Abstract
We previously analysed clinical and immunological parameters under Trichuris suis ova (TSO) therapy in four patients with secondary progressive multiple sclerosis. The serum Brain-derived neurotrophic factor (BDNF) levels of these four patients were assessed before, during and after therapy with TSO and showed significant decrease of BDNF during TSO therapy (p < 0.05).
Keywords: Multiple sclerosis, Trichuris suis ova, Brain-derived neurotrophic factor, Neuroprotection
Abstract
Nous avons précédemment analysé les paramètres cliniques et immunologiques sous thérapie par des œufs de Trichuris suis (TSO) chez quatre patients avec sclérose en plaques progressive secondaire. Les niveaux du facteur neurotrophique dérivé du cerveau (BNDF) chez ces quatre patients ont été mesurés avant, pendant et après la thérapie par TSO et ont montré une diminution significative du BNDF pendant la thérapie par TSO (p < 0.05).
Introduction
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin protein family, is an important modulator of neurotransmitter release and synaptic plasticity [11, 16] and has been hypothesized to play a role in the neuroprotective mechanisms of some MS therapies [1, 8, 13].
Correale and Farez could demonstrate beneficial immunomodulation by helminths in humans in an observational study of relapsing-remitting multiple sclerosis (RRMS) patients with community-acquired gastrointestinal infections [3]. They demonstrated that B cells isolated from these helminth-infected MS patients produced greater amounts of BDNF in vitro compared to those of normal subjects [4]. Fleming and colleagues reported favourable MRI and immunological results in MS patients after probiotic treatment with Trichuris suis ova (TSO) [5].
In the small trial presented previously we analysed the effects of TSO therapy on disease course and different immunological parameters in four patients with secondary progressive MS and found downregulation of Th1-associated cytokines [2]. With the present paper we analysed in this four patients the role of BDNF in MS therapy using TSO.
Material and methods
Four patients with secondary progressive MS without any other immunomodulatory or immunosuppressive therapy were treated for 6 months with TSO 2,500 eggs orally every 14 day. TSO were produced and provided by Ovamed GmbH, Barsbüttel, Germany, as a non-sterile aqueous suspension containing 2,500 embryonated alive TSO in 15 mL of sulphate stabilizer (0.05 mmol/l H2SO4). Patients were recruited from the Department of Neurology, Charité – Universitätsmedizin Berlin, and all fulfilled the 2005 McDonald criteria [12]. The study was approved by the local ethics committee. Blood for BDNF analysis was drawn in the morning, centrifuged (800 g for 15 min) and stored at −80 °C until the BDNF concentrations were determined. BDNF serum concentration was quantified by a modified ELISA (Promega Co., Madison, WI, USA) as previously described [15].
To evaluate effects of TSO therapy on BDNF serum levels we used a t-test for paired observations.
Serum from patients was available 1 month before (−1), at the start (0), and at months 1, 2, 3, 6 during therapy, as well as 1 and 4 months after TSO therapy.
