Abstract
Eudaimonic well-being—a sense of purpose, meaning, and engagement with life—is protective against psychopathology and predicts physical health, including lower levels of the stress hormone cortisol. Although it has been suggested that the ability to engage the neural circuitry of reward may promote well-being and mediate the relationship between well-being and health, this hypothesis has remained untested. To test this hypothesis, we had participants view positive, neutral, and negative images while fMRI data were collected. Individuals with sustained activity in the striatum and dorsolateral prefrontal cortex to positive stimuli over the course of the scan session reported greater well-being and had lower cortisol output. This suggests that sustained engagement of reward circuitry in response to positive events underlies well-being and adaptive regulation of the hypothalamic-pituitary-adrenal axis.
Keywords: well-being, rewards, neuroimaging
The concept of well-being—a sense of purpose and meaningful, positive engagement with life (Ryff, 1989)—has been of interest to philosophers since at least Aristotle. Aristotle termed this type of well-being eudaimonia and differentiated it from hedonic positive affect (PA), defined as the momentary experience of pleasure (see also Diener & Lucas, 1999). Aristotle was fundamentally interested in how people may increase their own degree of eudaimonic well-being, and at one point called eudaimonia the “highest of all goods achievable by human action” (Aristotle, 350 B.C./1925, p. 5). Supporting Aristotle’s interest in eudaimonic well-being, research has begun to uncover a link between well-being and measures of health (Pressman & Cohen, 2005). Individual differences in multiple types of well-being have been found to predict a variety of objective indices of health, including cardiovascular health (Boehm & Kubzansky, 2012); levels of stress, as measured by the hormone cortisol (Ryff, Singer, & Dienberg Love, 2004); and peripheral inflammation (Dockray & Steptoe, 2010). However, although there appears to be an important relationship between eudaimonic well-being and physical health, the neurobiology mediating this link is not understood.
The links between brain function and various health outcomes, including regulation of stress hormones, have been previously examined: Functional-neuroimaging studies have suggested that a network including the hippocampus, the amygdala, the prefrontal cortex (PFC), and the striatum may impact (or be impacted by) cortisol release (Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009; Pruessner et al., 2010; Strelzyk et al., 2012; Urry et al., 2006). These areas are also generally thought to be part of a circuit involved in the regulation of emotion (Ochsner & Gross, 2008). Therefore, the fact that their function is tightly linked with stress responses is not surprising. Of note, the ventral striatum, the amygdala, and the hippocampus may be particularly relevant for regulating cortisol release because they all directly connect to the paraventricular nucleus of the hypothalamus (Bubser & Deutch, 1999; Haber & Knutson, 2010), a key element of the hypothalamic-pituitary-adrenal axis.
In contrast, only recently have neuroscientists begun examining the other half of this association—the link between the brain and well-being. Two prior studies examining the neural correlates of eudaimonic well-being have found that surface electrophysiology measures of relative left anterior cortical activation at rest (Urry et al., 2004) and ventromedial prefrontal cortex (VMPFC) blood-oxygen-level-dependent signal in response to negative affective stimuli (van Reekum et al., 2007) predict well-being. In recent commentaries, Kringelbach and Berridge (e.g., Kringelbach & Berridge, 2009) have suggested that brain regions involved in basic reward processing, such as the ventral striatum and the PFC, may play an important role in well-being. However, this compelling hypothesis remains untested. Moreover, these predictions do not incorporate the fact that variability in the temporal dynamics of emotion critically contributes to health and well-being (Heller et al., 2009; Heller et al., 2013; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002; Solomon & Corbit, 1974), nor do they make predictions regarding how these temporal dynamics may be encoded in the brain.
Given that a variety of emotionally tinted events occur in an intermixed fashion over time, it may be particularly important for an individual to maintain engagement of reward circuitry in response to positive events, even in the midst of negative ones. Our prior work suggested that the inability to repeatedly engage reward circuitry in a sustained manner when presented with positive events intermixed with negative ones is associated with core clinical features of depression, such as reductions in positive affect (Heller et al., 2009). Thus, it is possible that sustained engagement of reward circuits may be important for well-being and protective against stress in healthy individuals. However, data linking sustained engagement of reward circuitry to well-being and objective measures of stress is lacking.
