrol. 2007;156:236–49. doi: 10.1159/000102089. [DOI] [PubMed] [Google Scholar]
- 24.Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526–31. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Swärd K, Valsson F, Odencrants P, Samuelsson O, Ricksten SE. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med. 2004;32:1310–5. doi: 10.1097/01.ccm.0000128560.57111.cd. [DOI] [PubMed] [Google Scholar]
- 26.Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004;65:1366–74. doi: 10.1111/j.1523-1755.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 27.Chater K, Kellum JA. Continuous vs. intermittent hemodialysis: with which spin will my patient win? Crit Care. 2007;11:313–4. doi: 10.1186/cc6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 33.Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–42. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 34.Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421–7. [PubMed] [Google Scholar]
- 35.Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–27. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 36.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–42. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 37.Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA, Jr, Proto-Siqueira R, et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340–4. doi: 10.1016/j.yexcr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.De Bari C, Dell’Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–21. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 39.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 40.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 41.Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36:1179–83. doi: 10.1590/s0100-879x2003000900006. [DOI] [PubMed] [Google Scholar]
- 42.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 43.Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci (Landmark Ed) 2009;14:4281–98. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- 44.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 45.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Andriolo G, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–54. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217:296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 48.Meirelles LDAS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–27. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Bassi EJ, de Almeida DC, Moraes-Vieira PM, Câmara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:329–42. doi: 10.1007/s12015-011-9311-1. [DOI] [PubMed] [Google Scholar]
- 50.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–25. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 51.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–77. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 52.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 53.Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R, et al. Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–13. doi: 10.1152/ajprenal.1996.271.2.F408. [DOI] [PubMed] [Google Scholar]
- 54.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–31. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 55.Bassi ÊJ, Moraes-Vieira PM, Moreira-Sá CS, Almeida DC, Vieira LM, Cunha CS, et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–45. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang N, Li Q, Zhang L, Lin H, Hu J, Li D, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012;7:e43768. doi: 10.1371/journal.pone.0043768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 58.Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–82. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Eliopoulos N, Zhao J, Bouchentouf M, Forner K, Birman E, Yuan S, et al. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol. 2010;299:F1288–98. doi: 10.1152/ajprenal.00671.2009. [DOI] [PubMed] [Google Scholar]
- 60.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25:3874–84. doi: 10.1093/ndt/gfq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SR, Lee SH, Moon JY, Park JY, Lee D, Lim SJ, et al. Repeated administration of bone marrow-derived mesenchymal stem cells improved the protective effects on a remnant kidney model. Ren Fail. 2010;32:840–8. doi: 10.3109/0886022X.2010.494803. [DOI] [PubMed] [Google Scholar]
- 62.Rampino T, Gregorini M, Bedino G, Piotti G, Gabanti E, Ibatici A, et al. Mesenchymal stromal cells improve renal injury in anti-Thy 1 nephritis by modulating inflammatory cytokines and scatter factors. Clin Sci (Lond) 2011;120:25–36. doi: 10.1042/CS20100147. [DOI] [PubMed] [Google Scholar]
- 63.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Y, Johnston P, Zhang B, Zakari A, Chowdhry T, Smith RR, et al. Kidney-derived stromal cells modulate dendritic and T cell responses. J Am Soc Nephrol. 2009;20:831–41. doi: 10.1681/ASN.2008030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y, Chen P, Zhang CB, Ko GJ, Ruiz M, Fiorina P, et al. Kidney-derived mesenchymal stromal cells modulate dendritic cell function to suppress alloimmune responses and delay allograft rejection. Transplantation. 2010;90:1307–11. doi: 10.1097/TP.0b013e3181fdd9eb. [DOI] [PubMed] [Google Scholar]
