Abstract
Background
The prevalence of kidney stone disease is rising along with increasing rates of obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome.
Objective
To investigate the associations among the presence and severity of T2DM, glycemic control and insulin resistance with kidney stone disease.
Design, Setting, and Participants
We performed a cross-sectional analysis of all adult participants in the 2007–2010 National Health and Nutrition Examination Survey (NHANES). A history of kidney stone disease was obtained by self-report. T2DM was defined by: self-reported history, T2DM-related medication usage, and reported diabetic comorbidity. Insulin resistance was estimated using fasting plasma insulin (FPI) levels and the homeostasis model assessment of insulin resistance (HOMA-IR) definition. We classified glycemic control using HbA1c and fasting plasma glucose levels (FPG).
Outcome measurements and statistical analysis
Odds ratios (OR) for kidney stone disease were calculated for each individual measure of T2DM severity. Logistic regression models were fitted adjusting for age, sex, race/ethnicity, smoking history, and Quételet’s (body mass) index (BMI) (model A) as well as laboratory values and components of metabolic syndrome (model B).
Results and Limitations
Correlates of kidney stone disease included a self-reported history of T2DM (OR 2.44, 95% confidence interval [CI] 1.84–3.25) and history of insulin use (OR 3.31, CI 2.02-5.45). Persons with FPG 100–126 and >126 mg/dL had increased odds of kidney stone disease, OR 1.28 (CI 0.95–1.72) and OR 2.29 (CI 1.68-3.12), respectively. Corresponding results for persons with HgbA1c 5.7-6.4% and ≥6.5% were ORs 1.68 (CI 1.17–2.42) and OR 2.82 (CI 1.98–4.02), respectively. When adjusting for patient factors, a history of T2DM, the use of insulin, FPI and HgbA1c remained significantly associated with kidney stone disease. The cross-sectional design limits causal inference.
Conclusions
Among persons with T2DM, more severe disease is associated with a heightened risk of kidney stones.
Keywords: diabetes mellitus, glycemic control, insulin resistance, kidney stone disease
Introduction
Kidney stone disease is a painful and costly disease. Hospitalizations, interventions and work-days lost for kidney stones impose a major economic burden, with total annual medical expenditures in the United States exceeding $2.1 billion in 2000 [1]. The lifetime prevalence of kidney stones is increasing, with data from the most recent National Health and Nutrition Examination Survey (NHANES, 2007–2010) reporting an 8.8% population prevalence, a substantial increase from the 5.2% prevalence reported in a prior NHANES cohort [2]. U.S population studies have similarly documented a rise in the prevalence of obesity, metabolic syndrome and type 2 diabetes mellitus (T2DM)[3, 4]. Recent epidemiologic studies have demonstrated a significant association between dietary and lifestyle factors and kidney stone disease. Analyses from the Health Professionals Follow-up Study and the Nurses’ Health Study I and II identified associations among obesity, T2DM and incident kidney stone disease [5, 6]. Pathophysiologic explanations for the increased risk of kidney stones in diabetics have largely focused on insulin resistance. Insulin resistance is associated with derangements in renal ammonium production, increased urinary acidification, hypocitraturia, and hypercalciuria, all of which can contribute to the development of uric acid and calcium stones [7, 8, 9, 10].
Type 2 diabetes mellitus is a leading cause of end-organ disease and death in the United States. There is a direct relation between the degree of hyperglycemia and the risk of complications of T2DM over time. Among persons with T2DM, poorer glycemic control and more pronounced insulin resistance are associated with the risk of microvascular disease, peripheral vascular disease, amputations, myocardial infarction, stroke, heart failure, and all-cause mortality [11].
Despite compelling epidemiologic data supporting the association of diabetes and kidney stones, little is known about how diabetic severity might modify the risk of kidney stone disease. In this study, we investigated associations among the presence and severity of T2DM, glycemic control and insulin resistance with kidney stone disease in a nationally representative sample. We hypothesized that more severe T2DM would be associated with higher odds of kidney stone disease.
Methods
Study population
NHANES is a program of studies conducted by the National Center for Health Statistics, which is a part of the Centers for Disease Control and Prevention (CDC). These surveys are used to assess the health and nutritional status of adults and children in the U.S. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical and physiological measurements, as well as laboratory tests. The NHANES 2007–2010 survey is the most recent collection, which includes kidney stone-related data.