Therefore, in a sample of adults of a wide age range (N = 64; mean age = 58.20 years), we examined whether individual differences in the sustained engagement of reward circuitry in response to affective stimuli were related to health and well-being.1 We used a linear-trend analysis across the five scan runs of the study to derive a statistic representing the rate of habituation of brain activity in response to affective stimuli. Because the distinction between eudaimonic well-being and hedonic PA has been of fundamental interest to philosophers since Aristotle (Aristotle, 1925) and modern researchers (Kringelbach & Berridge, 2009) alike, we had participants complete a measure of eudaimonic well-being (Ryff, 1989) approximately 3 years prior to the scan session and a measure of trait hedonic PA (Watson, Clark, & Tellegen, 1988). These self-report measures were complemented with indicators of diurnal fluctuations of the stress hormone cortisol. Approximately 1.5 years prior to the scan session, participants provided four saliva samples per day on 4 consecutive days while at home. Cortisol magnitude was extracted, and we calculated the area under the curve with respect to ground (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) as an indicator of total hormonal output. Lower total cortisol output over the course of the day has been found to predict higher levels of positive affect (Steptoe, Wardle, & Marmot, 2005) and lower levels of morbidity (Dekker et al., 2008).
Method
Participants
Seventy-two participants (45 females, 27 males; age range = 38–79 years, M = 58.20, SD = 11.42) constituting a representative sample of the midwestern United States from the MIDUS-II sample (http://www.midus.wisc.edu/) were scanned in a General Electric 3 Tesla scanner. There were several waves of data collection for the MIDUS study, of which the functional-MRI (fMRI) portion was the last. Therefore, there was some variability between assessments. Of the 72 participants, 8 participants were excluded because of excessive motion: Four participants had several large spikes (> 2 mm), 2 participants had severe drift (> 2.5 mm), and 2 participants had severe drift and large spikes in combination. For more details, see The MIDUS Project and Participants in the Method section of the Supplemental Material.
Self-report measurements
Participants completed a measure of psychological well-being (PWB; Ryff, 1989; Ryff & Keyes, 1995), the Positive And Negative Affect Schedule-Now (PANAS-Now, measuring current PA; for more details, see Self-Report Measurements in the Supplemental Material) scale as well as the trait PANAS-Gen scale (Watson et al., 1988). The PWB measure contained six subscales (measuring self-acceptance, positive relations with others, autonomy, environmental mastery, purpose in life, and personal growth) that have been hypothesized to account for the primary dimensions encompassing the PWB construct. The PWB measure is a highly reliable measure of eudaimonic well-being (6-week test-retest reliability for the six scales > 0.8; Ryff, 1989). The PWB measure also showed high stability: Using the reliable-change index (Christensen & Mendoza, 1986), we found that more than 95% of the sample showed no evidence of reliable increases or decreases on any of the six well-being scales over a 9- to 10-year period. Thus, the evidence suggested that there should be minimal change in PWB over the time lag between the fMRI scan and the PWB assessments.
To compute an overall PWB score, we calculated the mean score across the six subscales. PWB was measured during the first wave of data collection, which was conducted an average of 1,212 days prior to the fMRI session (SD = 367, minimum number of days = 348, maximum number of days = 1,669). In addition, the trait PANAS-Gen was administered. This scale asks participants to “Indicate to what extent you GENERALLY feel this way, that is, how you feel ON AVERAGE.” The PANAS-Gen was administered an average of 393 days before the fMRI session (though note that the median number of days between the completion of the PANAS-Gen and the fMRI session was 1 day, because only a subset of participants were recruited for the fMRI session 3–4 years later, whereas the majority were recruited to return the following day). For details about analyses contrasting the relation between sustained activity and both general and momentary hedonic well-being (i.e., the PA scale from the PANAS-Now), see Mediation Analysis in the Method section of the Supplemental Material.
Imaging task
Participants viewed 60 positive, 60 neutral, and 60 negative images for 4 s each over the course of five functional scan runs with a variable-length (4–16 s) intertrial interval. Images were taken from the International Affective Picture System (IAPS) set (Lang, Bradley, & Cuthbert, 2005), and their presentation order was randomized. Participants saw each image once during the scan session. Following 120 of the trials, a neutral male face (taken from the Surrey Set2) was presented for 500 msec either 1 or 3 s after the offset of the IAPS slide.3 Because our prediction regarding striatal engagement and well-being pertained to the neural response to the affective slides, we restricted our analysis to the IAPS-image-presentation epoch. For more details about the task and about image acquisition, see Imaging Task and Image Acquisition in the Method section of the Supplemental Material.
Image analysis
Using a 3-level approach in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Woolrich et al., 2009), a separate general linear model was computed for each run of echoplanar-imaging data. Subsequently, a fixed-effects general linear model was computed to combine the parameter estimates of the five runs for each participant. Sustained neural response was measured as the ability of participants to repeatedly engage reward circuitry over the scan session.