Results
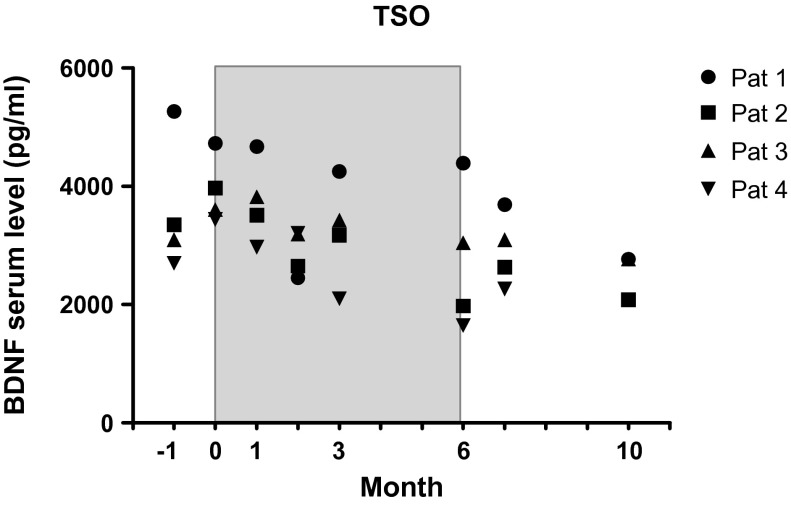
BDNF serum levels were within the range of previously published data on healthy controls [7]. When calculating means of BDNF levels from our four patients for each time point we saw a clear decrease under treatment and in the post-treatment period compared to the pre-treatment period (month −1: 3682.55 ± 1105.17; month 0: 3938.15 ± 572.08; month 1: 3744.98 ± 710.07; month 2: 2876.92 ± 381.10; month 3: 3238.18 ± 885.93; month 6: 2765.28 ± 1236.73; month 7: 2922.97 ± 615.90; month 10: 2826.03 ± 569.61). Statistical analysis of BDNF levels of different time points by t-test failed significance due to small sample size of this preliminary study.
Because the BDNF levels did not differ significantly between the two pre-treatment measurements, a mean pre-treatment BDNF level was computed at the individual level. The same procedure was applied for the two post-treatment levels, which also did not differ significantly from each other. A pairwise comparison of the four BDNF levels under treatment revealed statistically significant differences only between the first and the last assessment (p < 0.05). The BDNF levels after therapy were significantly lower than before therapy: pre-treatment: 3810.4 ± 831.1; post-treatment: 2875.5 ± 572.6, p < 0.02 (see Figure 1).
Figure 1.
BDNF serum levels of SPMS patients prior to (Month –1, 0), during (Months 1, 2, 3, 6) and subsequent to (Month 7, 10) therapy with TSO (shaded area).
Discussion
Taken together, we found a significant decrease in serum levels of BDNF in our four SPMS patients under therapy with TSO and speculate that TSO influences BDNF metabolism.
Assessment of serum BDNF levels in MS has yielded conflicting results to date [1, 6, 10]. Besides by neuronal secretion BDNF can also be produced by activated T cells, B cells, monocytes and macrophages and is influenced by handling of blood ex vivo [9]. Especially the BDNF production of immune cells in inflammatory brain lesions is discussed to be a result of body’s compensatory neuroprotective mechanism. In line with this understanding is the increase of BDNF levels during relapses in MS [6] and in stimulated immune cells of patients with higher inflammatory activity in the white matter [14].
Correale and colleagues showed increased production of BDNF and nerve growth factor in stimulated B cells from MS patients with a helminth infection compared to uninfected patients and controls. The authors discuss that these neurotrophic factors, produced by B cells, might have a possible immunoregulatory and probably immunosuppressive effect on T cells in helminth-infected MS patients.
An explanation for the opposite trends in BDNF levels shown in the study of Correale and ours may be several differences in study design: e.g. relapsing-remitting vs. secondary progressive MS, stimulated B cells vs. serum levels, natural infections vs. experimental TSO treatment, clinical observational vs. prospective clinical trial study design. In our case, the decreased serum BDNF levels under helminth therapy led us to speculate that reduced inflammatory activation of the CNS caused the decrease in serum BDNF levels. Because peripheral immune cells can also be a source of peripheral BDNF, one could discuss, that TSO induces reduced inflammatory activation of peripheral immune cells and consequently a reduced serum BDNF level.
One limitation of this pilot study is the small sample size of the trial, which was one of the first investigating TSO treatments in MS.
Although the precise relationship between serum BDNF levels and cerebral concentration of BDNF in humans remains unclear, our study does not suggest that BDNF might have a neuroprotective effect in MS patients treated with TSO.
Acknowledgments
We thank Silvia Saft for excellent technical assistance and James Ari Liebkowsky for language editing of the manuscript.