Participant health examinations, including biophysical measurements and blood and urine collections were conducted in the NHANES mobile examination center. Questionnaires were administered to NHANES participants both at home and in the mobile trailer. The current study included participants in NHANES 2007–2010 who were aged 20 years or older, underwent a health examination, and responded to the question “Have you ever had a kidney stone?”
Independent Variables – Diabetes and Severity Definitions
We used several methods to define the presence of T2DM: a self-reported history of DM, use of glucose-lowering medications (insulin or oral hypoglycemics), and self-reported diabetic comorbidities in the form of retinopathy. Insulin resistance was estimated using fasting plasma insulin levels and the homeostasis model assessment of insulin resistance (HOMA-IR) definition (fasting insulin (mg/dL) multiplied by fasting glucose (mg/dL) divided by a correction factor of 405). HOMA-IR is a method widely used in epidemiologic studies to quantify insulin resistance and beta-cell function. For fasting insulin and HOMA-IR, these measures were grouped into tertiles due to the absence of clinically relevant cut-off values and their skewed distributions. We categorized glycemic control using HbA1c and fasting plasma glucose levels. Participants were categorized as follows (<100 mg/dL, 100–126 mg/dL, ≥126 mg/dL) and HbA1c (<5.7%, 5.7–6.4%, ≥6.5%), according to cutoffs recommended by the American Diabetes Association.
Dependent Variable – Prevalent Kidney Stones
The primary outcome for our analysis was the participant response to the question “Have you ever had kidney stones?” during the medical questionnaire.
Other clinical characteristics
Age, sex, race/ethnicity, highest level of education, and household income were assessed by questionnaire. Individuals were grouped by age: 20–39 years, 40–59 years, and 60 years or older. We used the racial/ethnic group variable reported by NHANES, classified as “non-Hispanic white”, “non-Hispanic black”, “Mexican American”, “other Hispanic”, and “other/multiracial” and joined responses from the categories of “Mexican-American” and “Other Hispanic” into a single Hispanic category according to the analytic guidelines. We used household income as a measure of socioeconomic status, and re-categorized the income strata as $0–19,999, $20,000–34,999, $35,000–74,999, and ≥$75 000. Quételet’s (body mass) index (BMI) was calculated as weight in kilograms divided by height in meters squared, and categorized using the World Health Organization (WHO) cutoffs, as lean or normal weight (<24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Three or more consecutive blood pressure measurements were taken during the physical examination, with mean values of systolic and diastolic blood pressure used for our analysis. Of the many laboratory parameters measured in NHANES, we considered serum uric acid, calcium, high-density lipoprotein, and triglyceride concentrations as laboratory correlates of stone risk and/or metabolic syndrome/T2DM.
Statistical methods
All statistical analyses were performed using SAS v9.3 (SAS Institute Inc, Cary, North Carolina) and incorporated the recommended NHANES sample weights, strata and cluster design variables. Graphics were constructed using R v2.15. We utilized the sub-sample fasting sampling weights in the glucose and insulin laboratory collection component in order to appropriately account for NHANES’ complex survey structure and produce estimates that are representative of the total, non-institutionalized civilian US population. In order to produce estimates with greater statistical reliability, we combined two 2-year cycles of continuous NHANES (2007–2008 and 2009–2010).
We calculated the odds of kidney stone disease associated with each demographic factor and individually with each self-reported and calculated measure of diabetic severity. We then constructed two multivariable regression models to analyze each diabetic severity measure. In model A, we adjusted for age, sex, race/ethnicity, smoking history, and BMI. In model B we additionally included metabolic stone risk factors (serum uric acid and calcium levels), and selected metabolic syndrome traits (HDL <40mg/dL, triglyceride >150 mg/dL, and SBP>140 mmHg or DBP >90 mmHg). All statistical tests were 2-sided and a P value <0.05 was considered statistically significant.
Results
The unweighted patient sample included 12,153 participants; 12,110 (99.6%) responded to the question regarding history of kidney stone disease. The weighted population prevalence of T2DM (by self-report) was 8.6% ([CI], 8.1–9.1%).