In order to examine such sustained brain activity over the five scan runs, we performed a linear trend analysis in which we weighted scan run as −2, −1, 0, 1, and 2. This yielded a beta estimate corresponding to the slope of change in activity over the scan runs for the three conditions (positive, negative, and neutral). The product of this fixed-effects analysis was used in the group analysis. At the group level, we performed a multiple regression examining the relationship between sustained brain activity (i.e., the beta value obtained in the fixed-effects analysis) and mean PWB. Voxel-wise data were thresholded at p < .005 (k > 60 voxels), which corresponds to a significance level of p < .05 corrected for multiple comparisons based on Monte Carlo simulation across the whole brain. Monte Carlo simulations were conducted using AFNI’s AlphaSim program (http://afni.nimh.nih.gov/). Clusters found to be significant in the group analysis examining the relationship between PWB and sustained brain activity in response to positive images were used in subsequent analyses testing specificity and relationships with cortisol.
Following the initial group analysis, we performed several follow-up analyses examining the specificity of the finding of sustained brain activity in response to positive images and PWB. We extracted the mean sustained activity in the cluster in response to the valenced image and used these cluster statistics in follow-up analyses. To examine specificity of repeated striatal or dorsolateral prefrontal cortex (DLPFC) engagement with regard to valence, we performed simultaneous regression analyses including sustained neural activity in response to both positive and negative (or neutral) stimuli to predict PWB. To examine specificity with regard to sustained (relative to phasic) activity, we aggregated the mean signal across the entire scan session (as is commonly done in fMRI studies) and included both sustained and aggregated (striatal, DLPFC) measures in simultaneous regression analyses to test which measure accounted for greater variance in PWB and in cortisol. For more details, see Image Analyses in the Method section of the Supplemental Material.
Cortisol acquisition and analysis
Saliva samples were collected when participants first awoke (before they got out of bed), 30 min after they got out of bed, before they had lunch, and before they went to bed for 4 consecutive days. For additional details, and for details about the mediation-analysis method, see Cortisol Acquisition and Analysis and Mediation Analysis in the Method section of the Supplemental Material.
Results
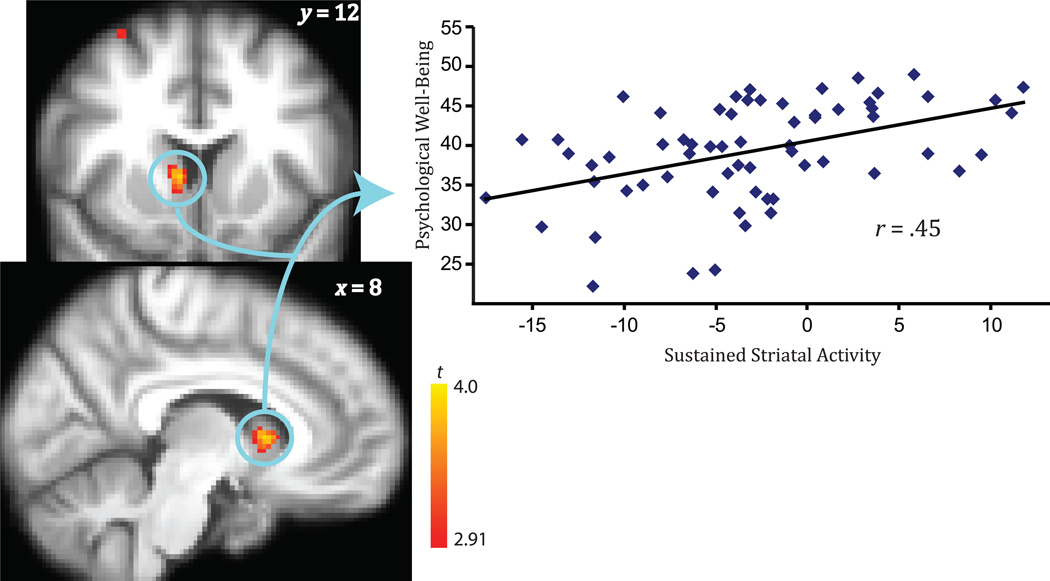
Increased well-being was associated with sustained engagement of brain activity in response to positive images in two regions (whole-brain analysis corrected for multiple comparisons): the striatum (in which the cluster extended into the ventral striatum; hereafter, we refer to this cluster as the striatum extending ventrally; r = .45, p < .001) and the right DLPFC (r = .47, p < .001; see Fig. 1, Fig. 2, and Table 1). The relationship between sustained engagement of the striatum and well-being remained significant after we controlled for individual differences in days between well-being assessment and fMRI scan session, b = 0.42, t(61) = 3.91, p < .001. The relationship between sustained engagement of the striatum and well-being remained after we controlled for sustained activity in response to neutral images, b = 0.35, t(61) = 2.91, p < .005. To further examine the valence specificity of the effect, we tested whether sustained striatal activity over the scan session in response to negative images predicted well-being. It did not (r = .15, p = .23), which suggests specificity of sustained striatal activity in response to positive stimuli predicting well-being. Indeed, the correlation of sustained striatal activity over the scan session to negative images was significantly lower than the correlation of sustained striatal activity over the scan session to positive images, t(61) = 2.38, p = .02. Because our sample included individuals whose ages spanned a wide range, we verified that controlling for age did not attenuate this relationship, b = 0.39, t(61) = 3.61, p < .001. We also tested whether striatal activity in response to the positive images aggregated across the scan session (as is customarily done in imaging studies) predicted well-being; in a simultaneous regression including measures of sustained and aggregated striatal activity to predict well-being, only sustained striatal activity predicted well-being, b = 0.42, t(61) = 3.94, p < .001, whereas aggregated striatal activity did not, b = 0.001, t(61) = 0.04, p = .97.