Cite this article as: Rosche B, Werner J, Benzel FJ, Harms L, Danker-Hopfe H & Hellweg R: Serum levels of brain-derived neurotrophic factor (BNDF) in multiple sclerosis patients with Trichuris suis ova therapy. Parasite, 2013, 20, 55.
References
- 1.Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. 2005. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. Journal of Neuroimmunology, 167(1–2), 215–218 [DOI] [PubMed] [Google Scholar]
- 2.Benzel F, Erdur H, Kohler S, Frentsch M, Thiel A, Harms L, Wandinger KP, Rosche B. 2012. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. Journal of Helmintholology, 86(3), 339–347 [DOI] [PubMed] [Google Scholar]
- 3.Correale J, Farez M. 2007. Association between parasite infection and immune responses in multiple sclerosis. Annals of Neurology, 61(2), 97–108 [DOI] [PubMed] [Google Scholar]
- 4.Correale J, Farez M, Razzitte G. 2008. Helminth infections associated with multiple sclerosis induce regulatory B cells. Annals of Neurology, 64(2), 187–199 [DOI] [PubMed] [Google Scholar]
- 5.Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD, Field AS, Boland J, Fabry Z. 2011. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Multiple Sclerosis Journal, 17(6), 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DR, Teixeira AL. 2009. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neuroscience Letters, 460(2), 130–132 [DOI] [PubMed] [Google Scholar]
- 7.Geisel O, Banas R, Schneider M, Hellweg R, Muller CA. 2013. Serum levels of brain-derived neurotrophic factor in patients with internet use disorder. Psychiatry Research, 209(3), 525–528 [DOI] [PubMed] [Google Scholar]
- 8.Jones JL, Anderson JM, Phuah CL, Fox EJ, Selmaj K, Margolin D, Lake SL, Palmer J, Thompson SJ, Wilkins A, Webber DJ, Compston DA, Coles AJ. 2010. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain, 133(Pt 8), 2232–2247 [DOI] [PubMed] [Google Scholar]
- 9.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. 1999. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? Journal of Experimental Medicine, 189(5), 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liguori M, Fera F, Patitucci A, Manna I, Condino F, Valentino P, Telarico P, Cerasa A, Gioia MC, Palma G, Quattrone A. 2009. A longitudinal observation of brain-derived neurotrophic factor mRNA levels in patients with relapsing-remitting multiple sclerosis. Brain Research, 1256, 123–128 [DOI] [PubMed] [Google Scholar]
- 11.Luhder F, Gold R, Flugel A, Linker RA. 2013. Brain-derived neurotrophic factor in neuroimmunology: lessons learned from multiple sclerosis patients and experimental autoimmune encephalomyelitis models. Archivum Immunologiae et Therapiae Experimentalis, 61(2), 95–105 [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. 2005. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Annals of Neurology, 58(6), 840–846 [DOI] [PubMed] [Google Scholar]
- 13.Thöne J, Ellrichmann G, Seubert S, Peruga I, Lee DH, Conrad R, Hayardeny L, Comi G, Wiese S, Linker RA, Gold R. 2012. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. American Journal of Pathology, 180(1), 267–274 [DOI] [PubMed] [Google Scholar]
- 14.Weinstock-Guttman B, Zivadinov R, Tamaño-Blanco M, Abdelrahman N, Badgett D, Durfee J, Hussein S, Feichter J, Patrick K, Benedict R, Ramanathan M. 2007. Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. Journal of Neuroimmunology, 188(1–2), 167–174 [DOI] [PubMed] [Google Scholar]
- 15.Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. 2007. Serum neurotrophins – a study on the time course and influencing factors in a large old age sample. Neurobiology of Aging, 28(9), 1436–1445 [DOI] [PubMed] [Google Scholar]
- 16.Zuccato C, Cattaneo E. 2009. Brain-derived neurotrophic factor in neurodegenerative diseases. Nature Reviews Neurology, 5(6), 311–322 [DOI] [PubMed] [Google Scholar]