Prevalence and Correlates of Kidney Stone Disease
The weighted overall prevalence of stone disease was 8.8% (95% confidence interval [CI], 8.1–9.5%). Table 1 shows demographic and other clinical correlates of kidney stone disease. In a series of crude and multivariable-adjusted regression models, we investigated the associations of self-reported and laboratory measures of diabetic severity with kidney stone disease (Table 2).
Table 1.
Patient factors and the odds of kidney stone disease.
| Demographics | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
|
| ||
| Age | ||
| 20–39 | 1.00 (referent) | 1.00 (referent) |
| 40–59 | 1.84 (1.25–2.71) | 1.88 (1.22–2.90) |
| ≥60 | 2.94 (2.05–4.23) | 2.70 (1.82–4.01) |
|
|
||
| Gender | ||
| Female | 1.00 (referent) | 1.00 (referent) |
| Male | 1.67 (1.30–2.14) | 1.78 (1.35–2.35) |
|
| ||
| Race | ||
| White, Non-Hispanic | 1.00 (referent) | 1.00 (referent) |
| Black, Non-Hispanic | 0.42 (0.27–0.65) | 0.39 (0.24–0.63) |
| Hispanic | 0.60 (0.46–0.74) | 0.56 (0.41–0.77) |
| Other/multiracial | 0.74 (0.48–1.15) | 0.99 (0.64–1.52) |
|
|
||
| Household Income | ||
| > $75,000 | 1.00 (referent) | 1.00 (referent) |
| $35,000 to $74,999 | 1.39 (0.92–2.08) | 1.50 (0.96–2.34) |
| $20,000 to $34,999 | 1.58 (1.03–2.43) | 1.77 (1.07–2.91) |
| $0 to $19,999 | 1.59 (1.07–2.36) | 1.95 (1.24–3.09) |
|
| ||
| Education | ||
| College graduate | 1.00 (referent) | 1.00 (referent) |
| Some College | 1.39 (1.05–1.83) | 1.41 (1.02–1.94) |
| High School/GED | 1.34 (1.00–1.79) | 1.25 (0.89–1.76) |
| 9ththough 11th | 1.43 (0.96–2.13) | 1.44 (0.96–2.18) |
| Less than 9thgrade | 1.26 (0.78–2.05) | 1.11 (0.64–1.94) |
|
| ||
| BMI | ||
| Normal | 1.00 (referent) | 1.00 (referent) |
| Overweight | 1.36 (0.98–1.89) | 1.14 (0.83–1.58) |
| Obese | 1.86 (1.38–2.51) | 1.67 (1.27–2.18) |
|
| ||
| Positive Smoking History | 0.98 (0.77–1.23) | 0.72 (0.55–0.94) |
Table 2.
Measures of T2DM severity and the odds of kidney stone disease.
| Unadjusted | Multivariable Model A | Multivariable Model B | |
|---|---|---|---|
| Diabetes Parameters | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
|
| |||
| Insulin use | 3.31 (2.02–5.45) | 2.25 (1.23–3.97) | 2.31 (1.34–3.97) |
|
| |||
| Oral hypoglycemic use | 1.03 (0.54–1.95) | 1.04 (0.54–2.02) | 1.07 (0.52–2.20) |
|
| |||
| Retinopathy | 1.56 (0.71–3.41) | 1.51 (0.69–3.33) | 1.43 (0.70–2.92) |
|
| |||
| Self-reported history of DM2 | 2.44 (1.84–3.25) | 1.76 (1.33–2.32) | 1.63 (1.23–2.16) |
|
| |||
| Fasting plasma glucose (mg/dL) | |||
| Normal (<100) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Prediabetes (100–126) | 1.28 (0.95–1.72) | 0.88 (0.66–1.18) | 0.88 (0.64–1.22) |
| Diabetes (>126) | 2.29 (1.68–3.12) | 1.28 (0.93–1.76) | 1.24 (0.91–1.70) |
|
| |||
| Fasting plasma insulin (uU/mL) | |||
| Tertile 1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 | 1.16 (0.82–1.64) | 1.07 (0.74–1.53) | 0.95 (0.65–1.39) |