Fig. 1.
Psychological well-being as a function of the ability to sustain activity in the striatum (extending into the ventral striatum) in response to positive stimuli (corrected for multiple comparisons; p < .005, k > 60). Brain images show areas of activation. Coordinates are shown in Montreal Neurological Institute format.
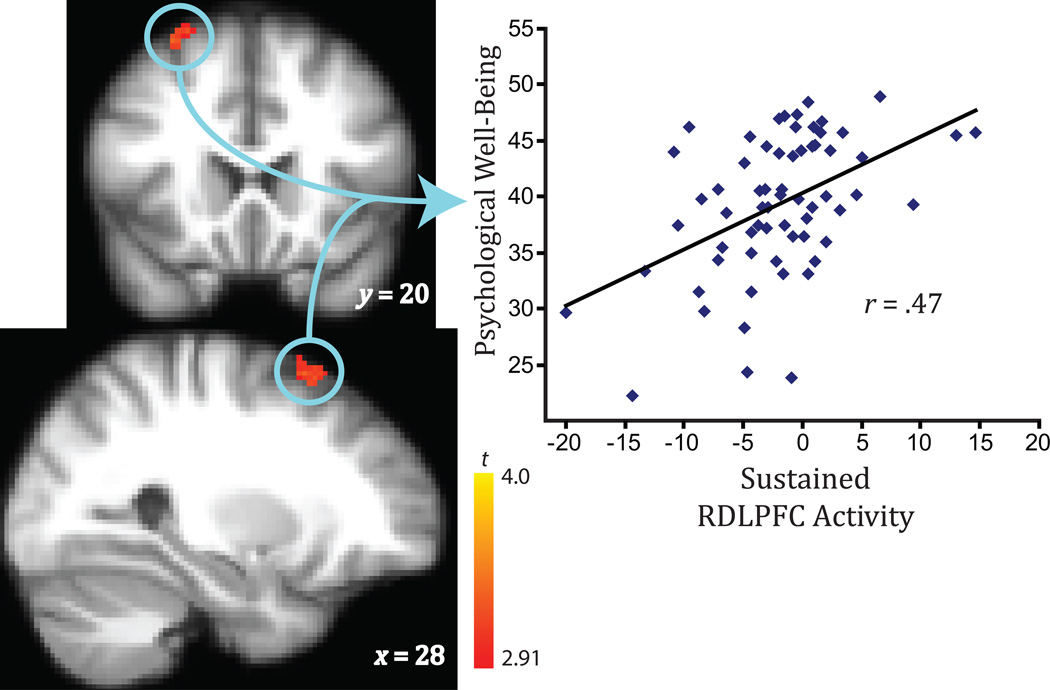
Fig. 2.
Psychological well-being as a function of the ability to sustain right dorsolateral prefrontal cortex (RDLPFC) activity in response to positive stimuli (corrected for multiple comparisons; p < .005, k > 60). Brain images show areas of activation. Coordinates are shown in Montreal Neurological Institute format.
Table 1.
Result of Voxel-Wise Correlation Between Sustained Brain Activity and Level of Eudaimonic Well-Being
| Region | Montreal Neurological Institute coordinates |
Cluster size | Maximum t value |
|---|---|---|---|
| Right DLPFC (Brodmann’s area 8/9) | x = 30, y = 14, z = 60 | 75 | 3.71 |
| Right striatum | x = 8, y = 12, z = 4 | 61 | 4.16 |
Note: “striatum” here refers to a cluster extending into the ventral striatum.