- 66.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11:1012–30. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 68.Sanz L, Santos-Valle P, Alonso-Camino V, Salas C, Serrano A, Vicario JL, et al. Long-term in vivo imaging of human angiogenesis: critical role of bone marrow-derived mesenchymal stem cells for the generation of durable blood vessels. Microvasc Res. 2008;75:308–14. doi: 10.1016/j.mvr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–8. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol. 2006;291:F902–12. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Park HC, Addabbo F, Ni J, Pelger E, Li H, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–89. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kucic T, Copland IB, Cuerquis J, Coutu DL, Chalifour LE, Gagnon RF, et al. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295:F488–96. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- 73.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–82. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 74.Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13(8B):2109–14. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–40. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 76.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–46. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 78.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–56. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 79.Park HC, Yasuda K, Ratliff B, Stoessel A, Sharkovska Y, Yamamoto I, et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol. 2010;298:F357–64. doi: 10.1152/ajprenal.00542.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–8. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 81.Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, Bussolati B, et al. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol. 2010;177:2011–21. doi: 10.2353/ajpath.2010.091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rabkin R, Fervenza F, Tsao T, Sibley R, Friedlaender M, Hsu F, et al. Hepatocyte growth factor receptor in acute tubular necrosis. J Am Soc Nephrol. 2001;12:531–40. doi: 10.1681/ASN.V123531. [DOI] [PubMed] [Google Scholar]
- 83.Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, et al. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22:287–97. doi: 10.3727/096368912X655019. [DOI] [PubMed] [Google Scholar]
- 84.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–73. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 85.Donizetti-Oliveira C, Semedo P, Burgos-Silva M, Cenedeze MA, Malheiros DM, Reis MA, et al. Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant. 2012;21:1727–41. doi: 10.3727/096368911X623925. [DOI] [PubMed] [Google Scholar]
- 86.Asanuma H, Vanderbrink BA, Campbell MT, Hile KL, Zhang H, Meldrum DR, et al. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res. 2011;168:e51–9. doi: 10.1016/j.jss.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cavaglieri RC, Martini D, Sogayar MC, Noronha IL. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–51. doi: 10.1016/j.transproceed.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 88.Liu X, Shen W, Yang Y, Liu G. Therapeutic implications of mesenchymal stem cells transfected with hepatocyte growth factor transplanted in rat kidney with unilateral ureteral obstruction. J Pediatr Surg. 2011;46:537–45. doi: 10.1016/j.jpedsurg.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 89.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–43. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 90.Shu T, Zeng B, Ren X, Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell. 2010;42:217–22. doi: 10.1016/j.tice.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–61. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 92.Kim MH, Cho GW, Huh YM, Kim SH. Transduction of human EPO into human bone marrow mesenchymal stromal cells synergistically enhances cell-protective and migratory effects. Mol Biol (Mosk) 2010;44:656–63. [PubMed] [Google Scholar]
- 93.Vanella L, Sanford C, Jr, Kim DH, Abraham NG, Ebraheim N. Oxidative stress and heme oxygenase-1 regulated human mesenchymal stem cells differentiation. Int J Hypertens. 2012;2012:890671. doi: 10.1155/2012/890671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38:426–35. doi: 10.1016/j.freeradbiomed.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 95.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011;300:F254–62. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu H, McTaggart SJ, Johnson DW, Gobe GC. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14:162–72. doi: 10.3109/14653249.2011.613927. [DOI] [PubMed] [Google Scholar]
- 97.Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–22. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 98.Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86:191–6. doi: 10.1159/000319366. [DOI] [PubMed] [Google Scholar]
- 99.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonventre JV. Microvesicles from mesenchymal stromal cells protect against acute kidney injury. J Am Soc Nephrol. 2009;20:927–8. doi: 10.1681/ASN.2009030322. [DOI] [PubMed] [Google Scholar]
- 101.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–35. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 102.Lindoso RS, Araujo DS, Adão-Novaes J, Mariante RM, Verdoorn KS, Fragel-Madeira L, et al. Paracrine interaction between bone marrow-derived stem cells and renal epithelial cells. Cell Physiol Biochem. 2011;28:267–78. doi: 10.1159/000331739. [DOI] [PubMed] [Google Scholar]
- 103.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–80. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He J, Wang Y, Sun S, Yu M, Wang C, Pei X, et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17:493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–97. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Behr L, Hekmati M, Lucchini A, Houcinet K, Faussat AM, Borenstein N, et al. Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif. 2009;42:284–97. doi: 10.1111/j.1365-2184.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