| Tertile 3 | 1.76 (1.41–2.19) | 1.42 (1.05–1.91) | 1.28 (0.93–1.77) |
|
| |||
| HbA1c (%) | |||
| Normal (<5.7) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Prediabetes (5.7–6.5) | 1.68 (1.17–2.42) | 1.33 (0.94–1.89) | 1.34 (0.92–1.94) |
| Diabetes (>6.5) | 2.82 (1.98–4.02) | 2.02 (1.41–2.89) | 1.91 (1.32–2.76) |
|
| |||
| HOMA-IR (FPI*FPG/405) | |||
| Tertile 1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 | 1.51 (1.07–2.15) | 1.36 (0.93–1.99) | 1.23 (0.84–1.78) |
| Tertile 3 | 2.11 (1.64–2.71) | 1.65 (1.20–2.29) | 1.50 (1.09–2.07) |
Model A: Adjusted for patient factors; age, gender, race, smoking history, and BMI
Model B: Adjusted for patients factors in Model A as well as for presence of HTNhypertension (BP>130/85mmHg), triglycerides >150 mg/DL, HDL <40 mg/DL, and serum measures of calcium and uric acid
For continuous variables: the OR for FPG, Insulin, and HOMA-IR is calculated per 10-unit change; the OR for HbA1c is calculated for 1-unit change
Unadjusted predictors of kidney stone disease included a self-reported history of diabetes (OR 2.44, [CI 1.84–3.25]) and a history of insulin use (OR 3.31, [CI 2.02–5.45]). Measures of both glycemic control (FPG and HbA1c) and insulin resistance (FPI and HOMA-IR) were significantly associated with a history of kidney stone disease when analyzed as both continuous variables and when categorized according to established clinical cutoffs. Participants with FPG 100 to 126 mg/dL and ≥126 mg/dL had increased odds of kidney stone disease, OR 1.28 [CI 0.95-1.72] and OR 2.29 [CI 1.68–3.12], respectively. Participants with HgbA1c values of 5.7–6.4% and ≥6.5% also had increased odds of kidney stone disease, (ORs of 1.68 [CI, 1.17–2.42] and 2.82 [1.98–4.02]), respectively. When fasting plasma insulin levels were evaluated by tertiles, there appeared to be a graded relation that was only statistically significant for patients in the top tertile. Participants with FPI in the highest tertile had almost twice the odds of kidney stones (FPI Tertile 3 OR 1.76 [CI, 1.41–2.19] compared with those in the lowest tertile. The unadjusted analysis of HOMA-IR demonstrated a similar graded effect; those persons with the highest elevations in HOMA-IR had over twice the odds of kidney stone disease (HOMA-IR Tertile 3 OR 2.11 [CI,1.64–2.71]). (Figure 1)
Figure 1.
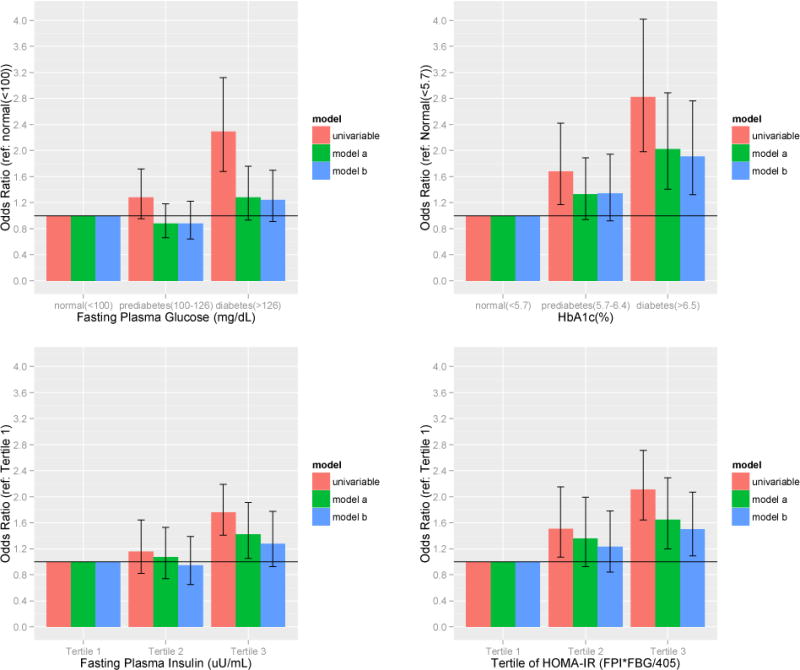
Odds ratios of kidney stone disease by biochemical measures of T2DM severity
In model A, when adjusting for patient factors (age, sex, race/ethnicity, BMI and smoking history), a history of DM, the use of insulin, HgbA1c, and the top tertile levels of FPI and HOMA-IR remained significantly associated with kidney stone disease. In model B, further adjustment for hypertension, dyslipidemia, and relative hyperuricemia and hypercalcemia did not materially change the association among individual measures of diabetes severity and the odds of kidney stone disease.