To assess the specificity of the relationship between sustained engagement of the striatum and eudaimonic well-being, we also assessed the relationship between trait hedonic PA and sustained engagement of the striatum in response to positive stimuli. There was a significant zero-order correlation between PANAS-Gen PA and sustained striatal activity, r = .31, t(62) = 2.58, p = .01. However, when including both PANAS-Gen PA and PWB in a simultaneous regression predicting sustained striatal activity, we found that PWB uniquely predicted sustained striatal activity, b = 0.50, t(61) = 2.85, p = .006, whereas PANAS-Gen PA did not, b = 0.24, t(61) = 0.143, p = .89 (results for current hedonic PA were identical; see Analyses With the PANAS-Now in the Results section of the Supplemental Material for details). This suggests that sustained engagement of the striatum in response to positive stimuli across trials is specifically related to eudaimonic well-being as opposed to hedonic PA per se.
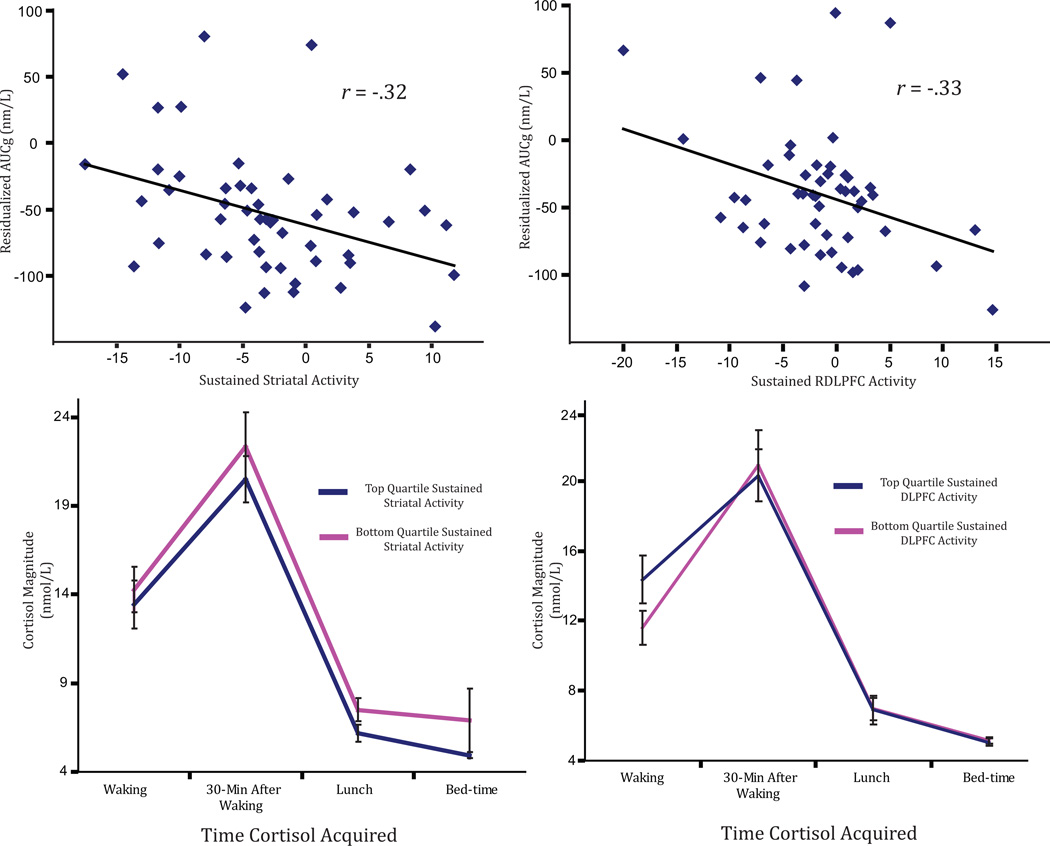
To extend these results, we tested whether sustained striatal activity predicted cortisol output over the course of a day. Because certain medications have been found to affect cortisol output, we additionally controlled for the number of blood pressure, cholesterol, depression, and corticosteroid medications participants were currently prescribed (Kirschbaum, Pirke, & Hellhammer, 1995). Sustained striatal activity in response to positive stimuli significantly predicted cortisol output, b = −2.43, t(44) = −2.23, p = .03, such that individuals with greater sustained striatal activity had lower daily cortisol output (Fig. 3). This effect was not attenuated when we controlled for age (p = .02), nor was it attenuated when we controlled for the number of days between cortisol assessment and fMRI session, b = −2.46, t(43) = −2.31, p = .03. Further suggesting specificity to positive stimuli, sustained striatal activity in response to negative stimuli did not predict cortisol output, b = −0.04, t(44) = −1.61, p = .11. Critically, sustained striatal activity in response to positive stimuli significantly mediated the relationship between well-being and cortisol (95% confidence interval = [−2.90, −0.10], p < .05), which suggests that sustained engagement of striatal activity may act as a protective factor in reducing daily cortisol output.4
Fig. 3.