Discussion
In this study, we found significant associations among individual measures of glycemic control (HbA1c and fasting plasma glucose), insulin resistance (fasting plasma insulin and HOMA-IR) and a history of kidney stones. We found the highest odds of kidney stones in persons with self-reported insulin use, HbA1c >6.5% and the highest tertiles of fasting insulin levels and HOMA-IR. Even after adjustment for various patient confounders these associations were largely preserved.
Of our measures of T2DM severity, HbA1c bore the strongest association with the odds of kidney stone disease. The more robust association of HbA1c over fasting glucose level is noteworthy (and expected) as HbA1c is a marker of longer-term glycemic control; one would anticipate considerable misclassification of T2DM severity by a single fasting plasma glucose.
Poor glycemic control is associated with numerous adverse health effects [12]; herein, we suggest that poorer glycemic control and more pronounced insulin resistance are associated with a higher odds of kidney stone disease as well. We estimate that among the approximate 3.1 million episodes of kidney stones in the US, 43% of cases, or 1.36 million may be attributable to T2DM. Of those, 680,000 may be attributed to poor glycemic control (HbA1c>6.5%). Given the rising rate of T2DM in the US and abroad, the national and global impact of T2DM on kidney stone disease is likely to intensify. A recent study by Shaw et al., projects a 20–70% increase in the prevalence of T2DM among developed and developing nations over the next 20 years, respectively [13].
The relation between T2DM and kidney stone disease has been largely explained by the effects of insulin resistance on urine pH and renal handling of ammonium and calcium. We utilized HOMA-IR as an adjunctive measure of insulin resistance in our study. HOMA-IR has been previously validated in patients with poorly controlled diabetes while on insulin therapy [14]. Studies suggest that HOMA-IR is a reliable parameter for the evaluation of insulin sensitivity in patients with T2DM.
Our analysis suggests that glycemic control is also associated with the pathogenesis of stone disease. Hyperglycemia and its resultant glycosuria have been implicated in altered renal handling of calcium, phosphorus and uric acid. Studies have demonstrated an increase in urinary calcium and phosphorus excretion in patients with T2DM [15, 16]. Recent investigations have also highlighted that stone formers with T2DM have increased urinary oxalate excretion [17, 18]. Both hyperoxaluria and hypercalciuria as a result of poor glycemic control may therefore lead to the formation of calcium oxalate stones. Additionally, uric acid has been found as the main component of stones in a significantly higher proportion of patients with T2DM [19]. Studies have shown that uric acid excretion is increased in the setting of hyperglycemia and glycosuria, a mechanism which may account for the increased formation of uric acid stones among diabetics [20, 21]. (Figure 2)
Figure 2.
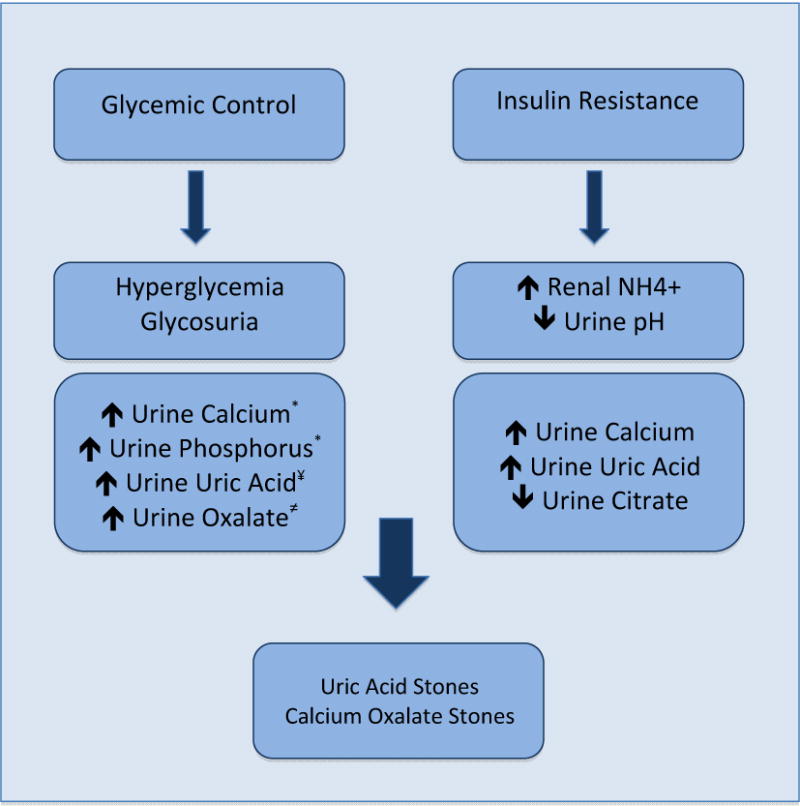
Mechanisms of Stone Formation in Diabetics [15–16*, 17≠, 20–21¥] NH4+ = ammonium.
Previous studies have evaluated the effect of metabolic syndrome [22] and T2DM on kidney stone disease. Kabeya and colleagues [23] investigated the role of metabolic syndrome traits, insulin resistance and glycemic control on kidney stone risk among a small cohort of patients presenting for a health check at their central hospital in Japan. This smaller study did not show any association of insulin resistance parameters with stone risk. Our examination of T2DM severity and metabolic syndrome traits in a nationally-representative sample of over 1,080 participants with kidney stone disease demonstrates a significant association among glycemic control as well as insulin resistance with kidney stone disease. Further, we were able to delineate a graded association among these markers of T2DM severity and kidney stone disease.
Strengths of this study include the use of data with broad external validity and national relevance. Also, the availability of laboratory measures of fasting plasma glucose, fasting plasma insulin, and HbA1c allowed us to assess the associations among these measures and kidney stone disease that would have been impossible using only data from questionnaires. This study also has important limitations; NHANES interview-questionnaire data are based on patient self-report and therefore subject to non-sampling errors such as recall and misclassification bias. For example, it is likely that some participants reported a history of DM due to type 1 DM and would be included in our analysis of the association of self-reported DM and kidney stones beDM. Additionally, due to the specific NHANES questionnaire format; we do not have details regarding the clinical severity or type of stone disease. While it is known there are many additional influences on stone disease including medications and dietary factors, we did not adjust for these heterogeneous components in our multivariable models. An additional limitation is its cross-sectional nature, allowing us to study only prevalent rather than incident stone disease. Prior studies by Taylor et al [24] and Curhan et al [25], have focused on incident stone disease in evaluating associations among dietary factors and kidney stones. However, while having stones may affect dietary habits (“reverse causality”), it is less plausible that it would create or worsen the severity of T2DM. Consequently, the use of prevalent rather than incident stone disease in our analysis is likely less critical. A randomized trial of different degrees of glycemic control would be necessary to tease out the issue of causality. However, a study of this nature would be quite impractical and potentially objectionable.
Kidney stones are associated with large direct and indirect costs to our healthcare system. The ability to identify and target underlying risk factors for stones can aid primary practitioners, nephrologists, and urologists in decreasing the incidence of the disease. In light of the mounting ‘epidemic’ of T2DM, kidney stone disease may become of increasing clinical concern, highlighting the need for further preventive measures.
Conclusions
Our study suggests that the severity of T2DM, as measured by glycemic control and insulin resistance, is an important risk factor for kidney stone disease. Future studies comparing treatment strategies in T2DM should aim to include kidney stones as an outcome of interest, to test the hypotheses generated here.