Sustained striatal activity (left) and dorsolateral prefrontal cortex (DLPFC) activity (right) in response to positive stimuli predicts total cortisol output over the day such that more sustained activity in the striatum (a cluster extending into the ventral striatum) in response to positive pictures predicts lower cortisol output – when controlling for medications currently prescribed. The analytic strategy utilized continuous distribution of sustained blood-oxygen-level-dependent signals, but for illustrative purposes, we have split the data into quartiles here. AUCg indicates Area Under the Curve with respect to ground. Error bars indicate standard errors of the means.
With regard to the significant association between sustained engagement of the dorsolateral prefrontal cortex (DLPFC) in response to positive images and well-being, the relationship remained after we controlled for sustained activity in response to neutral images, b = 0.55, t(61) = 4.40, p < .001, whereas sustained activity in response to neutral images did not predict well-being, b = −0.16, t(61) = −1.31, p = .19. For results from additional specificity analyses, see Analyses Addressing Specificity for the RDLPFC in the Supplemental Material.
To assess the specificity of the relationship between sustained DLPFC activity and well-being, we assessed the relationship between trait PA and sustained DLPFC activity in response to positive stimuli. There was a significant zero-order correlation between PANAS-Gen PA and sustained DLPFC activity, r = .32, t(62) = 2.69, p = .009. As with the striatum, when we included well-being and trait PA in a simultaneous regression to predict sustained DLPFC activity, well-being uniquely predicted sustained DLPFC activity, b = 0.42, t(61) = 3.00, p = .004, whereas PA did not, b = 0.20, t(61) = .14, p = .89. This suggests that sustained engagement of the DLPFC in response to positive stimuli is specifically related to eudaimonic well-being. To examine the total variance accounted for by well-being, we performed a simultaneous regression in which we included both sustained striatal activity and sustained DLPFC activity to predict well-being. The multiple correlation squared was .29, which suggests that 29% of the variance in well-being was accounted for by sustained striatal and DLPFC activity in response to positive images.
Sustained DLPFC activity in response to positive stimuli significantly predicted cortisol output over the course of the day, b = −2.50, t(44) = −2.04, p = .05, when we controlled for the medications known to affect cortisol output, such that individuals with greater sustained DLPFC activity had lower daily cortisol output. This effect was not attenuated when we controlled for age (p = .04) or for days between cortisol assessment and fMRI scan session, b = −2.71, t(43) = −2.25, p = .03. Again suggesting specificity to positive stimuli, sustained DLPFC activity in response to negative stimuli did not predict cortisol output, b = −0.91, t(44) = −0.84, p = .40. Using the same bootstrapping method that we used for the striatum cluster, we found that sustained DLPFC activity also mediated the relationship between well-being and cortisol (95% confidence interval = [−2.85, −0.05], p < .05).
Finally, given the possibility that subcortical activity mediates the relationship by which prefrontal engagement impacts behavior (Ochsner & Gross, 2008; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), we tested whether the sustained activity in the striatum cluster found in the group analysis mediated the relationship between sustained DLPFC activity and well-being. Indeed it did (95% confidence interval = [0.02, 0.31], p < .05), which suggests that sustained DLPFC engagement may only promote well-being to the degree that it effectively influences striatal activity for that individual.
Discussion
Nearly four decades ago, Solomon and Corbit (1974) suggested that elucidating the temporal dynamics of emotion could yield a more complete and nuanced understanding of affect. It is only recently that researchers have begun to empirically test this prediction. How the brain responds to the repeated occurrence of positive stimuli has been successfully examined to explain affective differences between depressed or at-risk individuals and healthy controls (Heller et al., 2009; Siegle et al., 2002). Whether similar dynamics may account for individual differences in well-being in healthy individuals had not been previously examined, but our findings suggest that they do. Such dynamics accounted for unique variance in well-being, given that activity aggregated across the scan session did not predict well-being. It is worth noting that this paradigm involved the presentation of positive images embedded within a stream of stimuli that included neutral and negative images. In our view, the use of this procedure enhances the ecological validity of these findings, given that in everyday life, individuals do not often encounter uninterrupted positive stimuli. Negative experiences intermix with positive ones, and the ability of individuals to repeatedly engage circuitry involved in reward and positive affect in the face of negative stimuli may be important for health and well-being.