Acknowledgments
Aviva E. Weinberg, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. Dr. Leppert is supported by Award Number DK089086 from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
Footnotes
Study concept and design: Weinberg, Leppert, Chertow.
Acquisition of data: Weinberg.
Analysis and interpretation of data: Weinberg, Leppert.
Drafting of the manuscript: Weinberg.
Critical revision of the manuscript: Weinberg, Leppert, Patel, Chertow.
Statistical analysis: Weinberg, Leppert.
Obtaining funding: n/a.
Supervision: Leppert.
References
- 1.Lotan Y. Economics and cost of care of stone disease. Adv Chronic Kidney Dis. 2009 Jan;16(1):5–10. doi: 10.1053/j.ackd.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Smith AC, Hanley JM, Saigal CS. Prevalence of Kidney Stones in the United States. European Urology. 2012 Jul;62(1):160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011 Jun 22;305(24):2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010 Jan 20;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney international. 2005;68(3):1230–5. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005 Jan 26;293(4):455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 7.Sakhaee K, Adams-Huet B, Moe OW, Pak CYC. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002 Sep;62(3):971–9. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamm LL. Renal handling of citrate. Kidney Int. 1990 Oct;38(4):728–35. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 9.Pak CYC, Sakhaee K, Moe O, Preminger GM, Poindexter JR, Peterson RD, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003 Mar;61(3):523–7. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 10.Cameron MA. Urine Composition in Type 2 Diabetes: Predisposition to Uric Acid Nephrolithiasis. Journal of the American Society of Nephrology. 2006 May 1;17(5):1422–8. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 11.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EAM, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009 Jan 20;53(3):298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Woo V, Shestakova MV, Ørskov C, Ceriello A. Targets and tactics: the relative importance of HbA, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int J Clin Pract. 2008 Dec;62(12):1935–42. doi: 10.1111/j.1742-1241.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010 Jan;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Okita K, Iwahashi H, Kozawa J, Okauchi Y, Funahashi T, Imagawa A, et al. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J. 2012 Nov 10; doi: 10.1507/endocrj.ej12-0320. [DOI] [PubMed] [Google Scholar]
- 15.Nagasaka S, Murakami T, Uchikawa T, Ishikawa SE, Saito T. Effect of glycemic control on calcium and phosphorus handling and parathyroid hormone level in patients with non-insulin-dependent diabetes mellitus. Endocr J. 1995 Jun;42(3):377–83. doi: 10.1507/endocrj.42.377. [DOI] [PubMed] [Google Scholar]
- 16.Thalassinos NC, Hadjiyanni P, Tzanela M, Alevizaki C, Philokiprou D. Calcium metabolism in diabetes mellitus: effect of improved blood glucose control. Diabet Med. 1993 May;10(4):341–4. doi: 10.1111/j.1464-5491.1993.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 17.Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010 Jun;183(6):2244–8. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Lange JN, Wood KD, Knight J, Assimos DG, Holmes RP. Glyoxal formation and its role in endogenous oxalate synthesis. Adv Urol. 2012;2012:819202. doi: 10.1155/2012/819202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005 Feb;20(2):468–9. doi: 10.1093/ndt/gfh594. [DOI] [PubMed] [Google Scholar]
- 20.Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986 Nov;62(733):1001–6. doi: 10.1136/pgmj.62.733.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotfredsen A, McNair P, Christiansen C, Transbøl I. Renal hypouricaemia in insulin treated diabetes mellitus. Clin Chim Acta. 1982 Apr 23;120(3):355–61. doi: 10.1016/0009-8981(82)90376-x. [DOI] [PubMed] [Google Scholar]
- 22.West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic Syndrome and Self-Reported History of Kidney Stones: The National Health and Nutrition Examination Survey (NHANES III) 1988–1994. American Journal of Kidney Diseases. 2008 May;51(5):741–7. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Kabeya Y, Kato K, Tomita M, Katsuki T, Oikawa Y, Shimada A, et al. Associations of insulin resistance and glycemic control with the risk of kidney stones. Intern Med. 2012;51(7):699–705. doi: 10.2169/internalmedicine.51.6426. [DOI] [PubMed] [Google Scholar]
- 24.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004 Dec;15(12):3225–32. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004 Apr 26;164(8):885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]