Our data demonstrate that the specific sustained engagement of reward circuitry in response to positive stimuli is associated with increased eudaimonic well-being measured several years previously. The import of this relation is amplified by the finding that sustained activity in the striatum extending ventrally and DLPFC activity both predicted levels of the stress hormone cortisol, such that more sustained activity in response to positive stimuli predicted lower levels of cortisol output (also measured on a different date). Critically, sustained activity in both these regions mediated the relationship between cortisol and well-being, which suggests a possible neurobiological mechanism through which well-being may influence health. In addition, that well-being and cortisol were measured on days other than the day of the fMRI session may reflect the strength of their associations with sustained brain activity, given that these associations appear to reflect stable interindividual differences lasting over the course of years.
The striatum is an area known to be involved in the anticipation and processing of reward, as well as reinforcement learning (Haber & Knutson, 2010; Kringelbach & Berridge, 2009). However, research has also supported a role for the striatum in coding punishment and negative affect (e.g., Roitman, Wheeler, & Carelli, 2005). The striatum receives dopaminergic projections from the ventral tegmental area, as well as efferents from the amygdala, the hippocampus, and the medial PFC, which make it well situated to assist in the assignment of salience to stimuli (Berridge & Robinson, 1998). Until now, habituation in the striatum has mostly been examined with reference to addiction processes (Di Chiara, 2002)—whereby a lack of habituation to drug cues is interpreted as part of the addiction process. However, the question of whether, in certain contexts, sustained engagement of this region to appetitive cues may also represent an adaptive and healthy process has not been widely discussed. Results from the mediation analyses presented here, showing that sustained activity in the striatum extending ventrally significantly mediated the relationship between sustained DLPFC activity and well-being, in addition to the work by Wager and others (Ochsner & Gross, 2008; Wager et al., 2008), provide initial evidence in favor of this hypothesis. For example, sustained connectivity between the striatum and distinct structures in the PFC or other limbic regions may predict whether such sustained striatal activity is adaptive (e.g., promoting well-being) or not (e.g., promoting addiction or craving processes). Our results suggest that a more nuanced view of how the striatum responds to the repeated occurrence of positive stimuli is needed.
The area of the DLPFC (Brodmann’s area 8/9) identified in the current study is not typically thought to be part of the affective network. In general, these regions of the DLPFC are more commonly found to be involved in studies of working memory (Owen, McMillan, Laird, & Bullmore, 2005) and attention (Corbetta, Patel, & Shulman, 2008). Nonetheless, this region is likely important in facilitating affect-cognition interactions: A meta-analysis has shown that the DLPFC is reliably engaged when regulating emotion (Kalisch, 2009).
Both the striatum and the DLPFC are also well positioned to influence cortisol output. The striatum has gamma-aminobutyric-acid (GABAergic) projections that extend directly to the hypothalamus (Haber & Knutson, 2010), and it could impact hypothalamic activity indirectly via its projections to the bed nucleus of the stria terminalis, the amygdala, and the hippocampus (Davis, Walker, Miles, & Grillon, 2010; Haber & Knutson, 2010). As a result, sustained engagement of the striatum may be one pathway by which cortisol output is regulated. Further work examining how the striatum responds to the repeated occurrence of positive stimuli in relation to cortisol output are required. The DLPFC, on the other hand, has no direct outputs to the hypothalamus; however, several studies have demonstrated a modulatory role for the DLPFC in cortisol output (Dedovic et al., 2009; Pruessner et al., 2010) and relations between PFC activity and diurnal cortisol output (Urry et al., 2006). Thus, the DLPFC may affect cortisol indirectly via its regulatory connections to limbic structures.
One limitation of the current study concerns the measure of hedonic positive affect. Although the PA scale of the PANAS is typically considered a measure of hedonic positive affect, recent research has challenged this notion. Specifically, a growing body of research has found that the PA scale from the PANAS correlates with trait anger and has suggested that high PA and approach motivation may not be solely related to positive valence (Harmon-Jones & Harmon-Jones, 2010). Future work should continue to address the dissociation between PA and anger and their neural correlates.
The fact that a neutral face was included on some of the trials in our study is worth noting. The addition of such a neutral face may have affected the processing of subsequent affective stimuli and the context of the experiment. However, the order of stimulus presentation was pseudorandomized across our participants and fully counterbalanced across valence conditions. This suggests that additional neutral stimuli may been averaged out. In addition, it is the case in life that affective stimuli are often followed by neutral stimuli. Therefore, for future studies, it will be worth exploring the temporal dynamics of brain activity in experimental contexts in which affective stimuli are and are not followed by neutral stimuli.
Examining the temporal dynamics of emotion and brain activity may yield a refined view of how emotion unfolds over time and unveil the individual differences relating to such variation. Our work adds additional evidence in support of this view by demonstrating that sustained striatal and DLPFC activity in response to positive stimuli—in the midst of negative and neutral stimuli—predicted well-being. Critically, sustained activity in these regions mediated the relationship between daily cortisol output and well-being. This suggests a neurobiological mechanism by which psychological constructs such as eudaimonia may ultimately improve physical health and quality of life.
Supplementary Material
Footnotes
Author Contributions
A. S. Heller, C. M. van Reekum, C. D. Ryff, and R. J. Davidson developed the study concept. C. M. van Reekum, C. D. Ryff, and R. J. Davidson contributed to the study design. Testing and data collection were performed by C. M. van Reekum, S. M. Schaefer, R. C. Lapate, and B. T. Radler. C.M. van Reekum and A. S. Heller analyzed and interpreted the data under the supervision of C. D. Ryff. and R. J. Davidson. A. S. Heller drafted the manuscript, and all authors provided critical revisions. All authors approved the final version of the manuscript for submission. A. S. Heller and C. M. van Reekum contributed equally to the work reported here and should be considered jointly as primary authors.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Although there are several timescales that could be used to examine the temporal dynamics of brain activity, in this report, the term “sustained” reflects the ability to engage brain activity across trials.
The research on which this report is based acknowledges the use of the Extended Multimodal Face Database and associated documentation. Further details about this software can be found in Messer, Matas, Kittler, Luettin, and Maitre (1999; http://www.ee.surrey.ac.uk/Research/VSSP/xm2vtsdb).
The neutral face was included for alternative analyses designed to examine affective coloring (i.e., the effect of a previous emotional stimulus on the perception of a subsequent neutral one). However, the effect of this stimulus was not the goal of the current analysis and is therefore not further mentioned here.
There was not a significant correlation between well-being and cortisol, B = −0.02, t(44) = −1.21, p = 0.23, although the association was in the expected direction.
References
- Ross D, editor. Aristotle. (350 B.C./1925) Nicomachean ethics. New York, NY: Oxford University Press; [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychological Bulletin. 2012;138:655–691. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Christensen L, Mendoza JL. A method of assessing change in a single subject: An alteration of the RC index. Behavioral Therapy. 1986;17:305–308. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Tiemeier H. Salivary cortisol is related to atherosclerosis of carotid arteries. Journal of Clinical Endocrinology & Metabolism. 2008;93:3741–3747. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Diener E, Lucas RE. Personality and subjective well-being. In: Kahneman D, Diener E, Schwarz N, editors. Well-being: The foundations of hedonic psychology. New York, NY: Russell Sage Foundation; 1999. pp. 213–229. [Google Scholar]
- Dockray S, Steptoe A. Positive affect and psychobiological processes. Neuroscience & Biobehavioral Reviews. 2010;35:69–75. doi: 10.1016/j.neubiorev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C. On the relationship of trait PANAS positive activation and trait anger: Evidence of a suppressor relationship. Journal of Research in Personality. 2010;44:120–123. [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. American Journal of Psychiatry. 2013;170:197–206. doi: 10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, USA. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences. 2009;13:479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Technical Report A-6) Gainesville: University of Florida; 2005. [Google Scholar]
- Messer K, Matas J, Kittler J, Luettin J, Maitre G. XM2VTSbd: The Extended M2VTS Database. Proceedings of the 2nd International Conference on Audio and Video-Based Biometric Personal Verification. New York, NY: Springer Verlag; 1999. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Lupien SJ. Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter award winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology. 1989;57:1069–1081. [Google Scholar]
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. Journal of Personality and Social Psychology. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer BH, Dienberg Love G. Positive health: Connecting well-being with biology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359:1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences, USA. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelzyk F, Hermes M, Naumann E, Oitzl M, Walter C, Busch HP, Schächinger H. Tune it down to live it up? Rapid, nongenomic effects of cortisol on the human brain. Journal of Neuroscience. 2012;32:616–625. doi: 10.1523/JNEUROSCI.2384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Davidson RJ. Making a life worth living: Neural correlates of well-being. Psychological Science. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1 Suppl.):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.